- *Corresponding Author:
- Hanhua Wang
Macau University of Science and Technology, Taipa, Macau 999078, China
E-mail: wanghh.123@163.com
| Date of Received | 02 October 2023 |
| Date of Revision | 11 April 2024 |
| Date of Accepted | 27 September 2024 |
| Indian J Pharm Sci 2024;86(5):1800-1809 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Qu zhi qiao (Qu Aurantii Fructus ) is mainly produced in Changshan, Quzhou, and is one of the new eight flavours of Zhejiang, containing flavonoids, volatile oils, alkaloids and coumarins, etc., which has the effect of regulating qi and stagnation and reducing swelling. There are many kinds of compounds in Qu Aurantii Fructus, so it is not comprehensive to evaluate the quality only by a few index components. Based on the quality marker theory, the quality markers of Qu Aurantii Fructus were predicted and analysed from the aspects of plant affinity, chemical composition, medicinal properties of traditional Chinese medicine, effectiveness such as traditional Chinese medicine and measurable composition. The chemical components of narirutin, naringin, naringenin, hesperidin, neohesperidin, limonene, beta-myrcene, limonin, synephrine, hesperidolactone hydrate, umbelliferon and marmine were used as candidate quality markers-markers of Qu Aurantii Fructus. Meanwhile, the potential targets and pathways of the quality marker and absorption, distribution, metabolism, excretion, and toxicity of the quality marker were predicted and analysed.
Keywords
Qu Aurantii Fructus, quality marker, chemical material basis, pharmacological action, quality control
Qu zhi qiao (Qu Aurantii Fructus (QAF)) is the dried immature fruit of Citrus aurantium (C. aurantium) Changshan-huyou Y.B. Chang, a cultivated variety of C. aurantium in the family of Rutaceae, commonly known as hu you pieces. It is mainly produced in Quzhou, Zhejiang Province, which is one of the new eight flavours of Zhejiang and six flavors of qu[1]. It is slightly cold in character, bitter, pungent and sour in taste, and enters the lung, spleen and large intestine meridians, with the efficacy of regulating qi and broadening the middle, moving stagnation and eliminating flatulence, which is mainly used for treating chest and hypochondria stagnation of qi, flatulence and pain, food accumulation that does not dissolve, phlegm and drink internalization, gastric prolapse, prolapse, and uterine prolapse, etc.,[2]. In 2016, QAF was included in the 2015 edition of Zhejiang Province Chinese Medicine Preparation Standards, and its proportion in the commodities of Aurantii Fructus (AF) gradually increased. Some scholars, comparing the plant basal origin, chemical composition and pharmacodynamics, believed that C. aurantium Changshan-huyou Y.B.Chang should be used as one of the basal origins of AF, in order to restore the legitimate status of QAF as AF medicinal use[2]. In recent years, the research and application of Quality Marker (Q-Marker) about Traditional Chinese Medicine (TCM) has been deepening, producing some theoretical and applied results[3-7]. In this paper, based on the Q-Marker theory in TCM, we predicted and analysed the Q-Markers of QAF to provide reference for the quality control of QAF.
Materials and Methods
Predictive analysis of Q-Marker based on evidence related to plant affinities:
Citrus L., habitually known as mandarin orange, is commonly found in a variety of plants such as grapefruit, mandarin, tangerine, orange, lemon, citron and kumquat, which are distributed in the tropical and subtropical regions[8]. The resources of Citrus and orange in China are extremely rich, mostly cultivated species. The 2020 edition of the Chinese Pharmacopoeia included TCM of Citrus sinensis which are Guangchenpi, Chenpi, Sihuaqingpi, Qingpi, Juye, Juluo, Juhe, Zhishi, Zhiqiao, Huajuhong, Foshou, Xiangyuan, Goujuli and Daidaihua. The main secondary metabolites of Citrus fruits are volatile oils and flavonoids. Volatile oils and flavonoids as the basis for the classification of Citrus fruits in the Brassicaceae family, are of great significance for plant resource research. It is of great significance to use volatile oils and flavonoids as the basis for classifying Citrus fruits of Rutaceae.
