- *Corresponding Author:
- Prapaporn Boonme
Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences & Drug Delivery System Excellence Center, Prince of Songkla University, Hat-Yai, Songkhla 90112, Thailand
E-mail: prapaporn.b@psu.ac.th
| Date of Received | 11 July 2020 |
| Date of Revision | 15 November 2020 |
| Date of Acceptance | 12 February 2021 |
| Indian J Pharm Sci 2021;83(1):84-92 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Microemulsions are widely used as potential drug delivery systems, especially through the dermal route as a means to avoid systemic side effects. Also, it is well known that the formation and characteristics of MEs depend on their composition. This study aimed to investigate the influence of various types and ratios of components which could dissolve diclofenac sodium on ME formation in term of size of ME regions through the construction of sixteen pseudoternary phase diagrams using the titration method. The data obtained were used to prepare oil-in-water and water-in-oil diclofenac sodium MEs. Two o/w and two w/o blank microemulsions were selected from the system providing the largest ME region and these were subsequently incorporated with 1 % w/w diclofenac sodium. Afterward, their physicochemical and drug release properties were assessed. The largest ME region was found in the system consisting of 2:1 Cremophor RH40:Span 80, ethylhexyl palmitate and 2:1 water:isopropanol. Characteristics of diclofenac sodium MEs were similar to those of their blank counterparts, with the exception of the drug-contained MEs having higher conductivity. Our findings indicated that compatibility of oil and surfactant structures was the crucial parameter for ME formation. Furthermore, the present research not only expanded the phase behavior studies of MEs using different blends of Cremophor RH 40 and Span 80 as surfactant and cosurfactant mixtures, but also reported the application of ethylhexyl palmitate in ME formulations. Incorporation of diclofenac sodium into four studied MEs did not affect ME type. Location of the drug, drug mobility and interfacial film rigidity in MEs were found to influence the release characteristics of the loaded drug.
Keywords
Diclofenac sodium, ethylhexyl palmitate, microemulsion, release kinetics, topical delivery
Microemulsions (MEs) have wide interest as potential drug delivery systems, especially through the dermal route as a means to avoid systemic side effects [1,2]. They are defined as systems mainly composed of aqueous phase, oil phase and surfactant. Besides, cosurfactants and cosolvents may be added in some systems to enhance ME formation. MEs are thermodynamically stable and optically isotropic liquids containing internal droplet diameters within the nano-size range. Their advantages include spontaneous formulation, aesthetic appearance, thermodynamic stability, ease of preparation and high capacity to incorporate as well as delivery both hydrophilic and lipophilic active compounds. It is generally recognized that MEs can be classified depending based on their microstructure into three types, namely water-in-oil (w/o), bicontinuous and oil-in-water (o/w). These microstructures are formed depending on the types and ratios of their components. Although MEs can spontaneously form, understanding the phase behavior or association structure formation in a system is important for formulation development. The relationship between the phase behavior and the composition in a system can be captured with the construction of a pseudoternary phase diagram [3-5].
Surfactants play a crucial role in the formation of MEs since they can reduce the interfacial tension and form interfacial film between aqueous and oil phases, resulting in stabilization of ME systems. Combining two surfactants is generally known to provide strong interfacial film around the internal droplets. A surfactant with higher hydrophilic-lipophilic balance (HLB) and another with lower HLB can be mixed and used for enhancement of interfacial film flexibility [3-5]. Nonionic surfactants are considered as minimal toxicity, low skin irritation potential and less toxic toward biological membranes than ionic ones. Furthermore, they can enhance skin penetration of the active ingredients in several pharmaceutical products [6]. Thus, blends of two nonionic surfactants, namely Cremophor RH 40 (RH40, HLB = 14-16) and Span 80 (S80, HLB = 4.3) in various ratios were investigated as surfactant mixtures (Smix) to formulate diclofenac sodium MEs in this study. Diclofenac sodium loaded MEs prepared with either RH40 or S80 as surfactant had previously been reported [7,8]; however, combining RH40 and S80 has never been investigated for ME formulations of this drug.
The selection of oil phase is also important since the hydrophobic tail of the oil influences the association structure formation [9]. In this work, oleic acid (OA), isopropyl myristate (IPM), isopropyl palmitate (IPP) and ethylhexyl palmitate (EP) were studied for their effects on ME formation. Furthermore, it has been observed that the size of ME region could be increased through mixing aqueous phase with a cosolvent [10]. Hence, ethanol (EtOH), isopropanol (IPA) and polyethylene glycol 400 (PEG400) were determined for their influences on ME formation when used as cosolvents in the aqueous phase. All components investigated are ingredients with generally recognized as safe (GRAS) status and widely used in topically pharmaceutical products.
Diclofenac sodium was selected as a model drug to investigate the effects of ME type and composition on the characteristics and release in the current study. It is a non-steroidal anti-inflammatory drug (NSAID) belonging to the phenylacetic acid derivative group. It is used worldwide to relieve the symptoms of painful and inflammatory conditions. Although diclofenac sodium is known as a safe NSAID, some serious gastrointestinal tract side effects limit its oral administration [11,12]. Hence, its topical formulation in form of MEs is an interesting alternative to its oral dosage forms. The proposed topical diclofenac sodium MEs formulations are expected to be easy to apply, increase patients’ compliance and drug efficacy as well as reduce systemic side effects. Diclofenac sodium MEs have been mostly formulated in w/o type in previous reports [8,13,14]. A comparison between w/o and o/w MEs containing diclofenac sodium was previously reported; however, in that work, construction of phase diagrams and preparation of formulations were performed at 70° [15].
