- *Corresponding Author:
- Deepthi Rapaka
Division of Pharmacology, Andhra University College of Pharmaceutical Sciences, Visakhapatnam-530 003, India
E-mail: deepthirapaka7@gmail.com
| Date of Submission | 19 February 2014 |
| Date of Revision | 02 February 2015 |
| Date of Acceptance | 06 September 2015 |
| Indian J Pharm Sci 2015;77(5):511-514 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Aging patients with diabetes are at higher risk of developing Alzheimer's disease. Emerging evidences demonstrate the role of brain insulin resistance, which is a key mediator in prediabetes and diabetes mellitus that may lead to Alzheimer's disease. Insulin and insulin-like growth factors regulate many biological processes such as axonal growth, protein synthesis, cell growth, gene expression, proliferation, differentiation, and development. Among these, the energy metabolism and synaptic plasticity are the major transduction processes regulated by insulin, which are the core objectives for learning and memory. It was also proposed that hyper insulinemia induced insulin resistance results in injury to the central nervous system by the activation of glycogen synthase kinase 3β which is the key ailment in the cognitive decline. Hence, the endogenous brain specific insulin impairments and signaling account for the majority of Alzheimer's abnormalities.
Keywords
Aging, Alzheimer’s disease, prediabetes, insulin resistance, glycogen synthase kinase 3β
As the human population is aging, the incidence of neurodegenerative diseases are also increasing. One such pathological disorder is Alzheimers Disease (AD) which affects the elderly population. As the average life expectancy increases, the number of AD patients are projected to increase by 27% by 2020, 70% by 2030, and nearly 300% by 2050 [1], unless there are better treatments for the reduction in progress of the disease.
Type 2 diabetes (T2D) also affects elderly, 25% of the population (60 y and above) had type 2 diabetes in 2007. If prediabetes is considered the prevalence is over 50% in persons of 60 y and above [2]. Epidemiological, cell biology and animal model evidences suggest the significant link between AD and diabetes. Many epidemiological evidences have also shown that prediabetes and diabetes are related to higher risk of dementia [3]. It was also evidenced that patients with T2D have a two to three‑fold increased risk for AD, referring to AD as “brain‑type diabetes” [4]. Findings from the prospective population based studies, meta‑analysis have shown an increased risk of cognitive impairment in people with diabetes [5,6]. More interest has been dedicated in the recent years to the effect of diabetes on brain, as it is found to be implicated in neurological complications. The high prevalence of T2D, prediabetes and its related conditions, their association with AD may have enormous impact on the public. In this review we would summarize the current brief understanding of correlation between prediabetes and AD.
Prediabetes and the risk of late onset AD
Diabetes milletus is mainly associated with insulin resistance or decreased insulin sensitivity. Diabetics on long run found to have specific cognitive deficits characterized by decreased execution [7,8], processing speed [9], verbal and non‑verbal memory [9,10] along with vascular depression where it severely impairs attention. Generally cognitive impairment occurs either in early stages of life during development of the nervous system or in late stages of life i.e.; in brain neurodegeneration (aging) [11]. The key organs of memory and emotions, hippocampus and amygdala were found to be atrophied in diabetes mellitus, which was very similar to the atrophy in AD [12]. Diabetes milletus may accelerate brain aging there by reducing the cognitive reserve and threshold development of AD symptoms; it may also interfere with amyloid and tau metabolism [13]. In a recent meta‑analysis study of “type 2 diabetes and dementia risk”, it was found that diabetics confer a relative risk of 1.46 for AD [6]. Researches suggest the risk of cognitive decline and neurodegeneration in prediabetes [14].
