- *Corresponding Author:
- C. Chinnarasu
Department of Chemistry and Food Technology, K. S. Rangasamy College of Technology, Tiruchengode, Namakkal, Tamil Nadu 637215, India
E-mail: chandrasekar.chinnarasu@gmail.com
| Date of Received | 05 November 2020 |
| Date of Revision | 07 May 2022 |
| Date of Acceptance | 10 February 2023 |
| Indian J Pharm Sci 2023;85(1):267-272 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Ultrasonic assisted extraction using green solvents such as ethanol, water and hydro-ethanol (50/50) have been used to evaluate the separation of bioactive compounds from Annona squamosa (Custard apple) leaves. The investigation of Fourier-transform infrared spectroscopy is being used to characterize bioactive substances. The hydro-ethanolic extract is rich in carboxylic acids, phytosterols and sugars such as arabinose, xylose and galactose, according to the bioactive substances screening results. When compared to other solvents such as water and ethanol, the binary mixture of hydro-ethanolic extract of leaves yielded a higher amount of extraction. Furthermore, the antioxidant activity of the extract was evaluated by 2, 2-Diphenyl -1- picrylhydrazyl radical assay. The hydro-ethanolic (50/50) extract of leaves exceeded other solvent extracts in terms of antioxidant activity.
Keywords
Custard apple leaves, green solvents, ultrasound, Fourier-transform infrared spectroscopy, carboxylic acids, sugars, antioxidant activity
Custard apple (CA) tree (Annona squamosa Linn.) is a small tree or shrub of the Annonaceae family, is widely cultivated in many areas of the world including India, America, Brazil and West Indies mainly for edible fruits[1-3]. Leaves of the CA tree are rich in secondary metabolites such as glycoside, alkaloids, sponins, flavonoids, tannis, carbohydrates, proteins, phenolic compounds, phytosterols, amino acids, alkyl ketones and sesquiterpenes[1-6]. Bioactive compounds from CA leaves have been associated with a range of diverse functional roles including antioxidant activity, antidiabetics, hepatoprotective, cyotoxic activity, genetoxicity and anti-tumor activity[6-9].
Extraction is one of the most used processes to recover bioactive compounds from plant species. Green extraction is an inevitable trend in phytochemicals research, concept of green extraction has been proposed by many researchers[10-15]. Green extractions mean utilization of greener solvents, low degradation of chemical compounds, minimizing the energy and waste and eliminate the environmental pollution[11-15]. Ultrasonic Assisted Extraction (UAE) offers several advantages over the conventional extraction methods, such as higher products yields and selectivity of bioactive compounds, in addition to less time and energy spent on extraction, easier operation and cost effective when compared to other modern extraction techniques[14-18].
Fourier Transform Infrared (FT-IR) spectroscopy is an analytical technique that provides information about the molecular vibration modes of the molecule structure and dynamics of complex biomolecules[19-22]. In addition, FT-IR spectroscopy is emerging as a powerful alternative approach due to its high value of sensitivity, no need reagent to do analysis, a high reproducibility, reliable low operation cost and requires a small quantity of the samples[22,23].
To the best of our knowledge, UAE of CA leaves using green solvents (water, ethanol and ethanol/ water (50/50)) haven’t been investigated so for. The aim of the present research work, UAE technique is used for the extraction of chemical constituents from leaves of CA. Various green solvents (water, ethanol and water-ethanol) are optimized for better extraction of phytochemicals from leaves of CA. FT-IR is used to analysis of chemical compounds of extract of CA leaves and then the antioxidant activity assay with performed with 2, 2-Diphenyl- 1-picrylhydrazyl (DPPH) using Ultra Violet-Visible (UV-Vis) spectroscopy.
Materials and Methods
Collection of plant leaves:
Leaves of CA collected in polyethylene bags from village Molayanoor (N 11.9278°; E 78.3359°) Dharmapuri, Tamil Nadu, India. Fig. 1 shows the image of CA tree. The leaves identified according to the details given in the reported literature[5]. The collected leaves were washed thoroughly with running tap water and kept at an open place for drying at room temperature. Then, the leaves were made into the fine powder using Samsung 550 W grinder. The powder samples were put into an airtight brown glass bottle before extraction into green solvents.
Solvents and reagents:
Ethanol (HPLC grade) and DPPH reagent were purchased from Sigma-Aldrich (Bengaluru, India). Double distilled Milli-Q grade water was used in the experiments.
