- *Corresponding Author:
- Shyamoshree Basu
Department of Pharmaceutical Technology, NSHM College of Pharmaceutical Technology, NSHM Knowledge Campus, Kolkata‑Group of Institutions, Kolkata-700 053, India
E‑mail: shyamoshree@gmail.com
| Date of Submission | 28-Feb-2011 |
| Date of Revision | 31-Aug-2012 |
| Date of Acceptance | 03-Sep-2012 |
| Indian J Pharm Sci, 2011, 73 (3): 300-302 |
Abstract
The aim of the present study is to prepare and evaluate mucoadhesive nasal gels of venlafaxine hydrochloride. Mucoadhesive nasal gels were prepared using polymers like carbopol 934 and sodium alginate and characterized in terms of viscosity, texture profile analysis, ex vivo drug permeation profiles and histopathological studies. The results show that values of viscosity, hardness and adhesiveness increase while those of cohesiveness decrease with corresponding increase in concentration of the polymers. Ex vivo drug permeation profiles showed that formulation containing 5% sodium alginate provided a better controlled release of the drug than the other formulations over a period of 12 h. Histopathological studies assured that gels containing different polymers did not produce any significant change in the nasal mucosae of goat even after 12 h permeation study. Mucoadhesive nasal gel of venlafaxine hydrochloride is a novel dosage form which delivers the drug directly into systemic circulation and provides controlled release of the drug.
Keywords
Mucoadhesive, nasal gel, novel dosage form, venlafaxine hydrochloride
Venlafaxine is a potent selective serotonin reuptake inhibitor, used in the treatment of general anxiety disorder. Owing to its short biological half‑life and rapid clearance from the body, no parenteral formulation of venlafaxine is possible. It is mainly administered via the oral route in the form of tablets. But it undergoes extensive hepatic metabolism giving bioavailability of 45% [1]. Moreover, pertaining to its high aqueous solubility it may lead to overdose after going into solution in vivo [2]. Wyeth developed an extended release formulation of venlafaxine for once daily administration, Effexor® XR capsules, containing coated pellets of venlafaxine. This single dose formulation was however, effective in overcoming nausea and other gastrointestinal disturbances associated with venlafaxine [3]. After oral administration, venlafaxine undergoes extensive presystemic metabolism in the liver. Based on the mass balance studies, it was found that about 92% of the single dose of venlafaxine is absorbed but the absolute bioavailability was found to be only 45% [3]. Various modified release solid oral dosage forms of venlafaxine hydrochloride (HCl) have been prepared and they offer advantages of improved patient compliance and decreased side effects [4‑6]. Most of the oral controlled release drug delivery systems are based on either gel forming matrix or coated formulations, or the combination thereof [7]. Numbers of patents have been granted for modified release venlafaxine HCl formulations. A European patent, assigned to Sherman et al., describes encapsulated extended release formulation of venlafaxine in the form of coated spheroids [3]. Sela prepared sustained release compositions containing coated nonpareil cores [8]. Molenda formulated capsules containing coated microgranules of venlafaxine [9]. A US patent describes an extended release composition of venlafaxine HCl in matrix tablet dosage form, in which the drug is mixed with a combination of hydrophilic and hydrophobic matrix forming components [10]. Seth prepared a sustained release formulation of venlafaxine by coating drug containing core with a mixture of water soluble and semipermeable film forming polymer [11]. A Canadian patent assigned to Bhattacharya et al., describes zero order sustained release dosage forms [12]. US patent assigned to Vaya et al., describes dual retard technique to effectively control the release rate of venlafaxine [13]. Patat et al., carried out a randomized, double‑blind, four‑way crossover, placebo‑controlled study of 16 healthy young men who were given either a single oral dose of 50 mg of conventional tablet of venlafaxine, 75 mg of extended release venlafaxine formulation, or an intravenous dose of 10 mg of venlafaxine, or a placebo at 1‑week intervals [14]. The absolute bioavailability of venlafaxine was between 40 and 45% and was similar for both the conventional and extended release formulations. However, the bioavailability of venlafaxine can be enhanced by administering it via the nasal route.