The term ‘prediabetes’ is a condition where the body tissues are exposed to abnormally high levels of insulin for extended periods, that may persist for many years. Insulin resistance develops gradually, even though enough insulin is still produced to overt diabetes, but results in impaired fasting glucose and/or impaired glucose tolerance [14,15]. Insulin resistance seems to increase Advanced Glycation End‑products (AGE), consequently oxidative stress by the formation of reactive oxygen species (ROS) by accelerating the aging process [16]. The resultant oxidative stress leads to increased damage of DNA, mitochondrial dysfunction, reduced ATP production. Impairment of brain insulin signaling appears to be the core of neurodegeneration cascade in late onset of AD [17,18] so the common link between prediabetes and late onset of AD is insulin resistance, which causes hyperinsulinemia [19]. Insulin, insulin‑like growth factors modulate the vital functions including survival and growth of neurons, gene expression, protein synthesis, myelin production and maintenance in oligodendrocytes, synapse formation and plasticity [20,21]. Consequently impaired signaling or resistance adversely affects a wide range of neuronal and glial functions.
Mechanisms leading to AD
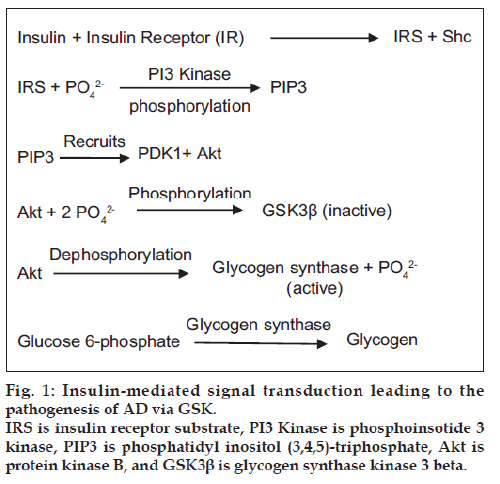
Even though brain constitutes 2% of the body weight, it consumes almost 25% of glucose [22], so the glucose metabolism is vital to the brain. Therefore disruptions in the glucose supply, transport or utilization results in neuronal damage and functional deficits. Hypoglycemia and hyperinsulinemia are accompanied by neuronal death and excitotoxicity in the developing brain, chronic hypoglycemia can lead to permanent brain damage [23] while hyperinsulinemia results in various functional decline in CNS [24,25]. Insulin resistance is a critical feature of diabetes and that may be detected years before the clinical onset of hyperglycemia [26,27]. Insulin resistance is due to reduced ability of insulin receptor to respond to insulin stimulation [28]. During this condition pancreatic beta cells secrete higher levels of insulin to compensate for the declined function of receptors resulting in hyperinsulinemia [29]. Insulin receptors are present throughout the brain, abundantly in the olfactory bulb, cerebral cortex, hippocampus, hypothalamus and the amygdala. The highest concentration of insulin receptor mRNA expression was found in the choroid plexus and cerebellum [30]. Binding of insulin to its receptor substrate activates the tyrosine kinase activity, leading to autophosphorylation of tyrosine residues, which initiates intracellular cascade [30,31]. This insulin mediated signal transduction controls the activity of many enzymes, including p85 regulatory subunit of phosphatidylinositol‑3‑kinase (PI3‑K) [32], this enzyme stimulates glucose transport [33] and inhibits apoptosis by activating Protein kinase‑B (Akt/PKB), which acts on glycogen synthase kinase‑3 (GSK‑3) [34], This GSK‑3 becomes inactive upon phosphorylation by Akt, thereby dephosphorylating glycogen synthase (active form), this enzyme increases the rate of conversion of glucose 6‑phosphate to glycogen [35] (fig. 1).
Although neurons are non‑insulin dependent but they are insulin responsive [36]. Insulin receptors are widely expressed in the brain as stated above. These receptors are more concentrated in the neurons, especially in the post‑synaptic regions [37]. Brain insulin signaling plays a key role not only in the regulation of food intake, body weight and reproduction but also in the learning and memory [38]. Defective insulin signaling or insulin resistance results in decreased cognitive ability and development of AD [39].
