Neha Kiroula, J. S. Negi*, K. Singh, Rakhee Rawat and Bhavna Singh
Department of Pharmaceutical Sciences, S Bhagwan Singh PG Institute of Bio-medical Sciences and research, Balawala, Dehradun-248 161, India.
- *Corresponding Author:
- J. S. Negi
Department of Pharmaceutical Sciences
S Bhagwan Singh PG Institute of Bio-medical Sciences and research
Balawala, Dehradun-248 161, India
E-mail: rx.jnegi@gmail.com
Received Date: 16 Sep 2015; Revised Date: 21 May 2016; Accepted Date: 26 May 2016
Abstract
In this research, ganciclovir-loaded gold nanoparticles were developed via glutathione surface modification. First citrate reduced gold nanoparticles were prepared from chloroauric acid. Further the surface of gold nanoparticles was also modified with glutathione. Then ganciclovir was loaded onto glutathione modified gold nanoparticles through the reaction between carboxyl group of glutathione and amino group of ganciclovir. Gold nanoparticles were characterized by photon correlation spectroscopy, transmission electron microscopy and infrared spectroscopy. The drug loading of 87.27±2.52% was observed for ganciclovir loaded gold nanoparticles. The particle size distribution of gold nanoparticles was ranged from 26.3 to 31 nm. Thus ganciclovir loaded gold nanoparticles were successfully prepared which may further improve the oral bioavailability of ganciclovir.
Keywords
Gold nanoparticles, ganciclovir, citrate reduced gold nanoparticles, glutathione modifies gold nanoparticles
Nanocarriers such as dendrimers, liposomes and polymeric nanoparticles have been successfully utilized for delivery of active drugs in order to achieve improved bioavailability, targeted delivery, toxicity reduction and improved bio-distribution[1-4]. Metal nanoparticles gain attention of researchers for both diagnostic and therapeutic applications[5-6]. More size monodispersity, light absorption and emission tunability with possibility of surface modification are some attractive features of metallic nanoparticles. Recently gold nanoparticles are also being widely investigated for therapeutic and diagnostic applications[7]. Gold nanoparticles (AuNPs) are having unique optical properties and uniform size distribution in nanometre range. The AuNPs also has strong binding affinity towards organic molecules having thiol and amine groups[8-10]. Drug conjugation with AuNPs can be achieved either by direct conjugation of drug with AuNPs or by drug conjugation with surface modified gold nanoparticles[11-14]. Also electrostatic or hydrogen bond attachment of drug with AuNPs may be a better strategy than covalent linkage[15]. Venkatpurwar et al.[16] reported attachment of doxorubicin with porphyran reduced AuNPs by electrostatic interaction. Mirza et al.[17] also reported direct conjugation of doxorubicin by electrostatic binding of protonated amine group of doxorubicin with negatively charged citrate reduced AuNPs.
In this paper we also tried to conjugate Ganciclovir (GCV) with AuNPs. Ganciclovir is a synthetic purine nucleoside analogue of guanine and is effective for treatment and chronic suppression of cytomegalovirus (CMV) retinitis in immune compromised patients and for prevention of CMV disease in transplant patients[18]. GCV is a weak base having poor oral bioavailability (6-7%) due to its highly polar nature[19]. Due to poor oral bioavailability, currently GCV is given orally at dose of 1000 mg thrice a day. Nanoparticles have been used as a physical approach to alter and improve the pharmacokinetic and pharmacodynamic properties of various types of drug molecules. Thus the major aim of present study is to develop AuNPs based formulation of poor bioavailable drug GCV which may further enhance its oral bioavailability as well as reduction in dose and dose related side effects.
In order to conjugate GCV to AuNps, surface modification of AuNps was required. Zhang et al.[23] also conjugate folic acid with glutathione (GSH) modified AuNPs. GSH is a natural tripeptide containing thiol, amino and carboxylic groups. Simpson et al.[20] found that glutathione coated AuNPs were having low immunogenicity and better biocompatibility. Thus present study also investigates the functionalization of AuNPs with glutathione for subsequent conjugation with GCV.
