- *Corresponding Author:
- Yang Liu
Center for Anesthesia and Perioperative Medicine, Xi’an Peoples Hospital, Xi’an, Shaanxi 710000, China
E-mail: liuyang19891024@163.com
| Date of Received | 14 September 2021 |
| Date of Revision | 14 August 2022 |
| Date of Acceptance | 07 February 2023 |
| Indian J Pharm Sci 2023;85(1):128-134 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of pristimerin on gefitinib resistance in lung cancer cells and its regulation on microRNA-936. Lung cancer cell HCC827 was cultured in vitro, lung cancer gefitinib resistant cell HCC827/gefitinib resistant was established and HCC827/gefitinib resistant cells were randomly assigned to control group, pristimerin-L group, pristimerin-M group, pristimerin-H group, gefitinib group, gefitinib+pristimerin group, gefitinib+microRNA-negative control group, gefitinib+microRNA-936 group, gefitinib+pristimerin+anti-microRNA negative control group and gefitinib+pristimerin+anti-microRNA-936 group. 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide was used to detect the inhibition rate of cell proliferation, as well as the median half-maximal inhibitory concentration; the expression amount of microRNA-936 was detected by quantitative reverse transcription-polymerase chain reaction; cell migration and invasion were detected by transwell chamber assay. Compared with HCC827 cells, the proliferation inhibition rate of HCC827/gefitinib resistant cells was significantly lower and the half-maximal inhibitory concentration value was significantly higher (p<0.05); compared with the control group, the inhibition rate of cell proliferation was increased, the half-maximal inhibitory concentration value was decreased and the expression of microRNA-936 was increased (p<0.05) in pristimerin-L group, pristimerin-M group and pristimerin-H group; compared with the gefitinib group, the inhibition rate of cell proliferation was higher and the number of migration and invasion cells decreased in the gefitinib+pristimerin group (p<0.05); compared with the gefitinib+microRNA negative control group, the gefitinib+microRNA-936 group showed higher cell proliferation inhibition rate and lower cell number in migration and invasion (p<0.05); compared with the gefitinib+pristimerin+anti-microRNA negative control group, the cell proliferation inhibition rate decreased and the migration and invasion cell numbers increased in the gefitinib+pristimerin+anti-microRNA-936 group (p<0.05). Pristimerin may enhance cell gefitinib sensitivity by inhibiting proliferation, migration and invasion of gefitinib resistant cells in lung cancer by up regulating microRNA-936 expression.
Keywords
Pristimerin, microRNA-936, lung cancer, gefitinib, drug resistance, proliferation, migration
Lung cancer is one of the common malignant tumors in the clinic and its main pathological type is non- small cell lung cancer, some patients are already at an advanced stage when diagnosed leading to the loss of optimal treatment timing, some patients are treated with chemotherapy and other modalities, but chemotherapy drugs have some toxic side effects and studies have shown that the generation of drug resistance is an important reason for reducing the efficacy of clinical treatment for lung cancer patients[1-3]. Therefore, it becomes a key research to explore the mechanism of the drug resistance of tumor cells and its reversal method. Pristimerin has anti-tumor, anti-inflammatory and other effects[4]. But its effect on gefitinib resistance in lung cancer cells is unknown. Studies have shown that microRNAs (miRNAs) can increase or decrease the sensitivity or resistance of chemotherapy drugs by regulating tumor cell drug resistance related gene expression[5]. MiR-936 expression is down regulated in laryngeal squamous cell carcinoma[6]. In this study, we mainly investigate the effect of pristimerin on gefitinib resistance in lung cancer cells and explore its regulatory effect on miR-936, aiming to provide a new direction for reversing gefitinib resistance.
Materials and Methods
Materials and reagents:
The lung cancer HCC827 cell strain was purchased from the American Type Culture Collection (ATCC) cell bank, United States of America (USA). Pristimerin was purchased from Sigma, USA; gefitinib was purchased from AstraZeneca, USA; 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) and Dimethyl Sulfoxide (DMSO) were purchased from Sigma, USA; Trizol reagent was purchased from Takara, Japan; Lipofectamine 2000 was purchased from Beijing Solarbio Science and Technology Co. Ltd.; reverse transcription and quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) kits were purchased from Tiangen Biotech, Beijing; miR-936 mimics, miRNA-Negative Control (NC), anti-miR-936 and anti-miR-NC were purchased from Shanghai GenePharma and transwell chambers were purchased from Corning, USA.
Method:
Experimental grouping: HCC827 cells were cultured in flasks until 80 % of the flask bottom was covered with cells, the culture medium was discarded and after 24 h incubation with medium containing 2.5 mg/ml gefitinib it was changed to medium containing 0.2 mm gefitinib, the cells were incubated for 7 d and incubated for 14 d in medium without gefitinib and subsequently the cells were incubated in medium containing 0.1, 0.5, 1, 5, 10, 20 μmol/l gefitinib in the culture medium for 28 d and after expansion of the culture, MTT assay was used to test the resistance of the cells to gefitinib and a stable phenotype of the resistant cell strain was obtained, namely HCC827/Gefitinib Resistant (GR) [7]. Logarithmic growth phase HCC827/GR cells were harvested and pristimerin of various concentrations was used (0.25, 0.5, 1 μmol/l) for treatment of 24 h[8] and were scored as pristimerin-L group, pristimerin-M group and pristimerin-H group, respectively. Meanwhile, normal cultured HCC827/GR cells were included as the control group. Subsequent experiments set up the gefitinib group (containing 0.5 μmol/l gefitinib for treatment of 24 h), gefitinib+pristimerin group (containing 1 μmol/l pristimerin and 0.5 μmol/l gefitinib for treatment of 24 h), gefitinib+miR-NC group (miR-NC transfected to HCC827/GR cells, containing 0.5 μmol/l gefitinib for treatment of 24 h), gefitinib+miR-936 group (miR-936 mimics transfected into HCC827/GR cells, containing 0.5 μmol/l gefitinib for treatment of 24 h), gefitinib+pristimerin+anti-miR- NC group (anti-miR-NC transfected to HCC827/GR cells, at a concentration of 1 μmol/l pristimerin and a concentration of 0.5 μmol/l gefitinib for treatment of 24 h), gefitinib+pristimerin+anti-miR-936 group (anti-miR-936 transfected into HCC827/GR cells, at a concentration of 1 μmol/l pristimerin and a concentration of 0.5 μmol/l gefitinib for treatment of 24 h).