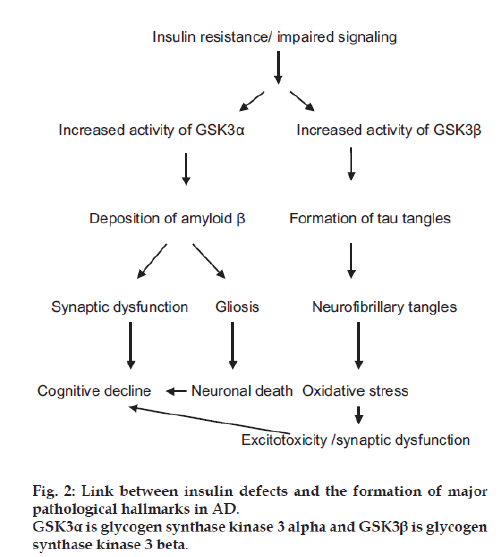
One of the key signaling molecule responsible for the pathogenesis of AD was GSK3, a multifunctional serine/threonine kinase. The activity of this signaling molecule is enhanced in impaired insulin signaling or insulin resistance, most commonly seen in prediabetics. Insulin and insulin like growth factors after a cascade of reactions generate the Akt which regulates the glucose and energy metabolism in normal physiological conditions, the GSK 3 upon activation produces two homologous isoforms GSK3 α and GSK3 β. The former one is implicated in amyloid precursor protein/amyloid β regulation, while the later one in Tau regulation. Increased GSK3 α activity was found to enhance the deposition of amyloid β extracellularly in the brain regions [40,41], mainly in the hippocampus as the activity of the GSK [42] was found to be more here, which is one of the major pathological hallmarks in AD, the amyloid β deposits impair synaptic functions and also promotes gliosis leading to cognitive decline and neuronal death.
Insulin resistance also promotes rise in the activity of GSK3 β [43] which promotes the hyperphosphorylation of the microtubule associated protein tau, thereby forming neurofibrillary tangles intracellularly which may induce oxidative stress leading to the excitotoxicity, synaptic dysfunction which leads to cognitive decline (fig. 2).
Future perspectives
Insulin resistance is the main pathogenic feature of metabolic syndromes like diabetes, hypertension, dyslipidemia, and obesity. The study of insulin resistance was mainly focused on metabolic tissues until yesterday. Evidences from researchers now indicate that insulin resistance also affects the nervous system. Studies revealed increased risk of AD in diabetes, as it makes neurons more vulnerable to amyloid and tau toxicity. The Akt signaling is the crucial and common feature in normal functioning of the cell, where it’s signaling seems to be highly decreased in insulin resistance. Insulin resistance is commonly observed in prediabetes also, where there is a major risk of developing diabetes. Screening and prophylaxis of prediabetics appears to be necessary in order to overcome the fore coming disorders like diabetes and AD. Literature clearly reveals the presence of insulin receptor mRNA in the choroid plexus, where the cerebrospinal fluid is being synthesized. So, a better biomarker can be obtained by further research, such that the progress of prediabetes can be reduced and studies will be needed to understand the contribution of hyperinsulinemia in developing AD. Further research is required to establish the molecular mechanism and signal transduction pathways common to both prediabetes and AD, for developing new therapeutic targets.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Yesavage JA, O’Hara R, Kraemer H, Noda A, Taylor JL, Ferris S, et al. Modeling the prevalence and incidence of Alzheimer’s disease and mild cognitive impairment. J Psychiatr Res 2002;36:281-6.
- Luchsinger JA. Diabetes, related conditions, and dementia. J Neurol Sci 2010;299:35-8.
- Luchsinger JA. Type 2 diabetes, related conditions, in relation and dementia: An opportunity for prevention? J Alzheimers Dis 2010;20:723-36.
- Accardi G, Caruso C, Colonna-Romano G, Camarda C, Monastero R, Candore G. Can Alzheimer disease be a form of type 3 diabetes? Rejuvenation Res 2012;15:217-21.
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol 2006;5:64-74.
- Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern Med J 2012;42:484-91.
- Munshi M, Grande L, Hayes M, Ayres D, Suhl E, Capelson R, et al. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care 2006;29:1794-9.
- Perlmuter LC, Hakami MK, Hodgson-Harrington C, Ginsberg J, Katz J, Singer DE, et al.Decreased cognitive function in agingnon-insulin-dependent diabetic patients. Am J Med 1984;77:1043-8.
- Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol Aging 2005;26:26-30.
- Roriz-Filho JS, Sá-Roriz TM, Rosset I, Camozzato AL, Santos AC, Chaves ML, et al. (Pre) diabetes, brain aging, and cognition. Biochim Biophys Acta 2009;1792:432-43.
- Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: A lifespan perspective. Lancet Neurol 2008;7:184-90.
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 2003;46:1604-10.
- Gasparini L, Xu H. Potential roles of insulin and IGF-1 in Alzheimer’s disease. Trends Neurosci 2003;26:404-6.
- Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology 2004;63:1187-92.
- Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 2004;63:658-63.
- Smith MA, Sayre LM, Monnier VM, Perry G. Radical AGEing in Alzheimer’s disease. Trends Neurosci 1995;18:172-6.
- de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: Relevance to Alzheimer’s disease. J Alzheimers Dis 2005;7:45-61.
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease – Is this type 3 diabetes? J Alzheimers Dis 2005;7:63-80.
- Tang J, Pei Y, Zhou G. When aging-onset diabetes is coming across with Alzheimer disease: Comparable pathogenesis and therapy. Exp Gerontol 2013;48:744-50.
- Wozniak M, Rydzewski B, Baker SP, Raizada MK. The cellular and physiological actions of insulin in the central nervous system. Neurochem Int 1993;22:1-10.
- D’Ercole AJ, Ye P, Calikoglu AS, Gutierrez-Ospina G. The role of the insulin-like growth factors in the central nervous system. Mol Neurobiol 1996;13:227-55.
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism. Relevance to functional brain imaging and to neurodegenerative disorders. Ann N Y Acad Sci 1996;777:380-7.
- Dunne MJ, Cosgrove KE, Shepherd RM, Aynsley-Green A, Lindley KJ. Hyperinsulinism in infancy: From basic science to clinical disease. Physiol Rev 2004;84:239-75.
- Stolk RP, Pols HA, Lamberts SW, de Jong PT, Hofman A, Grobbee DE. Diabetes mellitus, impaired glucose tolerance, andhyperinsulinemia in an elderly population. The Rotterdam Study. Am J Epidemiol 1997;145:24-32
- Stolk RP, Breteler MM, Ott A, Pols HA, Lamberts SW, Grobbee DE, et al. Insulin and cognitive function in an elderly population. The Rotterdam Study. Diabetes Care 1997;20:792-5.
- Bell GI. Lilly lecture 1990. Molecular defects in diabetes mellitus. Diabetes 1991;40:413-22.
- Taylor SI. Lilly lecture: Molecular mechanisms of insulin resistance. Lessons from patients with mutations in the insulin-receptor gene. Diabetes 1992;41:1473-90.
- Le Roith D, Zick Y. Recent advances in our understanding of insulin action and insulin resistance. Diabetes Care 2001;24:588-97.
- Heidenreich KA, Zahniser NR, Berhanu P, Brandenburg D, Olefsky JM. Structural differences between insulin receptors in the brain and peripheral target tissues. J Biol Chem 1983;258:8527-30.
- Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim BiophysActa 2009;1792:482-96.
- Kahn CR, White MF. The insulin receptor and the molecular mechanism of insulin action. J Clin Invest 1988;82:1151-6.
- White MF, Kahn CR. The insulin signaling system. J Biol Chem 1994;269:1-4.
- Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab 2012;23:133-41.
- Lam K, Carpenter CL, Ruderman NB, Friel JC, Kelly KL. The phosphatidylinositol 3-kinase serine kinase phosphorylates IRS-1. Stimulation by insulin and inhibition by Wortmannin. J Biol Chem 1994;269:20648-52.
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 1997;275:661-5.
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995;378:785-9.
- Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 2009;30:586-623.
- Abbott MA, Wells DG, Fallon JR. The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J Neurosci 1999;19:7300-8.
- van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: Learning to survive. Prog Neurobiol 2006;79:205-21.
- de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB Rep 2009;42:475-81.
- Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem 2008;104:1433-9.
- Maurer K, Hoyer S. Alois Alzheimer revisited: Differences in origin of the disease carrying his name. J Neural Transm 2006;113:1645-58.
- Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, et al. The role of GSK-3 in synaptic plasticity. Br J Pharmacol 2008;153 Suppl 1:S428-37.