Materials and Methods
GCV was obtained as gift sample from Unimark Remedies Ltd. (Vapi, India). Chloroauric acid (HAuCl4) and sodium citrate were purchase from Sigma-Aldrich (Bangalore, India). GSH was purchased from Hi Media Ltd. (India). Other chemical and solvents were also of analytical grades.
Synthesis of citrate-reduced gold nanoparticles (citrate-AuNPs):
Citrate-AuNPs were synthesized using HAuCl4 and sodium citrate according to the Turkevich method[21,22]. Briefly, 50 ml of 0.58 mM HAuCl4 was boiled in a conical flask under continuous stirring and 1 ml of 52.6 mM sodium citrate was added in boiled solution and heated to another 15 min. The solution was further allowed to cool at room temperature and kept for next 24 h.
Surface modification of synthesized citrate-reduced gold nanoparticles by GSH (AuNPs-GSH):
After the completion of synthesis of citrate-reduced gold nanoparticles, surface modification was done on freshly prepared citrate-AuNPs. Surface modification was done by GSH. Briefly 300 μl of 0.05 M solution of GSH was added to 50 ml of citrate reduced gold nanoparticle and kept for next 12 h under stirring[23] The resulted suspension was further dialysed (12 kD) for next 12 h to remove excess GSH molecules.
Preparation of GCV loaded AuNPs-GSH:
The stock solution of GCV was prepared in 0.1 N NaOH and treated with GSH modified gold nanoparticles. The appropriate amount of drug solution was added to AuNPs-GSH and pH was maintained in range of 5-8. This solution was kept under stirring at room temperature for next 24 h.
UV analysis of gold nanoparticles:
The UV analysis was determined by scanning the freshly prepared citrate-reduced gold nanoparticles, GSH-loaded citrate-reduced gold nanoparticles, and drug loaded citrate-reduced gold nanoparticles using the UV-Vis spectrophotometer (Systronics, Ahemdabad, India) which ranges from 400-800 nm[24].
Drug loading:
Briefly 4 ml of 0.01 M GCV solution in 0.1 N NaOH was added to 6 ml of AuNPs-GSH. The pH was maintained between 5-8 using 0.1 N NaOH. After 24 h time period under stirring, the conjugate dispersion was centrifuged at 10000 rpm in a cooling centrifuge. Drug loading was calculated by estimating the GCV content of the supernatant.
The drug concentration in supernatant was determined by a reverse-phase high performance liquid chromatography (RP-HPLC) method with UV detection. The HPLC system was Waters having a C18 reverse phase analytical column (Waters, 250×4.6 mm). According to the UV-visible absorption profile of GCV, UV detection was determined at 254 nm. The mobile phase consisted of 5% acetonitrile (v/v) and 95% 100 μM sodium phosphate (v/v). The flow rate of mobile phase was maintained at 1 ml/min[25] and the injection volume was 20 μl. The percentage drug loading of GCV in GCV loaded AuNPs-GSH was estimated by the following formula, (Total amount of GCV added – amount of GCV in supernatent)/(Total amount of AuNPs added)×100.
FTIR analysis:
Fourier transform infrared (FTIR) spectra were obtained by Thermonicolet Nexus spectrometer. Samples were pressed into potassium bromide (KBr) pellets and recorded at frequencies from 4000 to 200 cm-1 with resolution of 4 cm-1.
Chemical test identification for GCV:
GCV loaded AuNPs-GSH were centrifuged by cooling centrifuge at 10 000 rpm. The supernatant was removed and nanoparticles were redispersed in distilled water. 1 ml of 1 M NaOH and 1.5 ml of M potassium permanganate were added to nanodispersion. Reaction mixture was allowed to stand for 10 min after vigorous shaking. The mixture was than scanned in UV spectrophotometer[26].