This study aimed to investigate the phase behavior of various nonionic systems to prepare o/w and w/o MEs for incorporating diclofenac sodium at room temperature (25±2°). Additionally, physicochemical properties of the selected blank and diclofenac sodium MEs were characterised. In vitro drug release from the selected formulations via dialysis membrane was evaluated.
Materials and Methods
Diclofenac sodium was purchased from PC Drug Center Co., Ltd. (Bangkok, Thailand). Cremophor RH 40 (RH40, polyoxyl 40 hydrogenated castor oil), Span 80 (S80, sorbitan monooleate), oleic acid (OA), isopropyl myristate(IPM), isopropyl palmitate (IPP), ethylhexyl palmitate (EP), isopropanol (IPA) and polyethylene glycol 400 (PEG 400) were acquired from JKK Chemical LP (Bangkok, Thailand). Acetronitrile, methanol, ethanol (EtOH) and acetic acid were obtained from RCI Labscan Co. Ltd. (Bangkok, Thailand). Sodium chloride, anhydrous di-sodium hydrogen orthophosphate and potassium dihydrogen orthophosphate were procured from Univar Australia Pty Ltd. (New South Wales, Australia) and used for preparation of isotonic phosphate buffer solution pH 7.4 (PBS). All chemicals were pharmaceutical or analytical grade and used without modification. Distilled water was prepared in-house and used throughout the experiment.
Solubility study of diclofenac sodium in various components:
The solubility of diclofenac sodium in RH40, S80, OA, IPP, IPM, EP, IPA and water was determined. An excess amount of diclofenac sodium was added into 2 ml of each component. Subsequently, each mixture was shaken at room temperature for 72 h to attain equilibrium. The suspensions were then centrifuged at 15000 rpm for 30 min. The supernatants were filtered through a 0.45-μm nylon membrane filter. The drug concentrations in the filtrates were determined by high performance liquid chromatography (HPLC) technique after appropriate dilution.
Determination of the effects of various components on ME formation:
In order to find out the existence region of MEs, pseudoternary phase diagrams were constructed by titration method at room temperature. The mixture of RH40 and S80 at a weight ratio of 1:2, 1:1 or 2:1 was designated as Smix. Four oils (i.e., OA, IPM, IPP and EP) were separately investigated. Briefly, Smix and oil were mixed at the weight ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2 and 9:1. The blend of Smix and oil was then titrated drop-by-drop with aqueous phase under vigorous agitation and appearance of the obtained mixture was observed. Aqueous phase was either water or mixture of water and a cosolvent (i.e., IPA, PEG400 or EtOH) at a determined ratio. All component amounts providing clear MEs were recorded, calculated in term of percentage w/w and plotted on a triangular graph to define the ME region in the pseudoternary phase diagram of each system. The size of each ME region was evaluated as the percentage of the total area of the phase diagram by cut-and-weight method. Effects of various surfactant ratios, oil and cosolvent types as well as their ratios were investigated as shown in Table 1.
| System | Smix (RH40:S80) | Oil phase | Aqueous phase |
|---|---|---|---|
| S01 | 1:2 | OA | Water |
| S02 | 1:2 | IPM | Water |
| S03 | 1:2 | IPP | Water |
| S04 | 1:2 | EP | Water |
| S05 | 1:1 | OA | Water |
| S06 | 1:1 | IPM | Water |
| S07 | 1:1 | IPP | Water |
| S08 | 1:1 | EP | Water |
| S09 | 2:1 | OA | Water |
| S10 | 2:1 | IPM | Water |
| S11 | 2:1 | IPP | Water |
| S12 | 2:1 | EP | Water |
| S13 | 2:1 | EP | 4:1 Water:IPA |
| S14 | 2:1 | EP | 2:1 Water:IPA |
| S15 | 2:1 | EP | 2:1 Water:PEG400 |
| S16 | 2:1 | EP | 2:1 Water:EtOH |
Table 1: Composition Of The Studied Systems
Preparation of blank and diclofenac sodium MEs:
Four points were selected from the largest ME region for preparation of different blank MEs. Two points were for o/w MEs while other two points were for w/o MEs according to weight ratios of oil and aqueous phases. The four blank MEs were designated as F1, F2, F3 and F4 and were prepared by simply mixing the indicated amounts of the various components at room temperature. In order to prepare diclofenac sodium MEs, 1 % w/w diclofenac sodium and 99 % w/w blank MEs were mixed by magnetic stirring for 30 min at room temperature until the drug was completely dissolved. These produced four diclofenac sodium MEs designated as F1-DS, F2-DS, F3-DS, and F4-DS.