Predictive analysis of Q-Marker based on chemical composition:
The research on the mechanism of action and material basis of chemical components of TCM is an important foundation for the safety, efficacy and quality control of TCM, and has become the core of modernization of TCM. Chemical composition is the material basis for TCM to exert its efficacy, which can objectively reflect the safety and effectiveness of TCM. The chemical composition of TCM is affected by the variety of TCM, the growing environment, the time of harvesting, the place of use, the processing technology, the storage condition and the deep processing of TCM. At present, domestic and foreign scholars have isolated and identified more than 100 compounds from QAF, mainly including flavonoids, volatile oils, triterpenoids, coumarins and alkaloids, as well as heavy metal elements such as Aluminium (Al), Frsenic (As), Barium (Ba), Beryllium (Be), Cadmium (Cd), Cobalt (Co), Chromium (Cr), Iron (Fe), Manganese (Mn), Molybdenum (Mo), Lead (Pb), Antinomy (Sb), Selenium (Se), Tin (Sn), Strontium (Sr), Thallium (Tl) and Vanadium (V)[9] as shown in Table 1-Table 3[10-15].
Predictive analysis of Q-Marker based on TCM properties:
The theory of medicinal properties of TCM is the core of TCM theory and the basis for guiding clinical drug use. QAF is slightly cold, bitter, acuminate, acid, lung, spleen and large intestine. Bitter taste, can relieve, dry, and firm, its material base includes alkaloids, volatile oils, glycosides, quinones and flavonoids. Pungent taste, can disperse, move, pass, warm, rise, moisten and melt, its material base is mostly volatile oil, glycosides, alkaloids and amino acid components. The substance basis of sour taste is mostly flavonoids. Combined with the reported chemical composition of QAF, flavonoids, volatile oil and alkaloids can be used as reference for selecting Q-Marker.
Results and Discussion
The basic purpose of the evaluation of the effectiveness of TCM is to evaluate the effect of TCM on the body. QAF has the functions of regulating qi, regulating stagnation and eliminating distension. These traditional functions are mainly reflected in the effects on gastrointestinal tract, antiinflammatory, anti-bacterial and anti-oxidation. Therefore, exploring various pharmacological activities of QAF has become one of the important bases for analysing Q-Marker of QAF.
Ma et al.[16] used the rat model of functional dyspepsia to detect the intestinal propulsion rate, gastric residual rate, plasma gastrin, motilin and somatostatin contents, and observed the gastric antrum histopathology. The results showed that the qi-regulating effect of QAF on the model rats was better than Jiangzhiqiao, Chuangzhiqiao and Xiangzhiqiao. Feng et al.[17] used multiple regression analysis and bivariate correlation analysis to correlate the pharmacodynamics data of QAF methanol extract on the model of small intestinal charcoal advance and in vitro ileal contraction with the relative peak area of the common peak of fingerprint, and analyzed the correlation of spectrum effect. The results showed that, narirutin, naringin, naringenin, hesperidin and neohesperidin in methanol extract might be the effector substances in QAF to regulate gastrointestinal motility, naringin and neohesperidin showed a very significant correlation and were closely related to the regulation of gastrointestinal motility.
The flavonoids and coumarin compounds contained in QAF have many phenolic hydroxyl groups, which have significant antioxidant activity. Liu et al.[18] investigated the scavenging ability of total flavonoids from QAF on 2, 2-Diphenyl- 1-Picrylhydrazyl (DPPH) and 2, 2-Azinobis (3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) free radicals, and found it had strong antioxidant activity. Wang et al.[19] used DPPH, ABTS free radical scavenging experiments and total reducing power experiments to investigate the antioxidant activities of different samples of quartiers steamed, boiled, vinegar fried, wine fried, bran fried, bran made and honey made, and found that the samples of quantiers boiled, bran made and bran fried had better DPPH free radical scavenging ability. Boiled, steamed, and gluten-prepared samples showed better ABTS radical scavenging ability.
Li et al.[20] observed the effects of QAF extract on the transneutrophy-fluorescent zebrafish (Danio rerio) model induced by copper sulphate pentahydrate and the mouse (Mus musculus) model induced by Lipopolysaccharide (LPS), and found that QAF extract could both reduce the levels of inflammatory factors and increase the levels of anti-inflammatory factors. Xu et al.[21] found that flavonoid fractions of QAF could inhibit the inflammatory response of RAW264.7 cells induced by LPS, and the mechanism might be related to inhibiting p65 activation and reducing the expression of inflammatory factors. Jiang et al.[22] established Rattus norvegicus model of non-alcoholic fatty liver disease in rats induced by obesity, and found that liver lesions in rats were significantly reduced by oral administration of QAF extract, and the mechanism might play a role by inhibiting phosphorylated Nuclear Factor Kappa B (NF-κB) and Mitogen-Activated Protein Kinase (MAPK) pathways.