Particle size and zeta potential determination:
The average particle size and polydispersity index (PDI) of nanopartices were determined by photon correlation spectroscopy (PCS) technique using Beckman coulter Delsa™ Nano Common. 1 ml nanoparticles dispersion was diluted up to 10 ml with distilled water to reduce opalescence during particle size measurement[27]. Beckman coulter Delsa Nano Common was also used for zeta potential measurement of nanoparticles.
TEM analysis:
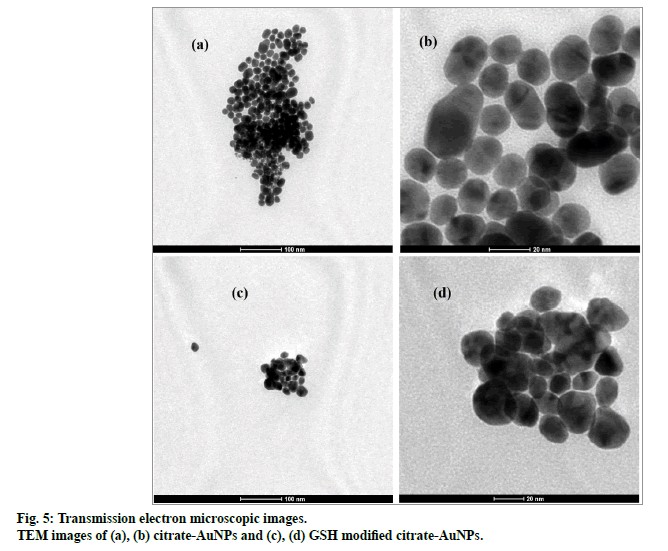
All samples for TEM analysis were examined in TECHNAI G2 S-TWIN and images were obtained at 120 Hz, respectively. A single drop of gold nanoparticles dispersion was deposited on a copper grid and then dried in air before imaging. Both Citrate- AuNPs and GCV loaded AuNPs-GSH were analysed for TEM images.
In vitro drug release of the conjugates:
The release study was carried out using dialysis bag of 12-15 kD cutoff. The suspension was filled inside the dialysis bag and tied at both the end. The bag was suspended in a beaker containing 200 ml release media[28]. The conjugates were examined in 0.1 N HCl for first 2 h and in phosphate buffer pH 6.8 for next 5 h with rotation speed at 50 rpm. 5 ml volume of dissolution media was withdrawn after given time interval and replaced with fresh medium. Samples were determined and the concentrations of the drug were also determined using HPLC.
Results and Discussion
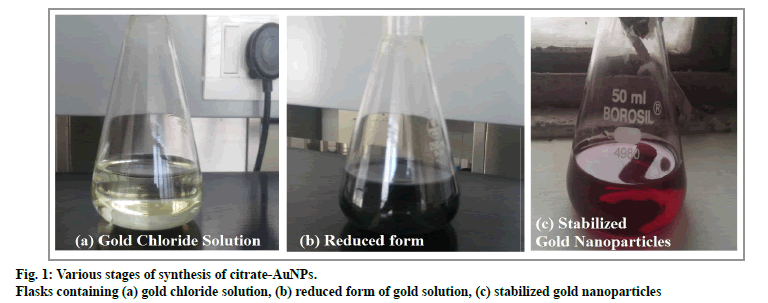
Colloidal dispersion of gold nanoparticles was prepared by reduction and stabilization of HAuCl4 with sodium citrate. The formation of ruby coloured dispersion was an indicator of nanoparticles formation[29]. Fig. 1 shows various stages of citrate-AuNPs formation, characterized by colour change from yellow to dark black and finally to ruby red colour. Different sizes of gold nanoparticles exhibit different colors. Ruby red color of dispersion indicates gold nanoparticles size is less than 50 nM. Formation of blue or black gold dispersion associated with larger size nanoparticles formation[30]. Further, Gold nanoparticles exhibit a unique surface plasmonic resonance (SPR) in visible region. The SPR depends on the particle size distribution of AuNPs. The maximum wavelength for citrate-AuNPs was observed at 523.9 nm which indicates small size distribution as well as good stability of nanoparticles[29].