Characterisation of blank and diclofenac sodium MEs:
The blank and diclofenac sodium MEs were visually observed for clarity, colour, phase separation and precipitation. The isotropic property of the prepared samples was observed under a polarized microscope (Olympus BX61, Japan). ME type was identified by combination of three techniques, i.e., dilution test, conductivity measurement and refractive index measurement. Dilution test was performed by dropping each sample into water and then observing for miscibility. Conductivity and refractive index values of the samples were measured using an electrical conductivity meter (Five Easy, Mettler Toledo, Switzerland) and a refractometer (Abbe 60/74 Refractometer, Bellingham & Stanley Ltd, UK), respectively. Pure water and oil were also measured for their refractive index values. Particle size and polydispersity index (PdI) values of the samples were determined without any dilution to avoid changing from MEs to other association structures by a zeta potential analyzer (Zetasizer Nano series, Nano ZS Red badge ZeN3600, Malvern Instruments Limited, UK). The pH values of the samples were evaluated by a digital pH meter (S20-K, Mettler Toledo, Switzerland). The rheological characteristics and viscosity values were performed by a rheometer (DV III Ultra Programmable Rheometer, Brookfield Engineering Laboratories, USA) using a spindle number SC4- 31 with five different shearing speeds from 20 to 100 rpm according to the detected % torque close to 100. All experiments were carried out in triplicate at room temperature.
In vitro release study of diclofenac sodium MEs:
The drug release profiles of F1-DS, F2-DS, F3-DS and F4-DS were studied in vitro by modified Franz diffusion cells (Hanson Model 57-6 M, Research Corporation, USA). Dialysis membrane with molecular weight cut-off (MWCO) of 3500 Dalton (Spectra/Por®3, Spectrum laboratories, Inc., USA) was used as a membrane model. It was cut into appropriate size and soaked in the receptor fluid for 1 h before placed between the donor and receptor chambers of the diffusion cell. The degassed PBS (12 ml) was used as receptor fluid and stirred at the speed of 300 rpm by a magnetic stirrer. Temperature of circulating bath was maintained at 37±0.5°. The sample (1.0 g) was applied to the donor chamber with diffusion area of 1.77 cm2. Aliquot of receptor fluid (1.0 ml) was collected after applying the sample for 0.5, 1, 2, 4, 6, 8, 10, 12 and 24 h. Fresh PBS of an equal volume was immediately to replace the receptor fluid. In vitro release study of each sample was carried out in five replications. All collected samples were analyzed for amounts of diclofenac sodium by HPLC method. The cumulative amount of released diclofenac sodium per unit area of the membrane (Q, μg/cm2) was calculated by Eqn. 1 and subsequently plotted against time to obtain the release profiles. The release data were also analyzed by three different kinetics models, namely zero order, first order and Higuchi model as shown in Eqn. 2-4, respectively.
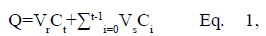 where Ct is the concentration of diclofenac sodium in the receptor
fluid at each sampling time (t), Ct is the concentration
of diclofenac sodium of the ith sample, and Vr and Vsare the volumes of the receptor fluid and the sample,
respectively.
where Ct is the concentration of diclofenac sodium in the receptor
fluid at each sampling time (t), Ct is the concentration
of diclofenac sodium of the ith sample, and Vr and Vsare the volumes of the receptor fluid and the sample,
respectively.
Zero order: Qt=Q0 - k0t Eq. 2, First order: lnQt=lnQ0 - kft Eq. 3, Higuchi model: Qt=kHt1/2 Eq. 4, where Qt is cumulative amount of diclofenac sodium released in time t, Q0 is initial amount of diclofenac sodium in the evaluated sample, and k0, kf and kH are release rate constants of zero order, first order and Higuchi model, respectively.
Analysis of diclofenac sodium:
The amounts of diclofenac sodium was quantitatively determined by HPLC as previously described with some modifications [13]. Analysis was performed using Shimadzu HPLC series Prominence-iLC-2030C 3D system (Shimadzu, Japan). A reverse-phase Luna®C18 column (5 μm particle size, 4.6×150 mm, Phenomenex, USA) with a guard column was used as a stationary phase. The mobile phase consisted of acetonitrile and 0.5 % acetic acid (60:40 v/v) at a flow rate of 1.0 ml/min. The injection volume was 20 μl and the detecting wavelength was set at 276 nm. The analytical technique was validated according to the International Conference on Harmonisation (ICH), guidelines for drug quantitative analysis. In brief, the analysis technique was confirmed for selectivity. The calibration curve between concentrations of diclofenac sodium standard solutions and peak areas was in the linearity. Additionally, the percent relative standard deviation (Percentage RSD) were less than 2 % for both intra-day and inter-day [16].
Statistical analysis:
One-way ANOVA followed by Post Hoc Multiple Comparison analysis was employed to analyze the data obtained from in vitro release study. The p value <0.05 was considered as difference that is statistically significant.