Nasal route has been explored widely for delivery of a large number of drug molecules, owing to its rich vasculature and thin epithelial lining due to which when a drug is administered via nasal route it directly reaches systemic circulation and provides rapid onset of action [15‑17]. Mucoadhesive nasal gels are the most prominent noninvasive dosage forms through which a drug can reach systemic circulation directly without undergoing first pass effect and this enhances underlying bioavailability of the drug [18,19].
Aboelwafa and Basalious [20] prepared novel controlled release venlafaxine HCl three‑layer tablet. In vivo study suggests that optimized polyethylene oxide three‑layer formulation developed in this work may be comparable to the marketed capsule in the form of coated pellets that are reported to suffer from low productivity, long processing time and high cost.
The aim of the present study is to develop nasal gels of venlafaxine HCl using mucoadhesive polymers and to characterize them in terms of viscosity, texture profile analysis, mucoadhesive strength, ex vivo drug permeation profiles and histopathological study of nasal mucosae. Till date no nasal formulation of venlafaxine has been developed. Therefore, mucoadhesive nasal gel of venlafaxine will be a novel dosage form that will deliver the drug slowly into systemic circulation and provide desired therapeutic effect for a long period of time.
Materials and Methods
Venlafaxine HCl was obtained as a gift sample from Jubilant Organosys, Noida, India. Sodium alginate and carbopol 934P were purchased from S.D. Fine Chemicals, Mumbai, India. Sodium taurocholate was obtained from Loba Chemie Pvt. Ltd, Mumbai, India.
Preparation of mucoadhesive nasal gels
Mucoadhesive nasal gels were prepared by dissolving venlafaxine HCl in nasal solution (0.65% NaCl, 0.04% KH2PO4, 0.09% K2HPO4 and 0.02% benzalkonium chloride) (pH 6) in a constant stirring condition [21]. Required amounts of polymers (sodium alginate, Carbopol 934P and mixture of Carbopol 934P and sodium alginate) were added to the solution and stirred on a magnetic stirrer until a uniform solution was obtained which was kept at 4° overnight to allow complete swelling so that a homogenous gel was formed. Composition of nasal gels are displayed in Table 1. Formulation of these gels are based on two variables having three levels.
| Formulation code | Venlafaxine HCl (mg) | Carbopol 934 (% w/v) | Sodium alginate (% w/v) |
|---|---|---|---|
| F1 | 37.50 | 3.00 | - |
| F2 | 37.50 | 4.00 | - |
| F3 | 37.50 | 5.00 | - |
| F4 | 37.50 | - | 3.00 |
| F5 | 37.50 | - | 4.00 |
| F6 | 37.50 | - | 5.00 |
| F7 | 37.50 | 1.50 | 1.50 |
| F8 | 37.50 | 2.00 | 2.00 |
| F9 | 37.50 | 2.50 | 2.50 |
| HCl=Hydrochloride | |||
Table 1: Composition Of Nasal Gels
Determination of viscosity