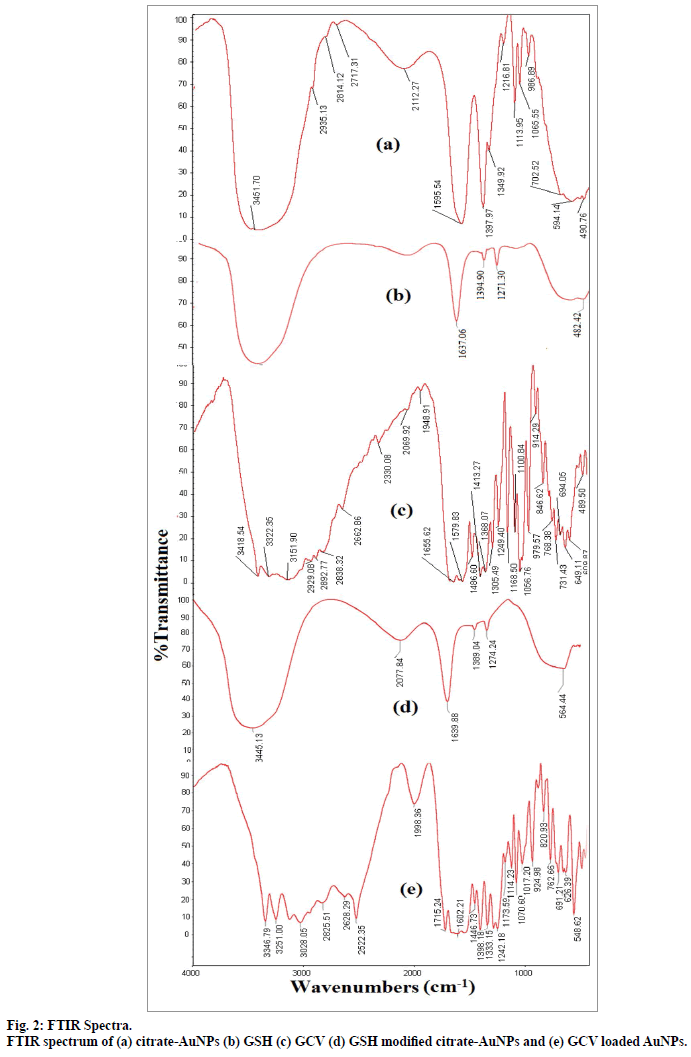
The FTIR spectra fig. 2 of citrate reduced gold nanoparticles depicted characteristic bands of citrate at 3451.71, 1595.54, 1397.97 cm-1. Presence of signal at 3451.71 cm-1 was assigned to the stretching vibration of OH group and the band at 1595.54 cm-1 was indicative of C=O stretching of carboxylate ions of citrate.
The surface of citrate-AuNPs was further modified by GSH. GSH consists of thiol groups due to presence of cysteine moiety[23]. Citrate ions were replaced by GSH molecules through strong Au-S bond. Similar thiol capping was also reported by several authors[31,32]. Further, change in color of gold suspension was observed with gradual addition of 0.05 M GSH solution. No change in ruby colour of gold suspension was observed upto 300 μl addition of 0.05 M GSH solution. Further addition of GSH solution was resulted in colour change from ruby to blue, which might be due to the aggregation of AuNPs. The surface modification with GSH was further confirmed by FTIR of GSHAuNPs. FTIR spectra of pure GSH and GSH-AuNPs were compared in fig. 2. The spectrum of GSH-AuNPs was identical to pure GSH spectrum which suggested presence of GSH molecule at gold nanoparticles surface.