Results and Discussion
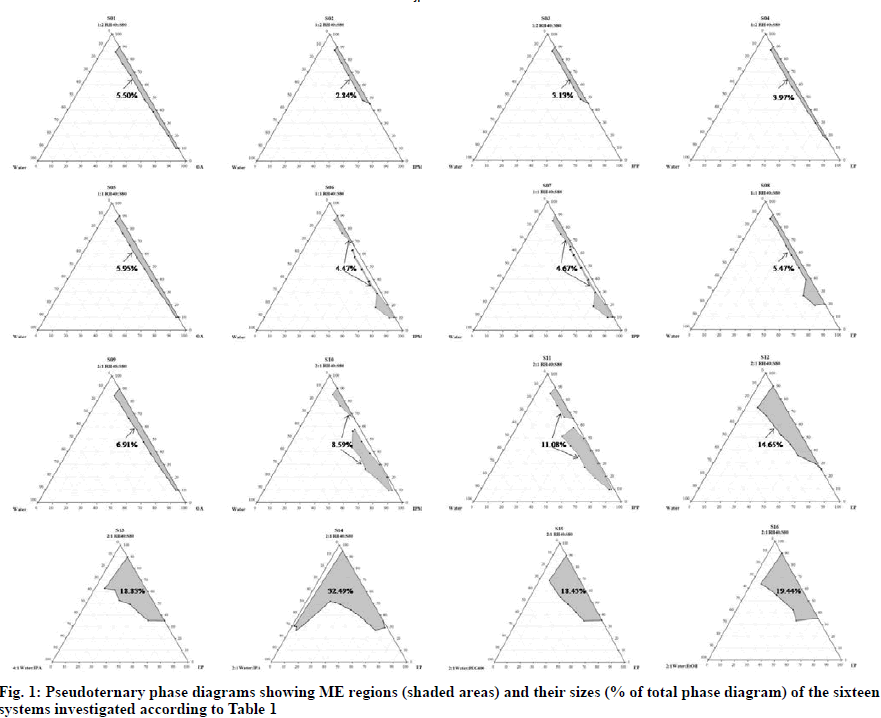
Solubility values of diclofenac sodium in different components are presented in Table 2. It can be seen that the drug was more soluble in hydrophilic than in hydrophobic components due to its intrinsic hydrophilicity. As illustrated in fig. 1, when compared with identical oil and aqueous phases, the sizes of ME regions obtained from the systems containing 2:1 RH40:S80 (total HLB of 10.8-12.1) as Smix were larger than those composed of 1:2 RH40:S80 (total HLB of 7.5-8.2) and 1:1 RH40:S80 (total HLB of 9.2- 10.2). This increase in ME region might be explained by the fact that the 2:1 RH40:S80 could provide the highest enhancement in partitioning of the studied oils at the interfacial film. Affinity between a surfactant or a surfactant blend and oil phase were reported to influence the ME formation since oil lipophilicity and oil penetration in the surfactant palisade layer affect the surfactant layer curvature of self-organized structures [10,17-20]. When 2:1 RH40:S80 and water were used as Smix and aqueous phase, respectively, it was observed that the sizes of ME region followed the order: EP (C24H48O2)>IPP(C19H38O2)>IPM (C17H34O2)>OA (C18H34O2 with a C–C double bond). A possible explanation could be related to the solubilization capability or compatibility between chain structure (length and bond-type) of oil and chain arrangement of Smix [21]. Certainly, differences in the phase behaviors could be the result of not only the geometric parameters but also chain stiffness and branching. Hence, the mutual compatibility between the hydrophobic tail of the surfactant and alkyl chain of oil could affect the insertion of oil into the surfactant film, leading to different spontaneous curvature [22]. Although the largest ME region was obtained when water was used as the aqueous phase, i.e., the system consisting of 2:1 RH40:S80 as Smix and EP as oil phase (S12); nevertheless, it was only 14.65 % which was not sufficient to generate the different ME types and incorporate the drug. Therefore, a cosolvent such as IPA, PEG400 or EtOH was added into the aqueous phase of this system to increase the size of the ME region. When water and cosolvent were adjusted to a ratio of 2:1, it can be seen that ME regions of the systems containing the studied cosolvents (S14, S15 and S16) were larger than the one without a cosolvent (S12). The ME region of the system with IPA as cosolvent (S14) was larger than those with PEG400 (S15) or EtOH (S16). Furthermore, it should be noted that when IPA was included in aqueous phase as a cosolvent, it enlarged ME region an amount-dependent manner. The system with 2:1 water:IPA (S14) yielded a larger ME region compared to that with 4:1 water:IPA (S13).
| Component | Solubility (mg/mL)* |
|---|---|
| RH40 | 18.12±0.01 |
| S80 | 2.32±0.01 |
| OA | 15.54±0.14 |
| IPM | 0.22±0.01 |
| IPP | 0.17±0.01 |
| EP | 0.15±0.01 |
| IPA | 9.21±0.01 |
| Water | 20.640.04 |
*mean±SD, (n = 3).
Table 2: Solubility Of Diclofenac Sodium In Various Components
Thus, adding the proper amount of IPA as a cosolvent to the ME systems could enhance the ME region, which is attributable to the reduction in interfacial tension of the interfacial film layer and dielectric constant of aqueous phase [23].
Among the sixteen studied systems, S14 provided the largest ME region which was expected to form o/w and w/o ME types. Therefore, the obtained data not only expanded the phase behavior studies of MEs using different blends of RH40 and S80 as Smix, but also demonstrated the application of EP in ME formulations. EP is a mixture of esters formed by the reaction of 2-ethylhexyl alcohol with palmitic acid. It is an important nontoxic raw material typically used as a skin conditioning agent and emollient in cosmetics [24,25]; however, there is no report of its application in the preparation of MEs. EP could be used to prepare low skin-irritating risk organo gels [26]. Nanoemulsions composed of EP as oil phase were reported for improvement of skin moisturizing [27].