Wang et al.[1] confirmed through in vitro tests that different components of QAF had α-glucosidase inhibitory effects, which were enhanced with the increase of flavonoid content in each component. The components of QAF with higher flavonoid content had the effect of promoting glucose consumption in HepG2 cells. Zhen et al.[23] took diabetic db/db mice as a model and found that QAF extract could significantly improve the oxidative damage of kidney in db/db mice with type 2 diabetes, and the mechanism may be related to promoting the expression of antioxidant genes and improving the antioxidant capacity of kidney tissue. Using animal models, Wang et al.[24] found that QAF extracts could alleviate insulin resistance in mice with type 2 diabetes, and the mechanism may be closely related to the inhibition of liver lipid accumulation. Using diabetic db/db mice and INS-1 pancreatic beta cells as models, Wang et al.[25] observed the protective effects of naringenin and hesperetin on pancreatic beta cells in a highsugar environment and their mechanisms. The results showed that naringenin and hesperetin inhibited histone acetylation by regulating p300 inactivation mediated by protein kinase signalling pathway, thus protecting pancreatic beta cells and delaying the progression of diabetes.
Ye et al.[10] adopted the golden hamster model of hyperlipidemia and found that QAF flavonoids had the effect of reducing blood lipid, which may be achieved by improving lipid levels, reducing oxidative stress and inflammatory reaction, and activating Peroxisome Proliferator-Activated Receptor Alpha (PPAR-α) and PPAR-Gamma (γ). Ying et al.[26] fed rats with high-fat diet to establish a pharmacokinetic and pharmacodynamics study model of QAF administration, and found that QAF flavonoids can play a role in lowering blood lipid by reducing intestinal lipid absorption, inhibiting Hydroxymethylglutaryl-Coenzyme A (HMG-CoA) reductase activity, and regulating pancreatic lipase activity.
Wang et al.[27] used a mouse model of liver fibrosis induced by carbon tetrachloride and found that QAF extract could significantly alleviate the degree of liver injury, reduce the levels of serum alanine aminotransferase and glutamic oxalacetic aminotransferase in mice with liver fibrosis, and significantly reduce the infiltration of liver inflammatory cells. Meanwhile, the expression of tumour necrosis factor-α, interleukin-18 and interleukin-1beta genes in liver tissue of mice was significantly decreased, and the degradation of liver Inhibitor of Nuclear Factor Kappa B Alpha (NF-IκBα) protein, p-IKKα/β, p-p65, Nucleotidebinding Oligomerization Domain (NOD)-Like Receptor Protein 3 (NLRP3) and cleaved caspase-1 protein expression were inhibited.
Through in vitro antibacterial experiments, Ye et al.[28] found that the volatile oil of Changshan grapefruit, the original plant of QAF, had stronger inhibitory effect on fungi than bacteria, and showed high sensitivity to Trichophyton gypsum and Trichophyton rubrum. Liu et al.[29] established an asthma model in BALB/c mice and found that total flavonoids from QAF had anti-inflammatory and anti-oxidative stress effects on asthmatic mice infected with Respiratory Syncytial Virus (RSV), and the mechanism may be related to NF-κB signalling pathway.
In conclusion, flavonoids such as narirutin, naringin, naringenin, hesperidin, neohesperidin, and volatile oils such as limonene and β-laurene in QAF can be considered as candidate Q-Markers for QAF.
For Q-Marker, in addition to the requirement of active ingredients, it is necessary to have a simple and feasible method for content determination. Huang et al.[30] established High-Performance Liquid Chromatography (HPLC) for simultaneous determination of the content of narirutin, naringin, hesperidin, neohesperidin, luteolin, nobiletin and tangeretin in Qubriand Aurantii, which was simple and accurate. Feng et al.[9] established an accurate, reliable, simple and efficient HPLC method for simultaneous determination of the content of erioxoside, narirutin, naringin, naringenin, neohesperidin, hesperidin, hydrohesperolide, luteolin, hesperolide, nobiletin, tangeretin and orange peel olactone in Qu Aurantii. Ye et al.[31] established HPLC for the simultaneous determination of the content of neohesperidin, hesperidin, narirutin and naringin in QAF, and compared the content of four flavonoids in QAF and its related species (seed ou orange, seedless ou orange and huyou). In conclusion, these components could act as potential Q-Markers of QAF.