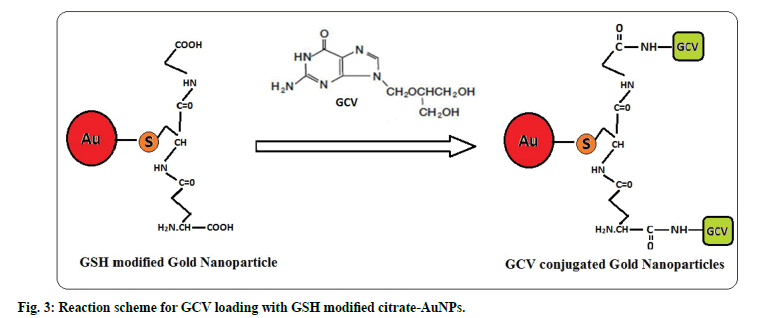
Further, GSH-AuNPs was treated with GCV solution while the pH was kept at 6.7. At this pH the amine groups of GCV remains protonated and carboxyl groups of GSH remain deprotonated. Drug loading onto GSH-AuNPs was achieved through electrostatic attraction between cationic amine group of GCV and anionic carboxyl groups of GSH (fig. 3). The drug loading of 87.27±2.52% (n=3) was observed for GCV-GSH-AuNPs. Loading of GCV was also confirmed by chemical test. Formation of green color with characteristic band at 602 nm confirmed the presence of GCV. This GCV loading on to GSHAuNPs was further confirmed by FTIR (fig. 2). All the characteristic peaks of GCV were also found in GCV loaded GSH-AuNPs. Venkatpurwar et al.[16] also reported possibility of hydrogen bonding in addition to electrostatic interaction between protonated amine group and anionic gold nanoparticles. GCV-GSHAuNPs were also kept at room temperature in closed glass vial to study stability of nanoparticles for 24 h. No colour change was observed visually for GCVGSH- AuNPs. Also no significant change in absorption wavelength was observed in SPR spectrum of GCVGSH- AuNPs which further prove the stability of GCV loaded gold nanoparticles.
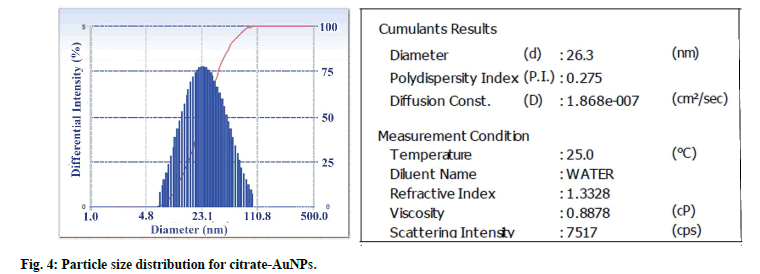
Particle size distribution of gold nanoparticles was determined by photon correlation spectroscopy (fig. 4). The average hydrodynamic diameter of citrate-AuNPs (n=3) was found 26.3±0.59 nm with PDI of 0.275. GSH-AuNPs (27.25±0.98 nm) and GCV loaded GSHAuNPs (31±1.3 nm) were having small increase in the hydrodynamic diameter. The surface modification with GSH and GCV might result in increase in electric double layer thickness which also increase diameter. This slight increase was also observed in SPR of gold nanoparticles. The red shift of 6 nm was observed for GCV loaded GSH-AuNPs. However, in TEM images no difference in particle size was observed this might be due to measurement of only electron dense gold core in TEM analysis (fig. 5)[22]. TEM micrograph of gold nanoparticles indicates that the gold nanoparticles were uniformly dispersed with spherical shape. Similar high order of uniformity (PDI=0.275) with gold nanoparticles was also reported earlier[11].
Zeta potential is considered as indicator of stability of nanoparticles. The zeta potential of citrate-AuNPs was –11.87 mV. This negative value was due to presence of three deprotonated anionic carboxyl groups of citrate ion. Further surface modification resulted in reduction in zeta potential to –10.26 mV due to replacement of citrate ion with GSH. GSH carries two deprotonated anionic carboxyl groups and one protonated cationic amine group. Thus cationic amine group nullified charge of one carboxyl group of GSH and in comparison to citrate-AuNPs, the GSH-AuNPs carries less negative charge[23]. With GCV loaded GSH-AUGNPs, higher value of –14.61 mV was observed. The protonated amine of group of GCV reacts with carboxyl group of GSH (pKa=2.12) and net negative charge might be due to presence of two anionic hydroxyl groups of GCV (pKaacidic=9.15, pKabasic=3.05). Thus both FTIR and zeta potential results confirmed the surface modification as well as GCV loading on to GSH-AuNPs.