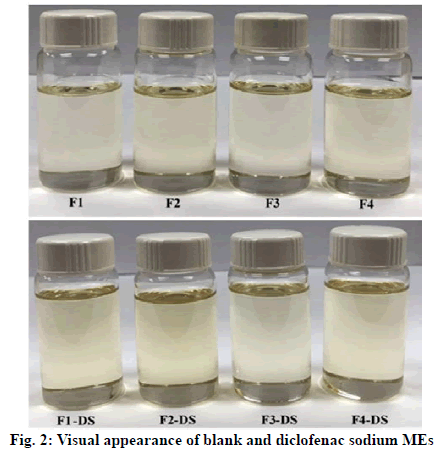
The system composed of 2:1 RH40:S80 as Smix, EP as oil phase and 2:1 water:IPA as aqueous phase was used to prepare four different blank MEs (F1, F2, F3 and F4) as described in Table 3. They were further incorporated with the investigated drug to obtain four 1 % w/w diclofenac sodium MEs, i.e., F1-DS, F2-DS, F3-DS and F4-DS, respectively. All blank and diclofenac sodium MEs were clear yellowish liquids as shown in fig. 2. Incorporation of diclofenac sodium into the blank MEs did not affect their visual appearance. No birefringence was observed when all obtained samples were examined under a polarized light microscope, indicating the isotropic property of the MEs (data not shown). Characteristics of the prepared samples were summarized in Table 4. The data indicated that F1, F1-DS, F2 and F2-DS were o/w MEs since they were miscible with water, had high conductivity and low refractive index close to that of water. In contrast, F3, F3-DS, F4 and F4-DS had the opposite properties, implying they were w/o MEs [28]. It was noted that addition of diclofenac sodium into the blank MEs increased the conductivity due to the presence of sodium salt; however, the drug incorporation did not affect the ME type. Similarly, it was previously reported that diclofenac sodium MEs showed higher conductivity values than their blank counterparts and loading diclofenac sodium into the formulations had no negative effect on stability of ME systems [8]. All blank and diclofenac sodium MEs had nano-size internal droplets. Their PdI values were high because the process of ME formation requires negative Gibbs free energy for spontaneity, resulting in high entropy and dynamic properties [29,30]. These observations are in accord with previous reports showing high PdI values of MEs [28,31]. The pH values of all formulations were quite neutral in the range of 6.5 to 8. These pH values are generally acceptable for skin application products and could provide positive effect on dermal absorption [32]. All samples had low viscosity with Newtonian flow, implying that they can spread easily on the skin. Apparently, viscosity values depended on the formulation components. F1 and F3 contained lower Smix amount than F2 and F4, and this led to lower viscosity values due to influence of intrinsic viscosity of surfactants [19].
| Formulation | Composition (% w/w) | |||||
|---|---|---|---|---|---|---|
| Oil phase | Smix | Aqueous phase | ||||
| EP | RH40 | S80 | Water | IPA | ||
| F1 | 5.00 | 23.33 | 11.67 | 40.00 | 20.00 | |
| F2 | 10.00 | 30.00 | 15.00 | 30.00 | 15.00 | |
| F3 | 60.00 | 23.33 | 11.67 | 3.33 | 1.67 | |
| F4 | 45.00 | 30.00 | 15.00 | 6.67 | 3.33 | |
Table 3: Composition Of The Selected Blank Mes
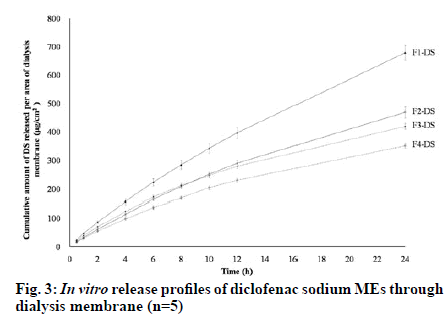
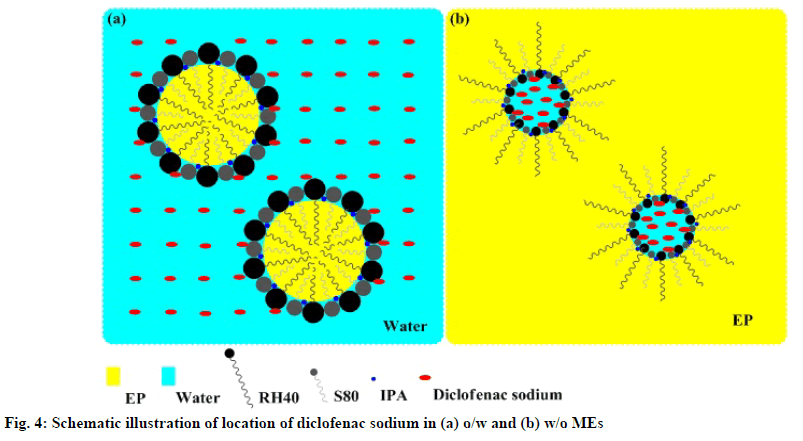
The release profiles of the studied diclofenac sodium MEs in fig. 3 showed that the drug could be slowly released from all MEs. From the obtained data, it was observed that the cumulative amounts of diclofenac sodium per area of dialysis membrane gradually increased with increasing time. Table 5 exhibits that the cumulative amount of diclofenac sodium released at 24 h (Q24) and release rate of F1-DS was significantly higher than other MEs (p<0.05). Although Q24 of F2- DS was not significantly different from that of F3-DS (p>0.05), F2-DS showed significantly faster release rate than F3-DS (p<0.05). Furthermore, Q24 and release rate of F2-DS and F3-DS were significantly higher than those of F4-DS (p<0.05). The amounts of diclofenac sodium released in the first 8 h followed the order: F1-DS>F3-DS≈F2-DS>F4-DS. After 8 h, the released amounts of diclofenac sodium followed the order: F1- DS > F2-DS > F3-DS > F4-DS. F1-DS and F2-DS were o/w MEs. Thus, it seems that diclofenac sodium being a hydrophilic drug is preferentially located in the external aqueous phase and near the hydrophilic head of nonionic surfactants of these