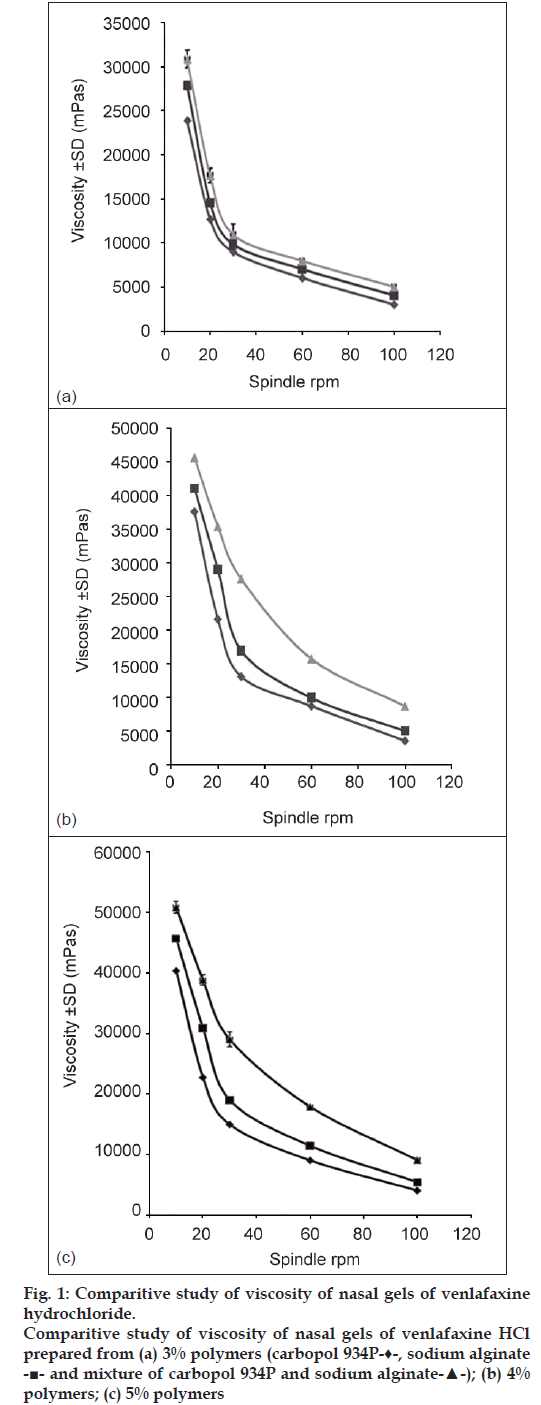
Viscosity of the nasal gels was studied using Brookfield Viscometer (DV II+Pro., Brookfield Engineering Labs, USA) at five different speeds of 10, 20, 30, 60 and 100 rpm, respectively using spindle M4 and cord no. 23 at 37±1° (fig. 1).
Texture profile analysis
Texture profile analyses of the prepared gels were performed using QTS‑25 Texture Analyser (Brookfield Engineering Labs., USA) to determine the mechanical parameters like hardness, cohesiveness and adhesiveness. An analytical probe of diameter 1.2 cm was depressed twice into each sample to a defined depth (15 mm), at a defined rate (30 mm/min), with a defined recovery period (15 s), between the end of the first compression and the beginning of the second.
A trigger force of 4 g was applied. At least six replicate analyses of each sample were performed at 37±1°. Data were collected and calculated by Texture pro software, version 2.1 (Biozon Food Innovations GmbH, Bremerhaven, Germany).
Evaluation of mucoadhesive strength
Mucoadhesive strength of each formulation was determined by measuring force required to detach nasal mucous membrane from the formulation using the same texture analyser. Freshly excised goat nasal membrane was attached to the upper probe of the instrument, and fixed amount of gel was kept below that. The upper probe was then lowered at a speed of 10 mm/min to touch the surface of the gel. A force of 0.1 N was applied for 5 min to ensure intimate contact between the membrane and the gel. The surface area of exposed mucous membrane was 1.13 cm2.
Ex vivo drug permeation study:
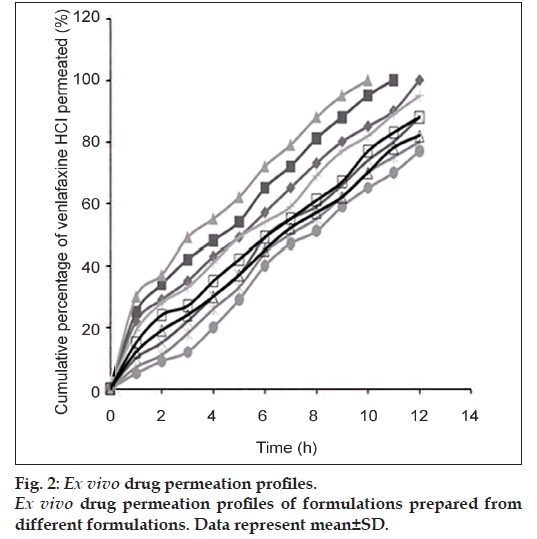
Ex vivo permeation study was conducted using a Franz diffusion cell containing 100 ml of phosphate buffer (pH 6, 0.1 M) using an excised goat nasal mucosa. The goat nose was obtained from local slaughterhouse within 15 min after the goat was sacrificed. After removing the skin, the nose was stored on ice cold phosphate buffer (pH 7.4, 0.05 M). The septum was fully exposed, and nasal mucosa was carefully removed using forceps and surgical scissors. The mucosal tissues were immediately immersed in Ringer’s solution [18]. The freshly excised nasal mucosa was mounted on the diffusion cell, and 100 μl of gel containing 37.5 mg venlafaxine was placed on it. Throughout the study, the buffer solution in the chamber was maintained at 37±1° by connecting the Franz diffusion cell with water bath. At predetermined time intervals, 1 ml of the samples was withdrawn at predermined time interval and replenished with an equal amount of phosphate buffer. The samples were appropriately diluted, filtered and absorbances were measured spectrophotometrically at 274 nm using Jasco V‑550 UV/Vis Spectrophotometer (Tokyo, Japan), taking phosphate buffer (pH 6) as the blank (fig. 2).