In drug release study, initially within two h, 11.68±2.1% drug was release in 0.1 N HCl. This high initial drug release might be due to high solubility of GCV in 0.1 HCl. However, after 5 h. only 42.87±3.3% drug was released. Researchers also proved that nanoparticles having size range below 300 nm are also absorbed well through GI tract via Payer’s patch[33,34]. The purpose of in vitro release study was also to check the stability of drug conjugation with gold nanoparticles. This suggest, GCV conjugated gold nanoparticles have enough time to get absorbed and remain circulated for longer durations. Although complete fraction of drug was not released in PBS buffer under in-vitro conditions but higher drug concentration might be achieved in actual in vivo conditions under enzymatic environment.
Surface modification of citrate reduced gold nanoparticles was achieved by removal of citrate ions by GSH molecules through Au-S bonding. GCV was successfully loaded with surface modified gold nanoparticles. The FTIR and zeta potential characterization of gold nanoparticle confirmed the GCV loading onto gold nanoparticles. Further low drug release profile indicates that GCV loaded gold nanoparticles remain available for absorption across the GI tract. This may further improve the bioavailability of GCV through gold nanoparticle mediated drug delivery.
Acknowledgements
Authors are grateful to AIIMS, Delhi for providing TEM facility and IIT, Roorkee for providing FTIR facility.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Oliveira JM, Salgado AJ, Sousac N, Mano JF, Reis RL. Dendrimers and derivatives as a potential therapeutic tool in regenerative medicine strategies-A review. Prog Pol Sci 2010;35:1163-94.
- Evjen TJ, Nilssen RA, Rögnvaldsson S, Brandl M, Fossheim SL. Distearoylphosphatidyl ethanolamine-based liposomes for ultrasound-mediated drug delivery. Eur J Pharm Biopharm 2010;75:327-33.
- Mo R, Sun Q, Li N, Zhang C. Intracellular delivery and antitumor effects of pH-sensitive liposomes based on zwitterionicoligopeptide lipids. Biomaterials 2013;34:2773-86.
- Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging.Nanomed-Nanotechnol 2012;8:147-66.
- Alivisatos AP, Johnsson KP, Peng XG, Wilson TE, Loweth CJ, Bruchez MP, et al. Organization of 'nanocrystal molecules' using DNA. Nature 1996;382:609-11.
- Alivisatos P. The use of nanocrystals in biological detection. Nat Biotechnol 2004;22:47-52.
- Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 2008;60:1307-15.
- Sperling RA, Rivera Gil P, Zhang F, Zanella M, Parak WJ. Biological applications of gold nanoparticles. ChemSoc Rev 2008;37:1896-1908.
- Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 2004;104:293-346.
- Duncan B, Kim C, Rotello VM. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. J Control Release 2010;148:122-7.
- Bao Q, Geng D, Xue J, Zhou G, Gu S, Ding Y,et al. Glutathione-mediated drug release from tiopronin-conjugated gold nanoparticles for acute liver injury therapy. Int J Pharm 2013;446:112-8.
- Pissuwan D, Niidome T, Cortie MBC. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J Control Release 2011;149:65-71.
- Vigderman L, Zubarev ER. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules.Adv Drug Deliv Rev 2013;65:663-76.
- Rana S, Bajaj A, Mout R, Rotello VM. Monolayer coated gold nanoparticles for delivery applications.Adv Drug Deliv Rev 2012;64:200-16.
- Gibson JD, Khanal BP, Zubarev ER. Paclitaxel-functionalized gold nanoparticles. J Am ChemSoc 2007;129:11653-61.