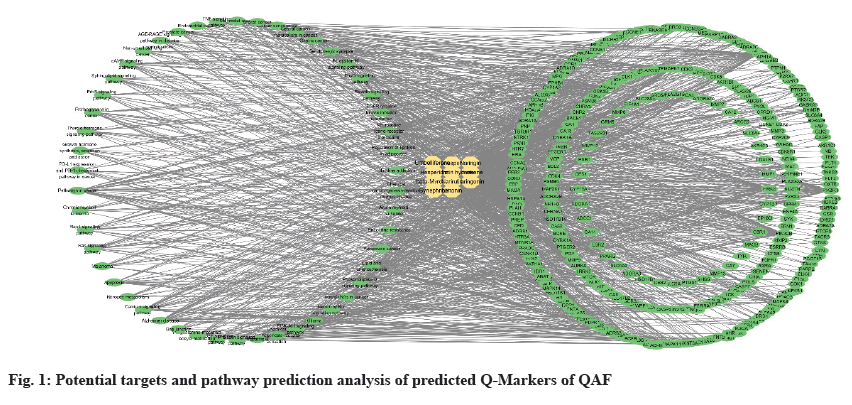
Network pharmacology has been widely used in the field of active ingredient discovery and evaluation through the multi-level interaction of "ingredient-target-disease" to explain the material basis and mechanism of medicinal effect of TCM[32,33]. Through Traditional Chinese Medicine Systems Pharmacology (TCMSP) and SwissTargetPrediction database, 276 potential Q-Markers (narirutin, naringin, naringenin, hesperetin, hesperidin, neohesperidin, nobiletin, tangeretin, limonene, β-myrcene, limonin, simfulin, hesperolide hydrate, umbelliferide, grapholide, hesperolide, and malmin etc.,) were obtained. Database for Annotation, Visualization and Integrated Discovery (DAVID) database was used for Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of targets, and 50 signalling pathways were found. Cytoscape software was used to construct the componenttarget- pathway network, as shown in fig. 1. There are 368 nodes in the figure, including 1378 connecting lines. The circle on the left represents the pathway, the circle on the right represents the target point, the diamond in the middle represents the predicted Q-Markers of QAF, and the connecting line represents the correlation between them. The larger the degree value, the larger the node. The density of connecting lines in the figure can directly reflect the important targets and pathways of predicted Q-Markers of QAF, and also reflect that these Q-Markers exert the corresponding drug effects through the interaction of multi-targets and multi-pathways.
| S. No | Compounds | Formula | CAS number | Structural formula |
|---|---|---|---|---|
| 1 | Rhoifolin[2] | C27H30O14 | 17306-46-6 | 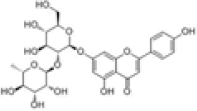 |
| 2 | Isorhoifolin[2] | C27H30O14 | 552-57-8 | 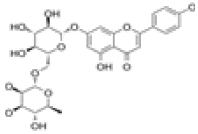 |
| 3 | Poncirin[2] | C28H34O14 | 14941-08-3 | 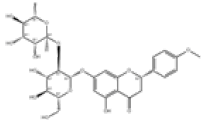 |
| 4 | Hesperetin[2] | C16H14O6 | 520-33-2 | 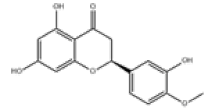 |
| 5 | 3,3',4',5,6,7,8-heptamethoxyflavone[2] | C22H24O9 | 1178-24-1 | 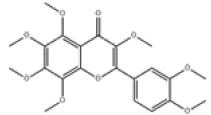 |
| 6 | Eriocitrin[10] | C27H32O15 | 13463-28-0 | 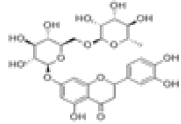 |
| 7 | Narirutin[10,11] | C27H32O14 | 14259-46-2 | 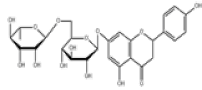 |
| 8 | Naringin[10,11] | C27H32O14 | 10236-47-2 | 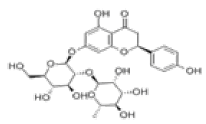 |
| 9 | Naringenin[10] | C15H12O5 | 480-41-1 | 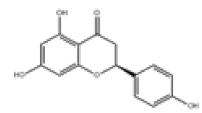 |
| 10 | Hesperidin[10,11] | C28H34O15 | 520-26-3 | 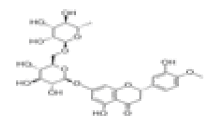 |
| 11 | Neohesperidin[10,11] | C28H34O15 | 13241-33-3 | 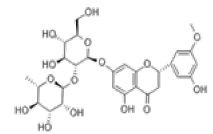 |
| 12 | Luteolin[10,11] | C15H10O6 | 491-70-3 | 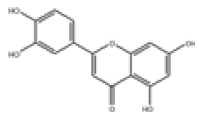 |