MEs. Conversely, diclofenac sodium is preferentially located in the internal aqueous phase and near the hydrophilic head of nonionic surfactants of w/o F3-DS and F4-DS. Hence, diclofenac sodium in F3-DS and F4-DS had to partition from the internal hydrophilic phase into the external hydrophobic phase before diffusing through dialysis membrane, resulting in lower release amounts and rates than that in F1-DS and F2-DS. Schematic illustration of the location of diclofenac sodium in the different types of MEs is presented in fig. 4. The findings of this study were consistent with those of previous works which reported that the location of active ingredient in MEs influences in vitro drug release rates and amounts [33-36]. When compared with the same o/w ME type, the aqueous phase amounts of F1-DS and F2-DS were 60 % and 45 %, respectively. The higher aqueous phase content in F1-DS could lead to easier drug mobility from external aqueous phase of ME through dialysis membrane. When compared within the same w/o ME type, the Smix amounts of F3-DS and F4-DS were 35 % and 45 %, respectively. Higher Smix amount affected stronger rigidity of interfacial film which could impede drug diffusion. Therefore, F3-DS provided more drug released amount and rate than F4-DS.
| Sample | Dilution test with water | Conductivity (μS/cm)* | Refractive index* | Type | Particle size (nm)* | PdI* | pH* | Viscosity at 100 rpm (cps)* | Rheological flow |
|---|---|---|---|---|---|---|---|---|---|
| Water | - | - | 1.3330±0.0001 | - | - | - | - | - | - |
| F1 | miscible | 111.37±2.60 | 1.4025±0.0001 | o/w | 155.2±0.2 | 0.677±0.006 | 6.73±0.04 | 141.4±4.44 | Newtonian |
| F1-DS | miscible | 317.13±3.65 | 1.4049±0.0001 | o/w | 151.9±13.0 | 0.805±0.170 | 7.23 ± 0.02 | 135.87±7.91 | Newtonian |
| F2 | miscible | 75.69±5.33 | 1.4200±0.0001 | o/w | 246.8±7.0 | 0.512±0.015 | 6.90±0.03 | 196.13±2.83 | Newtonian |
| F2-DS | miscible | 188.81±0.85 | 1.4210±0.0001 | o/w | 215.8±5.4 | 0.553±0.028 | 7.33 ± 0.02 | 196.06±6.34 | Newtonian |
| F3 | immiscible | 0.41±0.01 | 1.4511±0.0001 | w/o | 45.9±5.8 | 0.522±0.060 | 7.48±0.04 | 128.24±1.12 | Newtonian |
| F3-DS | immiscible | 1.90±0.01 | 1.4525 ± 0.0001 | w/o | 57.0±1.3 | 0.304±0.026 | 7.97±0.04 | 154.04±0.35 | Newtonian |
| F4 | immiscible | 1.37±0.06 | 1.4495 ± 0.0001 | w/o | 141.7±4.0 | 0.999±0.002 | 7.43±0.01 | 242.32±8.16 | Newtonian |
| F4-DS | immiscible | 5.04±0.38 | 1.4515±0.0001 | w/o | 141.3±5.4 | 1.000±0.000 | 7.85±0.02 | 240.35±4.65 | Newtonian |
| EP | - | - | 1.4460±0.0001 | - | - | - | - | - | - |
*mean±SD, (n = 3).
Table 4: Characteristics Of Blank And Diclofenac Sodium Mes
The release parameters of diclofenac sodium from the four MEs are summarized in Table 5. Release kinetics of F1-DS apparently fitted best with the zero order model while that of F2-DS, F3-DS and F4-DS seemed to be best fitted with the Higuchi model. Although F1-DS and F2-DS were o/w MEs, their components and physical properties were different as presented in Tables 2 and 3. Therefore, their release kinetics followed different models. F1-DS release was without directly concentration dependent following the zero order model. However, F2-DS release followed the Higuchi model and was mainly attributed to higher surfactant amount and higher viscosity compared with F1-DS. Hydrophilic diclofenac sodium could interact with hydrophilic head of RH40 and S80. High viscosity of formulations could obstruct drug diffusion [37]. In w/o F3-DS and F4-DS, diclofenac sodium was located in the internal aqueous phase. Therefore, the drug had to be released from matrix systems, i.e., diffusing internal droplets to continuous medium of MEs before passing through the membrane. This situation caused the release kinetics of F3-DS and F4-DS to follow the Higuchi model [38]. The results indicated that release characteristics of diclofenac sodium from MEs were related to location of the drug, drug mobility and interfacial film rigidity of MEs.