Histopathological study
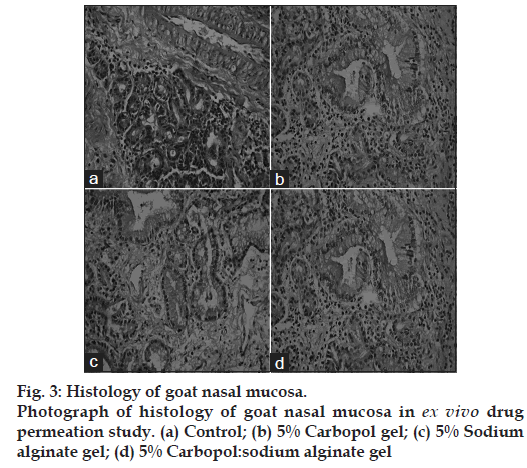
Histological study of excised nasal mucosa was conducted by comparing a fresh mucosa with the mucosae used for 5 h in the ex vivo permeation study to detect if any significant histological change has occurred during the experiment. After permeation study, nasal mucosa was cleared off the gel, sectioned with a rotary microtome (Model 1090 A, The Western Electric and Scientific Works, Ambala Cantt, India) and fixed in 10% formalin solution. The sectioned tissue was then stained with Hematoxylin and Eosin. Another normal mucosa was taken as a control and treated similarly. Tissue sections were observed under Olympus CKX41 optical microscope (Olympus Optical Co. Ltd., Tokyo, Japan). The photographs were taken with an Olympus‑SC 35 camera (fig. 3).
Statistics
Data were analysed by one way ANOVA followed by Tukey’s HSD (honestly significant difference) test using Vassar stat software (USA). P<0.01 has been considered to be significant statistically.
Results and Discussion
fig. 1 shows the viscosity profile of the nasal gels with respect to five different speeds of spindle rpm at 37±1°. All the formulations are observed to exhibit pseudoplastic flow.
Texture profile analysis measures the mechanical properties of a gel like hardness, cohesiveness and adhesiveness. They are determined with the help of QTS‑25 Texture Analyser and results are tabulated in Table 2. Maximum hardness and adhesiveness were exhibited by gel prepared with 5% carbopol 934 and sodium alginate (33.00±0.21 g and –94.77±0.75 g, respectively) while hardness and adhesiveness exhibited by gel prepared with 3% Carbopol 934 were 15.00±0.51 g and –45.03±5.02 g, respectively. Maximum cohesiveness was shown by gel prepared with 5% sodium alginate (0.94±0.04) while minimum cohesiveness was shown by gel prepared with 3% Carbopol 934 (1.10±0.05). Cohesiveness reduced with increase in amount of mucoadhesive agents. With increase in amount of dispersed solids, that is the mucoadhesive agents, semisolid nature of the gels increased, which caused the gel to become less coherent [22,23].