| 13 | Nobiletin[10,11] | C21H22O8 | 478-01-3 | 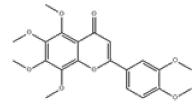 |
| 14 | Tangeretin[10,11] | C20H20O7 | 481-53-8 | 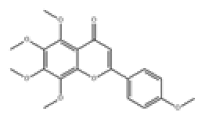 |
Table 1: The Flavonoids in QAF
| S.No | Compounds | Formula | CAS number | Structural formula |
|---|---|---|---|---|
| 1 | Limonene[13] | C10H16 | 5989-27-5 |  |
| 2 | β-myrcene[12,13] | C10H16 | 123-35-3 |  |
| 3 | DL-limonene[12,13] | C10H16 | 138-86-3 | 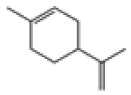 |
| 4 | β-caryophyllene[13] | C15H24 | 87-44-5 | 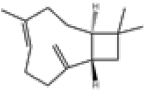 |
| 5 | α-caryophyllene[13] | C15H24 | 6753-98-6 | 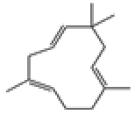 |
| 6 | Germacrene D[13] | C15H24 | 23986-74-5 | 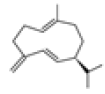 |
| 7 | γ-elemene[13] | C15H24 | 339154-91-5 | 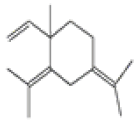 |
Table 2: The Volatile Oil in QAF
| S.no | Compounds | Formula | CAS number | Structural formula |
|---|---|---|---|---|
| 1 | Limonin[14] | C26H30O8 | 1180-71-8 | 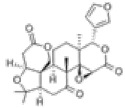 |
| 2 | Nomilin[14] | C28H34O9 | 1063-77-0 | 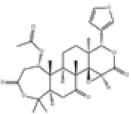 |
| 3 | Deacetylnomilin[14] | C26H32O8 | 3264-90-2 | 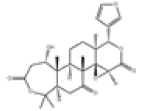 |
| 4 | Poncimarin[14] | C19H22O5 | 55916-48-8 | 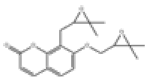 |
| 5 | Synephrine[15] | C9H13NO2 | 94-07-5 | 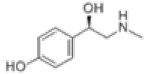 |
| 6 | N-methyltyramine[15] | C9H13NO | 370-98-9 | 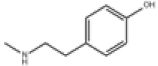 |
| 7 | Fabiatrin[2] | C21H26O13 | 18309-73-4 | 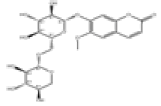 |
| 8 | Meranzin hydrate[10] | C15H18O5 | 5875-49-0 | 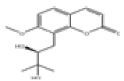 |
| 9 | Meranzin[10] | C15H16O4 | 23971-42-8 | 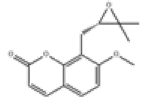 |
| 10 | Auraptene[10] | C19H22O3 | 495-02-3 |  |
| 11 | Umbelliferone[2] | C9H6O3 | 93-35-6 | 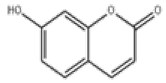 |
| 12 | Eriocitrin[2] | C27H32O15 | 13463-28-0 | 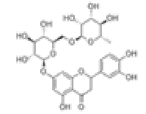 |
| 13 | Marmin[10] | C19H24O5 | 14957-38-1 | 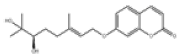 |
Table 3: Other Compounds in QAF
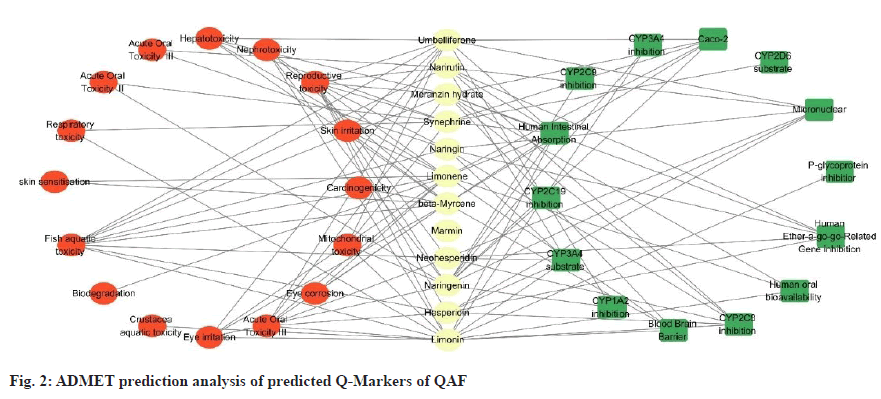
From the admetSAR-2.0 database (http://lmmd. ecust.edu.cn/admetsar2/), relevant information about potential Q-Markers of QAF was collected, and Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) parameters of these compounds were predicted, including human intestinal absorption, blood-brain barrier, Caco-2 cell permeability and acute oral toxicity, and their pharmacokinetic and toxicological properties were analysed. The specific results were showed in fig. 2, where the lines indicated positive correlations.