UAE:
Phytochemicals separated from leaves of CA using UAE carried out according to given literature with slight modification[10]. 5 gm moisture-free plant leaves powder was extracted at room temperature and 50° using 50 ml of different green solvents such as water, ethanol and hydro-ethanol (50:50 v) for 30 min of extraction time. The obtained extract was centrifuged using Thermo scientific centrifuge (ST 40R, USA) at 10 min. Then the supernatant was separated and stored at brown glass bottle at 4°.
Recovery percentage of extraction:
UAE of CA leaves extract yield (%) were calculated by the ratio between weight of the extract (Mextract) in without solvent and the weight of the sample (Msample) calculated by the equation 1.
X0=Mextract/Msample×100 (1)
FT-IR analysis:
Functional groups of the plant extract were identified by FT-IR with Attenuated Total Reflectance (ATR) analysis (Make: Agilent; model: Cary 660 Series). The spectra were recorded in the ATR crystal mode with angle of incidence were 45°. 0.5 μl CA leaves extract was loaded on the diamond crystal of the ATR device of the FT-IR spectrometer. Prior to the analysis, the ATR crystal was cleaned with water, wiped and dried out. A background was recorded with a clean crystal before the start of the measurements and before every new sample. FT-IR spectra of CA leaves extract were recorded between 4000 and 500 cm-1 at a resolution of 2 cm-1. The FT-IR measurements were carried out at room temperature (20°). All the spectra were in absorbance units and no ATR alteration was applied.
Antioxidant activity of plant extracts using DPPH:
The antioxidant activity of the extract of CA leaves was evaluated using DPPH radical assay using our previous study with slight modification[11-13]. CA leaf extracts have been added to 3.9 ml of a 6×10-5 mol/l DPPH in ethanol solution at different concentrations. DPPH absorbance was read using a UV-Vis spectrophotometer at 515 nm at 0 min and every 2 min until the reaction attained steady state. A calibration curve determined by linear regression with Equation 1 has been used to calculate CDPPH (DPPH concentration) in the reaction conditions.
Absorbance=12 709.CDPPH+0.002 (1)
DPPH percentage remaining was calculated using Equation 2.
Percentage DPPH remaining=CDPPHt/CDPPHo×100 (2)
Efficient concentration providing 50 % inhibition (EC50) was calculated graphically using a non-linear fitting curve by plotting the sample concentration vs. the percentage DPPH remaining on steady state. Antioxidant activity was expressed as Antioxidant Activity Index (AAI) was calculated considering the final concentration of DPPH and the EC50 of the tested compound in the reaction as follows Equation (3);
AAI=Final concentration of DPPH (μg/ml)/EC50 (μg/ml) (3)
DPPH final concentration was calculated respect to the concentration of DPPH in the reaction medium. The antioxidant activity is showed very strong when AAI≥2.0, strong antioxidant activity when AAI is between 1.0 and 2.0, and moderate antioxidant activity when AAI is between 0.5 and 1.0 and poor when AAI≤0.5[24]. The DPPH assays were calculated in triplicate.
Results and Discussion
In this study UAE of CA leaves using different green solvents such as water, ethanol and ethanol:water (50:50) was compared. UAE is an efficient technique that is widely used in separation of bioactive compounds from plant species, because of inexpensive, high extraction yield and simple operating procedure[10,16]. The selection of green solvents is a significant aspect that affects the separation of bioactive compounds from plant species without negative impact of human health and environment. The solvents are chosen based on “Generally Recognized As Safe (GRAS)”. The extraction yields of the CA leaf extracts are shown in Table 1. The highest extraction yield (21.72 %) was obtained with hydro-ethanol mixture (1:1) when compared to water and ethanol extract. The use of binary mixture of an ethanol and water, which improve the extraction efficiency, due to organic solvents enhances the solubility of the chemical substances and water increases the desorption of chemical substances[11]. Temperature is one of the significant factors in UAE of plant materials. As can be shown in Table 1, the highest extraction yield was obtained with three different solvents (hydroethanol, water and ethanol) at 50° when compared to room temperature. Higher temperature leads to an increase of the extraction yield due to increase material porosity, higher salvation and mass transfer when extraction temperature increased[25,26].