| Formulation code | Hardness (g) (gs) | |||
|---|---|---|---|---|
| Adhesiveness | Cohesiveness | Mucoadhesive strength (g) | ||
| F1 | 15.00 ± 0.51 | −45.03 ± 5.02 | 1.10 ± 0.05 | 9.63 ± 1.02 |
| F2 | 21.00 ± 0.36 | −69.36 ± 8.56 | 1.06 ± 0.04 | 13.22 ± 1.05 |
| F3 | 27.05 ± 0.32 | −80.36 ± 7.98 | 0.96 ± 0.03 | 16.39 ± 0.89 |
| F4 | 18.09 ± 0.0 | −74.67 ± 4.89 | 1.02 ± 0.03 | 11.80 ± 0.85 |
| F5 | 24.50 ± 0.11 | −85.78 ± 3.78 | 0.99 ± 0.05 | 14.05 ± 0.95 |
| F6 | 31.00 ± 0.20 | −53.50 ± 0.33 | 0.94 ± 0.04 | 17.00 ± 0.87 |
| F7 | 20.50 ± 0.11 | −76.25 ± 0.20 | 1.05 ± 0.11 | 13.50 ± 0.95 |
| F8 | 26.00 ± 0.02 | −88.50 ± 0.50 | 1.01 ± 0.05 | 15.00 ± 1.05 |
| F9 | 33.00 ± 0.21 | −94.77 ± 0.75 | 0.98 ± 0.04 | 19.25 ± 0.55 |
Table 2: Evaluation Of Mucoadhesive Nasal Gels
Mucoadhesive strength of the nasal gels was measured with QTS‑25 Texture Analyser. Values of mucoadhesive strengths are tabulated in Table 2. Nasal gels prepared with 3% carbopol 934 showed minimum strength while gels prepared with 5% mixture of carbopol 934 and sodium alginate showed maximum strength. Mucoadhesive strength increased with increase in amount of polymer used [24].
Ex vivo permeation study of venlafaxine from the nasal gels was conducted using phosphate buffer (pH 6.0) in Franz diffusion cells for a period of 12 h using goat nasal mucosa. fig. 2 shows that ex vivo drug permeation profiles through nasal mucosa of goat. Among them, F6 showed sustained release of venlafaxine for 12 h, and the drug release kinetics of formulation F6 is provided in Table 3. Ex vivo drug permeation plots show that with increase in polymer concentration from 3 to 5%, cumulative percentage of drug release decreased, but in case of carbopol gels, the results were different. Since, carbopol 934P has penetration enhancing effect by opening the tight junctions of the nasal mucosa, as the amount of carbopol in the gel increased, there was a marked increase in amount of drug permeation. However, gels prepared with 5% sodium alginate provided sustained release of the drug for 12 h period. From Table 3 it is observed that nasal gels prepared with 5% sodium alginate exhibit zero order release kinetics.
| Zero-order kinetics | First-order kinetics | Higuchi kinetics | Korsmeyer et al . kinetics | Hixon–crowell kinetics |
|---|---|---|---|---|
| y=6.769x+3.384 | y=–0.052x+2.07 | y=24.08x-18.57 | y=1.431x+0.412 | y=0.289x+1.255 |
| r2=0.99 | r2=0.96 | r2=0.891 | r2=0.923 | r2=0.855 |
Table 3: Drug Release Kinetics Of Formulation F6
Photographs of nasal mucosae are displayed in fig. 3. Fig 3a shows the histology of a normal nasal mucosa of goat, fig. 3b shows the histology of a goat nasal mucosa after conduction of 12 h ex vivo drug permeation study using 5% carbopol gel. Fig 3c shows the histology of a goat nasal mucosa after conduction of 12 h ex vivo drug permeation study using 5% sodium alginate gel. Fig 3d shows the histology of a goat nasal mucosa after conduction of 12 h ex vivo drug permeation study using 5% carbopol:sodium alginate gel.
Histopathological study does not show any significant alteration of the mucosae after conduction of 12 h ex vivo drug permeation study. The photographs shows no necrosis or removal of epithelial layer even after 12 h permeation study when compared with the normal mucosa, hence, it can be inferred that no major histological change has occurred and confirms the safety of nasal gels for nasal administration. Also, there was no significant alteration of the nasal epithelium. Thus, the concentrations of polymers used in the study were safe for the nasal epithelium.
Further, a bioavailability study has to be conducted to determine the therapeutic effect of venlafaxine released from this gel formulation and dose of the administered gel can be controlled by delivering it from a suitable metered dose applicator.
Mucoadhesive nasal gel of venlafaxine HCl is a new formulation which will deliver the drug in a controlled manner to the blood directly over a prolonged period. Hence, a sustained release dosage form of venlafaxine is developed for treatment of anxiety disorders. From the above study, it can be concluded that 5% gel of sodium alginate produced desirable results in all aspects and this dosage form will be further used for in vivo study.