- Venkatpurwar V, Shiras A, Pokharkar V. Porphyran capped gold nanoparticles as a novel carrier for delivery of anticancer drug: In vitrocytotoxicity study. Int J Pharm 2011;409:314-20.
- Mirza AZ, Shamshad H. Preparation and characterization of doxorubicin functionalized gold nanoparticles. Eur J Med Chem 2011;46:1857-60.
- Markham A, Faulde D. Ganciclovir: an update of its therapeutic use in cytomegalovirus infection. Drugs 1994;48:455-60.
- Anderson RD, Griffy KG, Jung D, Dorr A, Hulse JD, Smith RB. Ganciclovir absolute bioavailability and steady state pharmacokinetics after oral administration of two 3000 mg/d dosing regimens in human immunodeficiency virus and cytomegalovirus seropositive patients. Clinical Therapeutics 1995;17:425-32.
- Simpson CA, Salleng KJ, Cliffel DE, Feldheim DL. In vivo toxicity, biodistribution, and clearance of glutathione-coated gold nanoparticles.Nanomed-Nanotechnol 2013;9:257-63.
- Enustun BV, Turkevich J. Coagulation of colloidal gold. J Am ChemSoc 1963;85:3317-28.
- Morais T, Soares ML, Duarte JA, Soares L, Maia S, Gomes P, et al. Effect of surface coating on the biodistribution profile of gold nanoparticles in the rat. Eur J Pharm Biopharm 2012;80:185-93.
- Zhang Z, Jia J, Lai Y, Ma Y, Weng J, Sun L. Conjugating folic acid to gold nanoparticles through glutathione for targeting and detecting cancer cells. Bioorg Med Chem 2010;18:5528-34.
- Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB. Chitosan Reduced Gold Nanoparticles as Novel Carriers for Transmucosal Delivery of Insulin. Pharm Research 2007;24:1415-26.
- Ren JG, Zou MJ, Gao P, Wang Y, Cheng G. Tissue distribution of borneol-modified ganciclovir-loaded solid lipid nanoparticles in mice after intravenous administration. Eur J Pharm Biopharm 2013;83:141-8.
- AL-Neaimy UI, Hamdon EA. The use of oxidation reaction for the spectrophotometric determination of ganciclovir in pharmaceutical formulations. Raf J Sci 2012;23:93-104.
- Negi JS, Chattopadhyay P, Sharma AK, Ram V. Development of solid lipid nanoparticles (SLNs) of lopinavir using hot self nano-emulsification (SNE) technique. Eur J Pharm Sci 2013;48:231-9.
- Shah M, Chuttani K, Mishra AK, Pathak K. Oral solid compritol 888 ATO nanosuspension of simvastatin: optimization and biodistribution studies. Drug DevInd Pharm. 2011;37:526-37.
- Burda C, Chen X, Narayanan R, El-Sayed MA. Chemistry and Properties of Nanocrystals of Different Shapes. Chem Rev 2005;105:1025-1102.
- Hu M, Chen J, Li ZY, Au L, Hartland GV, Li X,et al. Gold nanostructures: engineering their plasmonic properties for biomedical applications. ChemSoc Rev 2006;35:1084-94.
- Hong R, Han G, Fernández JM, Kim BJ, Forbes NS, Rotello VM. Glutathione-mediated delivery and release using monolayer protected nanoparticlecarriers. J Am ChemSoc 2006;128:1078-9.
- Chompoosor A, Han G. Rotello VM. Charge dependence of ligand release and monolayer stability of gold nanoparticles by biogenic thiols. Bioconjugate Chem. 2008;19:1342-5.
- Das S, Chaudhury A. Recent advances in lipid nanoparticles formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011;12:62-76.
- Awaad A, Nakamura M, Ishimura K. Imaging of size-dependent uptake and identification of novel pathways in mouse Peyer’s patches using fluorescent organosilica particles. Nanomed-Nanotechnol 2012;8:627-36.