| Solvent | Temperature | Yield % | Antioxidant activity (µg DPPH/µg Extract) |
|---|---|---|---|
| H2O | 50 | 16.42 | 0.74 |
| C2H5OH | 50 | 11.47 | 1.06 |
| H2O-C2H5OH (1:1) | 50 | 21.72 | 1.47 |
| H2O | room temperature | 15.23 | 0.72 |
| C2H5OH | room temperature | 10.52 | 0.98 |
| H2O-C2H5OH (1:1) | room temperature | 20.16 | 1.35 |
Table 1: Extraction Yield and Antioxidant Activity from Annona squamosa Leaves using Ultra Sound Assisted Extraction Methods
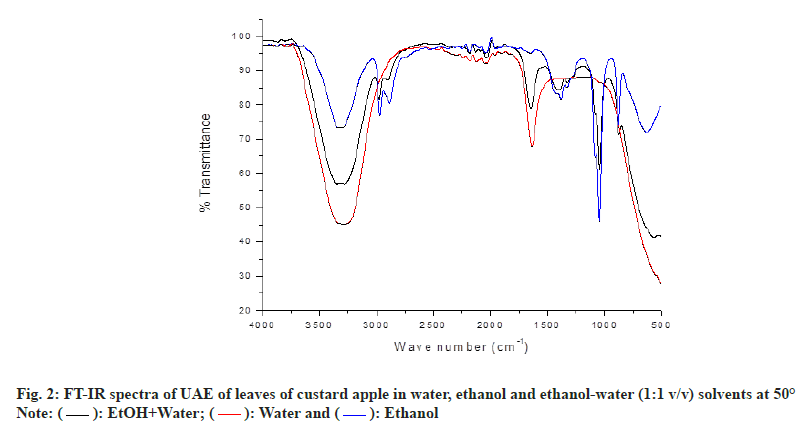
FT-IR is a dominant, versatile and non-destructive analytical technique used for characterization of chemical compounds and can give primary information on the molecular structure of organic substance in plant extract[19,27]. FT-IR spectroscopy is normally used for the tentative identification of functional groups. The functional characteristics in the extracts of Annona squamosa leaves with water, ethanol and hydro-ethanolic mixture (50:50) (v/v) are presented in fig. 2. This exposed the presence of a variety of characteristic functional groups through the absorption bands of the bioactive compounds in leaves of CA. The infrared spectra observed in the Annona squamosa leaf extract fall within the range between 4000 and 500 cm-1. In the spectra obtained for water extract of CA leaves, the broad and medium peak at 3291.97 cm-1 suggested the presence of O-H stretching vibration of carboxylic acids. The medium, sharp peak at 1636.61 cm-1 is assigned to the O-H bond related to water molecule[28].
As can be seen in the fig. 2, FT-IR spectrum of the ethanol extract of CA leaves the observed weak, broad peak at 3308.54 cm-1 might be due to the presence of O-H stretching vibration belonged to carboxylic acids[28]. The weak, sharp band at 2971.49 and 2886.37 cm-1 belongs to C-H asymmetric vibration of methoxy group and C-H stretching vibration of methylene group, respectively[29]. The weak, sharp band at 1382.03 cm-1 indicates the C-H deformation vibration of secondary alcohols (phytosterols). The medium, sharp peak found at 1045.16 cm-1 assigned to the aromatic C-H in-plane deformation vibrations of sugars such as arabinose, xylose and galactose. The observed weak, sharp peak at 879.82 cm-1 can be attributed to the presence of C-H bending vibration of substituted aromatic compounds. Weak, broad band at 631.79 cm-1 can be attributed to the presence of out-of-plane deformation vibration of alcohol[30,31]. Ethanol extract and hydro-ethanolic mixture (50:50) (v/v) extract of FT-IR spectrum were similar in peak shape but in absorption intensity is different (fig. 2). However, hydro-ethanol mixture (50:50) (v/v) extract shows one different characteristic absorption band (sharp, weak) for water molecule of O-H stretching at 1646.74 cm-1, these result is shown in fig. 2 [28].
CA leaf extracts were analyzed for their radical scavenging activity by DPPH radical assay. Antioxidant activity of the extract of CA leaves is shown in Table 1 and fig. 3. The antioxidant activity of ethanol-water extract has been significantly greater than the water and ethanol extracts.
This behavior has been discussed by many authors[11,32] and it can be explained by the use of a binary mixture, specifically a combination of an organic solvent and water, which improves extraction efficiency because of organic solvent increasing analytic solubility whereas water increases analyte desorption.
In the present study, ultrasound assisted extraction procedure has been developed that affords antioxidant rich extract from CA leaves using green solvents such as water, ethanol and hydro-alcoholic mixture. The water:ethanol (50:50) solvent extract of CA leaves exhibited the high antioxidant activity inhibiting the branching chain reaction of reactive free radicals. Hence, it is complementary that the ethanol:water extracts of leaves of CA with a higher amount of carboxylic acids, phytosterols and sugars including arabinose, xylose and galactose. The method reduces the extraction time and cost without negative impact of environment. It's also worth noting that the resulting antioxidant rich extract have desirable properties and could be ingested by people.