References
- Venlafaxine. Available from: http://www.wikipedia.org. [Last accessed 2011 Mar 20].
- Howell SR, Hicks DR, Scatina JA, Sisenwine SF. Pharmacokinetics of venlafaxine and O-desmethylvenlafaxine in laboratory animals. Xenobiotica 1994;24:315-27.
- Wyeth Laboratories. Effexor XR (venlafaxine hydrochloride) extended-release capsules prescribing information. Philadelphia, PA: 2006.
- Sherman DM, Clark JC, Lamer JU, White SA. Extended release formulation containing venlafaxine. EP Patent No, EP0797991B1; 2004.
- Sherman DM, Clark JC, Lamer JU, White SA, inventors. Extended release formulation containing venlafaxine. EP Patent No, EP1331003A1; 2003.
- Sherman DM, Clark JC, Lamer JU, White SA, inventors. Extended release formulation containing venlafaxine. US Patent No, US6274171B1; 2001.
- Pisek R, Zupan CS, Sugula ZM. Sustained release pharmaceutical composition comprising venlafaxine. PCT Int Appl WO 2006/010605 A2; 2006.
- Sela Y. Extended release compositions comprising as active compound venlafaxine hydrochloride. PCT Int Appl WO 03/041692; 2003.
- Molenda FA. Process for preparation of programmed liberation composition with venlafaxine and the resulting product. PCT Int Appl WO 02/102129; 2002.
- Sela Y, inventor. Extended release composition comprising as active compound venlafaxine hydrochloride. US Patent No, US2003190354; 2003.
- Seth P, inventor. Tablet comprising a delayed release coating. US Patent No, US6350471; 2002.
- Bhattacharya S, Pandita S, Mayank J, Kshirsagar R. Extended release coated microtablets of venlafaxine hydrochloride. CA255295 A1; 2005.
- Vaya N, Karan RS, Madkarni SS, Gupta VK. Novel drug delivery system. US Patent No, US2004096501; 2004.
- Patat A, Troy S, Burke J, Trocherie S, Danjou P, Le Coz F, et al. Absolute bioavailability and electroencephalographic effects of conventional and extended-release formulations of venlafaxine in healthy subjects. J Clin Pharmacol 1998;38:256-67.
- Ugwoke MI, Verbeke N, Kinget R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J Pharm Pharmacol 2001;53:3-21.
- Illum L. Nasal drug delivery: New developments and strategies. Drug Discov Today 2002;7:1184-9.
- Türker S, Onur E, Özer Y. Nasal route and drug delivery systems. Pharm World Sci 2004;26:137-42.
- Basu S, Chakrabatorty S, Bandyopadhyay AK. Development and evaluation of a mucoadhesive nasal gel of midazolam prepared with Linum usitatissimum L. seed mucilage.Sci Pharm 2009;77:899-910.
- Basu S, Bandyopadhyay AK. Development and characterization of mucoadhesive in situ nasal gel of midazolam prepared with Ficus carica mucilage. AAPS Pharm Sci Tech 2010;11:1223-31.
- Aboelwafa AA, Basalious EB. Optimization and in vivo pharmacokinetic study of a novel controlled release venlafaxine hydrochloride three-layer tablet. AAPS Pharm Sci Tech 2010;11:1026-37.
- Loyd V, Allen JR. Compounding nasal preparations. Current and Practical Compounding Information for the Pharmacist in SecundumArtem. Vol. 7. No. 1. Available from: http://www.perrigo.com/business/ pdfs/Sec%20Artem%207.1.pdf [Last accessed 19 October 2012].
- Bansal K, Rawat MK, Jain A, Rajput A, Chaturvedi TP, Singh S. Development of satranidazole mucoadhesive gel for the treatment of periodontitis. AAPS Pharm Sci Tech 2009;10:716-23.
- Tan YT, Peh KK, Al-Hanbali O. Effect of Carbopol and polyvinylpyrrolidone on the mechanical, rheological, and release properties of bioadhesive polyethylene glycol gels. AAPS Pharm Sci Tech 2000;1:E24.
- Harding SE. Mucoadhesive interactions. Biochem Soc Trans 2003;31:1036-41.