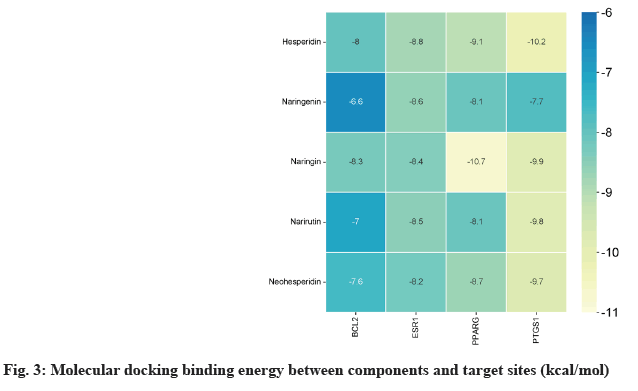
Narirutin, naringin, naringenin, hesperidin and neohesperidin were selected as targets for molecular docking with Prostaglandin-Endoperoxide Synthase 1 (PTGS1), PPARγ, B-Cell Lymphoma 2 (BCL2) and Estrogen Receptor 1 (ESR1) of nodes in the "medical-components-target-disease" network. the Three-Dimensional (3D) structures of the components (ligands) were downloaded from the PubChem database. The 3D structures of the key targets (receptors) were obtained from the Research Collaboratory for Structural Bioinformatics (RCSB) database. The ligands and receptors were repaired by the mgltools_ win32_1.5.6 software and saved as PDBQT files. The AutoDock Vina 1.1.2 software was used to test the binding affinity between the key components and the target proteins and calculate the binding energy. The final graphical visualization of the molecular docking was completed using Discovery Studio 2019.
The results were analysed, revealing that PPARγ exhibited the highest binding ability to naringin, with a binding energy of -10.7 kcal/mol. From a target perspective, ESR1, PPARγ, and PTGS1 showed strong affinity for each component, with binding energies exceeding -7 kcal/mol. In terms of the compounds hesperidin, naringin, narirutin, neohesperidin combined with various ingredients demonstrated favourable binding energies greater than 7 kcal/mol as shown in fig. 3.
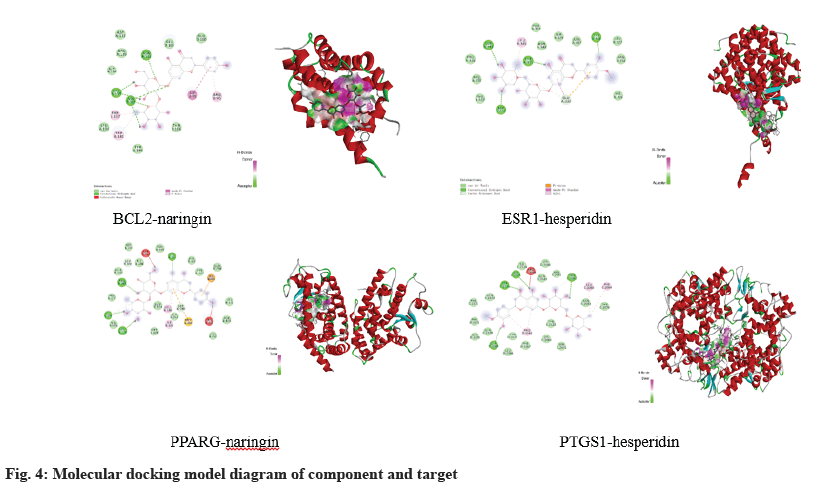
The compounds with the best binding energy for each target site were selected for visual analysis as shown in fig. 4. BCL2 binds to the active sites ASN136, VAL135, and ASN102 of naringin and ESR1 binds to the active sites SER341, TYR331, and TYR338 of hesperidin to form hydrogen bonds. The active sites LEU228, CYS285, ARG288 of PPARγ and naringin formed hydrogen bonds, and the active sites ASP2125, SER2126, THR2062 of PTGS1 and hesperidin formed hydrogen bonds.