Acknowledgements:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
References
- Zhu H, Chen L, Yu J, Cui L, Ali I, Song X, et al. Flavonoid epimers from custard apple leaves, a rapid screening and separation by HSCCC and their antioxidant and hypoglycaemic activities evaluation. Sci Rep 2020;10(1):8819.
[Crossref] [Google Scholar] [PubMed]
- Rabêlo SV, Costa EV, Barison A, Dutra LM, Nunes XP, Tomaz JC, et al. Alkaloids isolated from the leaves of atemoya (Annona cherimola×Annona squamosa). Rev Bras Farmacogn 2015;25:419-21.
- El-Chaghaby GA, Ahmad AF, Ramis ES. Evaluation of the antioxidant and antibacterial properties of various solvents extracts of Annona squamosa L. leaves. Arabian J Chem 2014;7(2):227-33.
- Kumar Y, Kumar SS. Two new tetrahydroisoquinoline analogs from Indian medicinal plant Annona squamosa. J Pharm Res 2013;7(6):510-5.
- Kalidindi N, Thimmaiah NV, Jagadeesh NV, Nandeep R, Swetha S, Kalidindi B. Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa Linn leaves. J Food Drug Anal 2015;23(4):795-802.
[Crossref] [Google Scholar] [PubMed]
- Basha SH, Subramanian S. Biochemical evaluation of antidiabetic and antioxidant potentials of Annona squamosa leaves extracts studied in STZ induced diabetic rats. Int J Pharm Sci Res 2011;2(3):643.
- Wang DS, Rizwani GH, Guo H, Ahmed M, Ahmed M, Hassan SZ, et al. Annona squamosa Linn: cytotoxic activity found in leaf extract against human tumor cell lines. Pak J Pharm Sci 2014;27(5):1559-63.
[Google Scholar] [PubMed]
- Gowdhami M, Sarkar BL, Ayyasamy PM. Screening of phytochemicals and antibacterial activity of Annona squamosa extracts. Int J Pharm Sci Invent 2014;3(7):30-9.
- Kumar Y, Chandra AK, Shruti S, Gajera HP. Evaluation of antidiabetic and antioxidant potential of custard apple (Annona squamosa) Leaf extracts: A compositional study. Int J Chem Stud 2019;7(2):889-95.
- Chandrasekar C. Extraction and characterization of novel polysaccharides from Annona squamosa fruit peel using ultrasound assisted extraction. Int J Pharm Pharm Res 2018;12:267- 78.
- Chinnarasu C, Montes A, Pereyra C, Casas L, Fernández-Ponce MT, Mantell C, et al. Preparation of polyphenol fine particles potent antioxidants by a supercritical antisolvent process using different extracts of Olea europaea leaves. Korean J Chem Eng 2016;33:594-602.
- Chinnarasu C, Montes A, Fernandez-Ponce MT, Casas L, Mantell C, Pereyra C, et al. Precipitation of antioxidant fine particles from Olea europaea leaves using supercritical antisolvent process. J Supercrit Fluids 2015;97:125-32.
- Chinnarasu C, Montes A, Fernandez-Ponce MT, Casas L, Mantell C, Pereyra C, et al. Natural antioxidant fine particles recovery from Eucalyptus globulus leaves using supercritical carbon dioxide assisted processes. J Supercrit Fluids 2015;101:161-9.
- Tiwari BK. Ultrasound: A clean, green extraction technology. Trends Analyt Chem 2015;71:100-9.
- Wen C, Zhang J, Zhang H, Dzah CS, Zandile M, Duan Y, et al. Advances in ultrasound assisted extraction of bioactive compounds from cash crops–A review. Ultrason Sonochem 2018;48:538-49.
[Crossref] [Google Scholar] [PubMed]
- del Mar Contreras M, Lama-Muñoz A, Espínola F, Moya M, Romero I, Castro E. Valorization of olive mill leaves through ultrasound-assisted extraction. Food Chem 2020;314:126218.
[Crossref] [Google Scholar] [PubMed]
- Brás T, Paulino AF, Neves LA, Crespo JG, Duarte MF. Ultrasound assisted extraction of cynaropicrin from Cynara cardunculus leaves: Optimization using the response surface methodology and the effect of pulse mode. Ind Crops Prod 2020;150:112395.
- Dzah CS, Duan Y, Zhang H, Wen C, Zhang J, Chen G, et al. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci 2020;35:100547.
- Patle TK, Shrivas K, Kurrey R, Upadhyay S, Jangde R, Chauhan R. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV-vis and FTIR spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 2020;242:118717.
[Crossref] [Google Scholar] [PubMed]
- Schwaighofer A, Montemurro M, Freitag S, Kristament C, Culzoni MJ, Lendl B. Beyond FT-IR spectroscopy: EC-QCL based mid-IR transmission spectroscopy of proteins in the amide I and amide II region. Analytical Chemistry. 2018; 90(11): 7072–9.