| Formulation | Q24(μg/cm2)* | Release rate (μg/cm2/h)* | Zero order model | First order model | Higuchi model | |||
|---|---|---|---|---|---|---|---|---|
| r2 | k0 (μg/cm2/h)* | r2 | kf(1/h)* | r2 | kH (μg/cm2/h1/2)* | |||
| F1-DS | 678.86±25.52 | 27.95±2.48 | 0.9876 | 27.95±2.48 | 0.7214 | 0.1252±0.0024 | 0.9786 | 154.64±14.54 |
| F2-DS | 469.42±19.71 | 19.50±1.82 | 0.9752 | 19.50±1.82 | 0.6974 | 0.1259±0.0026 | 0.9866 | 108.96±9.62 |
| F3-DS | 419.79±10.66 | 17.12±0.93 | 0.9521 | 17.12±0.93 | 0.6803 | 0.1128±0.0038 | 0.9968 | 97.36±5.55 |
| F4-DS | 351.92±8.25 | 14.45±0.77 | 0.9593 | 14.45±0.77 | 0.6887 | 0.1162±0.0055 | 0.9937 | 81.74±4.50 |
*mean±SD, (n = 5). Q24 was cumulative amount of diclofenac sodium released at 24 h. The k0, kf and kH were release constants of zero order, first order and Higuchi model, respectively.
Table 5: Release Parameters Of Diclofenac Sodium Mes
Based on the results obtained in the present investigation, the following conclusions can be drawn. Compatibility of oil and surfactant structures contributed to the crucial parameter for ME formation. The system consisted of 2:1 RH40:S80 as Smix, EP as oil phase and 2:1 water:IPA as aqueous phase provide the largest ME region and both o/w and w/o MEs could form according to oil and aqueous phase ratios. The characteristics of MEs with and without diclofenac sodium were similar; except for conductivity. In addition, the location of the drug, drug mobility and interfacial film rigidity in MEs were found to influence the release characteristics of the loaded drug.
Financial support and sponsorship:
The financial assistance from the Nanotec-PSU Center of Excellence on Drug Delivery System, Thailand is gratefully acknowledged
Acknowledgements:
Sincere thanks to Dr. Fredrick Nwude Eze for written English proof-reading.
Conflicts of interest:
Authors declare there is no conflicts of interest.
References
- Souto EB, Doktorovova S, Boonme P. Lipid-based colloidal systems (nanoparticles, microemulsions) for drug delivery to the skin: materials and end-product formulations. J Drug Del Sci Tech 2011;21(1):43-54.
- Lopes LB. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics 2014;6(1):52-77.
- Eccleston GM. Microemulsions. In: Swarbac J, Boylan JC, editors. Encyclopedia of Pharmaceutical Technology. New York: Marcel Dekker;1994;375-421.
- Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev 2000;45(1):89-121.
- Mehta SK, Kaur G. Microemulsions: thermodynamic and dynamic properties. In: Tadashi M, editor. Thermodynamics. Rijeka: Intech;2011;381-406.
- Pandey A, Mittal A, Chauhan N, Alam S. Role of surfactants as penetration enhancer in transdermal drug delivery system. J Mol Pharm Org Process Res 2014;2:113.
- Yang C, Shen Y, Wang J, Ouahab A, Zhang T, Tu J. Cationic polymer-based micro-emulgel with self-preserving ability for transdermal delivery of diclofenac sodium. Drug Deliv 2015;22(6):814-22.
- Kantarci G, Ozguney I, Karasulu HY, Arzik S, Guneri T. Comparison of different water/oil microemulsions containing diclofenac sodium: preparation, characterization, release rate, and skin irritation studies. AAPS PharmSciTech 2007;8(4):75-81.
- Wang W, Wei H, Du Z, Tai X, Wang G. Formation and characterization of fully dilutable microemulsion with fatty acid methyl esters as oil phase. ACS Sustainable Chem Eng 2015;3:443-50.
- Wuttikul K, Boonme P. Formation of microemulsions for using as cosmeceutical delivery system: effects of various components and characteristics of some formulations. Drug Deliv Transl Res 2016;6(3):254-62.
- Khazaeinia T, Jamali F. Effect of drug release rate on therapeutic outcomes: formulation dependence of gastrointestinal toxicity of diclofenac in the rat. Inflammopharmacol 2004;12(1):69-80.
- Francio VT, Davani S, Towery C, Brown TL. Oral versus topical diclofenac sodium in the treatment of osteoarthritis. J Pain Palliat Care Pharmacother 2017;31(2):113-20.
- Songkro S, Tanmanee N, Maneenuan D, Chuchome T, Lo NL, Boonme P. Investigation of enhancing effect of Glucam® P-20 on the in vitro skin permeation of diclofenac sodium microemulsions. Lat Am J Pharm 2012;31(5):734-42.