QAF is one of the authentic medicinal materials of AF, which is a major TCM for regulating qi with a medicinal history of 300 y. At present, the production of QAF is about 3000 time/y, accounting for one third of the total production of AF in China. With the increasing demand of QAF, the requirements of quality control are also increasing. It is difficult to meet the requirements of effectiveness and safety of QAF only using naringin and neohesperidin as the content determination index components. Based on the theory of Q-Marker of TCM, this paper predicted and analysed the Q-Marker of QAF from the aspects of plant relationship, chemical composition, medicinal properties, effectiveness and determinability of TCM. The components including narirutin, naringin, naringenin, hesperidin, neohesperidin, limonene, β-laurene, limonin, simfulin, hesperolide hydrate, umbelliferolide and malmin were used as candidate Q-Markers for QAF. The potential targets and pathways of Q-Markers and ADMET were predicted and analysed to ensure clinical efficacy and drug safety.
Data availability statement:
The data that support this study are available in the article and accompanying online supplementary material.
Acknowledgements:
Supported by General Scientific Research Project of Zhejiang Education Department (No: Y202147756), Scientific Research Project of Zhejiang Medical College (No: ZPCSR2020006).
Conflict of interests:
The authors declared no conflict of interests.
References
- Wang X, Wang SW, Fang YJ. Study on the hypoglycemic activity of different components of quzhiqiao in vitro and analysis of four flavonoids. Chin J Mod Appl Pharm 2017;34:1418-23.
- Zheng C, Zhao WL, Song JF. Demonstration and study of Qu Aurantii fructus medicinally used as Aurantii fructus. Chin J Mod Appl Pharm 2022;39(16)2096-102
- Liu CX. A new concept on quality marker of Chinese materia medica: Quality control for Chinese medicinal products. Chin Tradit Herb Drugs 2016:1443-57.
- Zhang TJ, Bai G, Liu CX. The concept, core theory and research methods of Chinesemedicine quality markers. Acta Pharm Sin 2019:187-96.
- Guo L, Gong M, Wu S, Qiu F, Ma L. Identification and quantification of the quality markers and anti-migraine active components in Chuanxiong Rhizoma and Cyperi Rhizoma herbal pair based on chemometric analysis between chemical constituents and pharmacological effects. J Ethnopharmacol 2020;246:112228.
- Chen L, Huang X, Wang H, Shao J, Luo Y, Zhao K, et al. Integrated metabolomics and network pharmacology strategy for ascertaining the quality marker of flavonoids for Sophora flavescens. J Pharm Biomed Anal 2020;186:113297.
[Crossref] [Google Scholar] [PubMed]
- Ren JL, Zhang AH, Kong L, Han Y, Yan GL, Sun H, et al. Analytical strategies for the discovery and validation of quality-markers of traditional Chinese medicine. Phytomedicine 2020;67:153165.
[Crossref] [Google Scholar] [PubMed]
- Tian T, Guo ZG, Zhou TM. Current status and prospects of research on authenticity traceability technology for Citrus products. Mod Food Technol 2023.
- Feng J, Hu W, Xu L, Li J, Wang S, Song J. Simultaneous determination of the contents of 12 flavonoids in quzhiqiao from different collection places by HPLC. Chin Pharm 2020:571-5.
- Ye A, Jiang J, Li R, Jin L, Wu X, Wang J. Study on hypolipidemic effect of pure total flavonoids from Qu Aurantii fructus on golden hamsters of hyperlipidemia and its potential mechanism. Chin J Mod Appl Pharm 2020;37(16):1938-46.
- Xu XZ, Zhao SQ, Wang LX. The functional components and functions contained in the peel of Changshan pomelo. Zhejiang Citrus 2014;31(1):10-12
- Xuemei Z, Xingqian Y, Dayuan Z. Study on the volatile oils and volatile components in the rind of Changshan Huyou pomelo cultivar. J Fruit Sci 2007;24(1).
- Tang RL, Ling M, Wu J. The current situation and progress of the development of Changshan pomelo. Sci Technol Innov Herald 2014;11(28):250-2
- He D, Shan Y, Wu Y, Liu G, Chen B, Yao S. Simultaneous determination of flavanones, hydroxycinnamic acids and alkaloids in citrus fruits by HPLC-DAD–ESI/MS. Food Chem 2011;127(2):880-5.
[Crossref] [Google Scholar] [PubMed]
- Lei MeiKang LM, Wang XiaoHong WX, Yang Jun YJ, Wang SiWei WS, Zhu ZiTong ZZ, Peng Fang PF, et al. Determination of 17 trace elements in Qu Aurantii fructus by microwave digestion-inductively coupled plasma mass spectrometry. J Food Safety Qual Inspection 2020;11(1):165-9.
- Ma Y, Chen H, Fang X, Wang X, Ge W. A Comparative study on regulating Qi effect of Quzhiqiao (Fructus Aurantii from Zhejiang) and Fructus Aurantii from the other three major producing areas on functional dyspepsia rats. Chin J Tradit Med Sci Technol 2021;28:722-6.