- Tiernan H, Byrne B, Kazarian SG. ATR-FTIR spectroscopy and spectroscopic imaging for the analysis of biopharmaceuticals. Spectrochim Acta A Mol Biomol Spectrosc 2020;241:118636.
[Crossref] [Google Scholar] [PubMed]
- Ramos S, Thielges MC. Site-specific 1D and 2D IR spectroscopy to characterize the conformations and dynamics of protein molecular recognition. J Phys Chem B 2019;123(17):3551-66.
- Akhgar CK, Ramer G, Z?bik M, Trajnerowicz A, Pawluczyk J, Schwaighofer A, et al. The next generation of IR spectroscopy: EC-QCL-based mid-IR transmission spectroscopy of proteins with balanced detection. Anal Chem 2020;92(14):9901-7.
- Scherer R, Godoy HT. Antioxidant activity index (AAI) by the 2, 2-diphenyl-1-picrylhydrazyl method. Food Chem 2009;112(3):654-8.
- Palma M, Barbero GF, Piñeiro Z, Liazid A, Barroso CG, Rostagno MA, et al. CHAPTER 2 Extraction of Natural Products: Principles and Fundamental Aspects. Natural Product Extraction: Principles and Applications. Royal Society of Chemistry 2013:58-88.
- Maran JP, Manikandan S, Nivetha CV, Dinesh R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab J Chem 2017;10:S1145-57.
- Santos EE, Amaro RC, Bustamante CC, Guerra MH, Soares LC, Froes RE. Extraction of pectin from agroindustrial residue with an ecofriendly solvent: Use of FTIR and chemometrics to differentiate pectins according to degree of methyl esterification. Food Hydrocoll 2020;107:105921.
- Agwuncha SC, Owonubi S, Fapojuwo DP, Abdulkarim A, Okonkwo TP, Makhatha EM. Evaluation of mercerization treatment conditions on extracted cellulose from shea nut shell using FTIR and thermogravimetric analysis. Mater Today Proc 2021;38:958-63.
- Wang QQ, Huang HY, Wang YZ. FTIR and UV spectra for the prediction of triterpene acids in Macrohyporia cocos. Microchem J 2020;158:105167.
- Alara OR, Abdurahman NH. GC–MS and FTIR analyses of oils from Hibiscus sabdariffa, Stigma maydis and Chromolaena odorata leaf obtained from Malaysia: potential sources of fatty acids. Chem Data Collect 2019;20:100200.
- Coates J. Interpretation of infrared spectra, a practical approach. 2006.
- Otero-Pareja MJ, Casas L, Fernández-Ponce MT, Mantell C, Martinez de la Ossa EJ. Green extraction of antioxidants from different varieties of red grape pomace. Molecules 2015;20(6):9686-702.
[Crossref] [Google Scholar] [PubMed]

 ): EtOH+Water; (
): EtOH+Water; ( ): Water and (
): Water and ( ): Ethanol
): Ethanol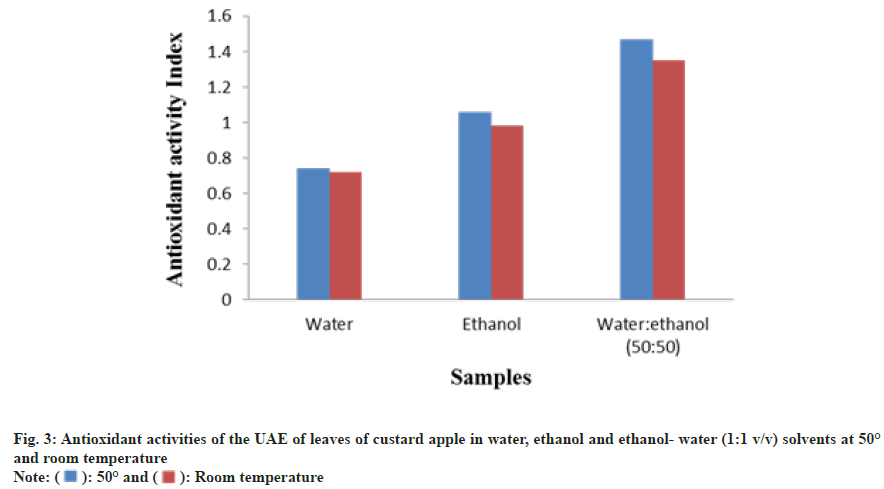
 ): 50° and (
): 50° and ( ): Room temperature
): Room temperature