- Thakkar PJ, Madan P, Lin SS. Transdermal delivery of diclofenac using water-in-oil microemulsion: formulation and mechanistic approach of drug skin permeation. Pharm Dev Technol 2014;19(3):373-84.
- Premarathne EPN, Karunaratne DN, Perera ADLC. Controlled release of diclofenac sodium in glycolipid incorporated microemulsions. Int J Pharm 2016;511(2):890-8.
- International Conference on Harmonization (ICH). Validation of Analytical Procedures: Text and Methodology (Q2R1) Geneva: ICH;2005.
- Kunieda H, Horii M, Koyama M, Sakamoto K. Solubilization of polar oils in surfactant self-organized structures. J Colloid Interface Sci 2001;236(1):78-84.
- Mahdi ES, Sakeena MHF, Abdulkarim MF, Abdullah GZ, Sattar MA, Noor AM. Effect of surfactant and surfactant blends on pseudoternary phase diagram behavior of newly synthesized palm kernel oil esters. Drug Des Devel Ther 2011;5:311-23.
- Roohinejad S, Oey I, Wen J, Lee SJ, Everett DW, Burritt DJ. Formulation of oil-in-water β-carotene microemulsions: effect of oil type and fatty acid chain length. Food Chem 2015;174:270-8.
- Hlaing NHE, Pakpayat N, Boonme P. Stability and release kinetics of natural oil microemulsions containing nicotinamide. J Cosmet Sci 2020;71(1):23-35.
- BayrakY, Iscan M. Studies on the phase behavior of the system non-ionic surfactant/alcohol/alkane/H2O. Colloids and Surfaces A: Physicochem Eng Aspects 2005;268(1-3):99-103.
- Mehta SK, Kaur G, Mutneja R, Bhasin KK. Solubilization, microstructure, and thermodynamics of fully dilutable U-type Brij microemulsion. J Colloid Interface Sci 2009;338(2):542-9.
- Ly HV, Longo ML. The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys J 2004;87(2):1013-33.
- Vyumvuhore R, Tfayli A, Manfait M, Baillet-Guffroy A. Vibrational spectroscopy coupled to classical least square analysis, a new approach for determination of skin moisturizing agents’ mechanisms. Skin Res Technol 2014;20:282-92.
- Fossa-Shirata MM, Maia-Campos PMBG. Influence of UV filters on the texture profile and efficacy of acosmetic formulation. Int J Cosmet Sci 2017;39:622-8.
- Burkhardt M, Noirez L, Gradzielski M. Organogels based on 12-hydroxy stearic acid as a leitmotif: dependence of gelation properties on chemical modifications. J Colloid Interface Sci 2016;466:369-76.
- Barreto SMAG, Maia MS, Benicá AM, Assis HRBS, Leite-Silva VR, Rocha-Filho PA, et al. Evaluation of in vitro and in vivo safety of the by-product of Agave sisalana as a new cosmetic raw material: development and clinical evaluation of a nanoemulsion to improve skin moisturizing. Ind Crops Prod 2017;108:470-9.
- Boonme P, Boonthongchuay C, Wongpoowarak W, Amnuaikit T. Evaluation of nicotinamide microemulsion on the skin penetration enhancement. Pharm Dev Technol 2016;21:116-20.
- Kreilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev 2002;54(Suppl 1):S77-S98.
- Rakshit AK, Moulik SP. Physicochemistry of w/o microemulsions: formation, stability, and droplet clustering. In: Fanun M, editor. Microemulsions: Properties and Applications. Boca Raton:CRC Press;2008:17-57.
- Acharya A, Sanyal SK, Moulik SP. Physicochemical investigations on microemulsification of eucalyptol and water in presence of polyoxyethylene (4) lauryl ether (Brij-30) and ethanol. Int J Pharm 2001;229(1-2):213-26.
- Martinez-Pla JJ, Martin-Biosca Y, Sagrado S, Villanueva-Camanas RM, Medina-Hernandez MJ. Evaluation of the pH effect of formulations on the skin permeability of drugs by biopartitioning micellar chromatography. J Chromatogr A 2004;1047(2):255-62.
- Spiclin P, Homar M, Valant AZ, Gasperlin M. Sodium ascorbyl phosphate in topical microemulsions. Int J Pharm 2003;256(1-2):65-73.
- Djordjevic L, Primorac M, Stupar M. In vitro release of diclofenac diethylamine from caprylocaproyl macrogolglycerides based microemulsions. Int J Pharm 2005;296(1-2):73-9.
- Mehta SK, Kaur G, Bhasin KK. Tween-embedded microemulsions - physicochemical and spectroscopic analysis for antitubercular drugs. AAPS PharmSciTech 2010;11(1):143-53.
- Songkro S, Lo NL, Tanmanee N, Maneenuan D, Boonme P
- Djekic L, Marković B, Micov A, Tomić M, Pecikoza U, Stepanović-Petrović R. Percutaneous delivery of levetiracetam as an alternative to topical nonsteroidal anti-inflammatory drugs: formulation development, in vitro and in vivo characterization. Drug Deliv Transl Res 2021;11(1):227-41.
- Siepmann J, Peppas NA. Higuchi equation: derivation, applications, use and misuse. Int J Pharm 2011;418:6-12.