- Feng JQ, Song JF, Zhao SQ. Study on spectrum-activity relationship of flavonoids in Qu Aurantii fructus for regulation of gastrointestinal motility. Chin J Mod Appl Pharm 2022;39(17):2241-45
- Liu XJ, Fang YJ, Xia DZ. Extraction process optimization and anti-oxidant activity of total flavonoids from Quzhou Aurantii fructus. Chin Tradit Patent Med 2020;42(7):1687-91.
- Wang L, Wang L, Wu T, Zhao S, Zheng M, Lu S. Effects of different processing methods on the contents of the pharmacodynamic index components and antioxidant activity of Citrus aurantium. Chin Pharm 2022:830-5.
- Li L, Zhang S, Xin Y, Sun J, Xie F, Yang L, et al. Role of Quzhou Fructus Aurantii extract in preventing and treating acute lung injury and inflammation. Sci Rep 2018;8(1):1698.
[Crossref] [Google Scholar] [PubMed]
- Xu X, Wang Y, Wang SW. Anti-inflammatory effect of the flavonoids from Quzhiqiao (Fructus Auranti) on LPS-induced RAW264.7 cells. Chin J Tradit Med Sci Technol 2019;26(4):529-32.
- Jiang J, Yan L, Shi Z, et al. Hepatoprotective and anti-inflammatory effects of total flavonoids of QuZhiKe (peel of Citrus changshan-huyou) on non-alcoholic fatty liver disease in rats via modulation of NF-κB and MAPKs. Phytomedicine 2019;64:153082.
- Zhen H, Wang S W, Zhong SY. Quzhike, extract of Citrus Changshan-Huyou, ameliorates renal oxidative damage in type 2 diabetic db/ db mice. Zhejiang J Integr Tradit Chin Western Med 2020;30(2):105-78.
- Wang W, Lan T, Zhen F. Effect of Quzhou Aurantii fructus extract on insulin resistance in type 2 diabetic mice. J Zhejiang Chin Med Univ 2022;46(9):936-44.
- Wang SW, Sheng H, Bai YF, Weng YY, Fan XY, Zheng F, et al. Inhibition of histone acetyltransferase by naringenin and hesperetin suppresses Txnip expression and protects pancreatic β cells in diabetic mice. Phytomedicine 2021;88:153454.
[Crossref] [Google Scholar] [PubMed]
- Ying Y, Wan H, Zhao X, Yu L, He Y, Jin W. Pharmacokinetic-pharmacodynamic modeling of the antioxidant activity of quzhou fructus Aurantii decoction in a rat model of hyperlipidemia. Biomed Pharmacother 2020;131:110646.
[Crossref] [Google Scholar] [PubMed]
- Wang SW, Lan T, Zheng F, Lei MK, Zhang F. Effect of extract of Quzhou Aurantii fructus on hepatic inflammation and NF-κB/NLRP3 inflammasome pathway in CCl_4-induced liver fibrosis mice. Zhongguo Zhong Yao Za Zhi 2021;46(6):1474-9.
- Ye XM, Ye XQ, Zhu DY. Preliminary study on the chemical composition and antimicrobial activity of volatile oil from grapefruit peel. China J Chin Mater Med 2003;11:94-6.
- Liu XJ, Jiang XQ, Fang YJ, Xia DZ, Wang SW, Zhong SY. Protective effect of total flavonoids from Fructus Aurantii on lung injury of asthma mice infected with RSV through NF-κB signaling pathway. Chin J Hospital Infect Dis 2021;31(22):3376-80.
- Huang WK, Yue C, Song JF, Ding GQ, Zhang WT, Zhao WL. Simultaneous determination of seven constituents in Citrus Changshanhuyou, YB Chang by HPLC. Chin J Mod Appl Pharm 2018;35:404-7.
- Ye YM, Hu SY, Xu XH. Determination and correlation analysis of the contents of four flavonoid glycosides in Citrus aurantium and its relatives. Zhejiang J Integr Tradit Chin Western Med 2022;32(8):752-5.
- Wen-Lu C, Jia-Na H, Xin-Ning Z, Ibarra-Estrada E, Li-Sheng W, Sha-Sha L, et al. Network biological modeling: A novel approach to interpret the traditional Chinese medicine theory of exterior-interior correlation between the lung and large intestine. Digital Chin Med 2020;3(4):249-59.
- World Federation of Chinese Medicine Societies. Guidelines for online pharmacology evaluation methods. World Chin Med 2021;16(4):527-32.