Cell proliferation inhibition rate was detected by MTT assay: HCC827 cells in logarithmic growth phase of each group and GR HCC827/GR cells were collected and seeded into 96-well plate (5×103/well), in which each well was added 20 μl MTT reagent, incubated for 4 h, the supernatant was discarded and 150 μl DMSO was added, and the relative absorbance value (Optical Density (OD)) at 490 nm was detected with a micro plate reader.
Cell gefitinib half-maximal Inhibitory Concentration (IC50) value was detected by MTT assay: HCC827 cells in logarithmic growth phase from each group and GR HCC827/GR cells were collected, digested with 0.25 % trypsin and cell suspension was prepared and seeded in 96-well plate (5×103/well) using 0.1, 0.5, 1, 5, 10 μmol/l gefitinib for treatment of 72 h and 15 μl of 5 mg/ml MTT reagent was added to each well for 4 h. The medium was discarded and 150 μl DMSO was added to each well, the relative absorbance value (OD) at 490 nm was detected by a micro plate reader and the gefitinib IC50 value at 50 % cell inhibition rate was calculated by Msvbvm50 software.
Expression levels of miR-936 in cells were detected by qRT-PCR: Logarithmic growth phase HCC827 cells and HCC827/GR cells were collected and total cellular Ribonucleic Acid (RNA) was extracted and reversely transcribed to synthesize complementary Deoxyribonucleic Acid (cDNA). The PCR amplification reaction system and reaction conditions were all operated according to the kit instructions to calculate the relative expression of miR-936.
Cell migration and invasion were detected by transwell assay:
For migration assay, HCC827/GR cells in logarithmic growth phase of each group were collected (5×104/ml) and were added to the upper chamber of a transwell chamber (200 μl/well), and the lower chamber was filled with medium containing 10 % fetal bovine serum (600 μl/well), incubated for 24 h, washed with Phosphate Buffered Saline (PBS), and fixed for 20 min in paraformaldehyde and 10 min in paraformaldehyde. For invasion assay, Matrigel was diluted using pre- chilled media and added to the upper chamber according to the protocol of 40 μl per well of density and incubated in the incubator for 5 h, and the follow-up steps are the same as cell migration assay.
Related protein expressions were detected by Western blot: HCC827/GR cells in logarithmic growth phase of each group were collected and total cellular protein was extracted, protein concentration was determined by Bicinchoninic Acid (BCA) assay, protein samples were taken for Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), membrane shifted, blocked, CyclinD1 (dilution 1:800), Matrix Metalloproteinase (MMP)-2 (dilution 1:1000), MMP-9 (dilution 1:800), p21 (dilution 1:800) primary antibody dilutions (Santa Cruz, USA), incubated at 24° for 24 h, washed with Tris Buffered Saline Tween® 20 Detergent (TBST) and secondary antibody dilutions (dilution 1:2000) were added (Abcam, USA), Electrochemiluminescence (ECL) was added drop wise and quantity one software was applied to detect the band gray value.
Statistical processing:
The data were analyzed by Statistical Package for the Social Sciences (SPSS) 21.0 and the measurement data were expressed in (X͞ ±s) and all conformed to normal distribution, independent samples t-test was used for comparison between two groups and one-way analysis of variance was used for comparison among multiple groups and p<0.05 was considered statistically significant.
Results and Discussion
After treatment by gefitinib at different concentrations, the inhibition rate of proliferation of HCC827/GR cells was decreased and the IC50 value was increased (p<0.05) compared with that of HCC827 cells, as shown in Table 1.
| Grouping | Gefitinib concentrations | IC50 (μmol/l) | |||||
|---|---|---|---|---|---|---|---|
| 0.1 μmol/l | 0.5 μmol/l | 1 μmol/l | 5 μmol/l | 10 μmol/l | 20 μmol/l | ||
| HCC827 | 11.51±1.14 | 28.65±2.71 | 42.15±4.22 | 59.32±5.84 | 71.36±7.14 | 83.62±8.41 | 2.11±0.24 |
| HCC827/GR | 3.12±0.31* | 8.45±0.82* | 11.63±1.18* | 17.39±1.72* | 25.47±2.53* | 34.98±3.25* | 78.62±7.33* |
| t | 21.305 | 21.403 | 20.895 | 20.662 | 18.174 | 16.184 | 31.297 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 compared with HCC827 group
Table 1: Effect of Gefitinib on the Inhibition Ratio of HCC827/GR in Lung Cancer HCC827 Cells and Lung Cancer GR Cells (X͞±s, n=9)
After treatment with different concentrations of gefitinib, compared with the control group, the inhibition ratio of cell proliferation was increased and the IC50 values were significantly decreased in the pristimerin-L group, pristimerin-M group and pristimerin-H group, while the inhibition ratio of cell proliferation and the IC50 values were statistically significant (p<0.05) in the pristimerin-L group, pristimerin-M group and pristimerin-H group, as shown in Table 2.
| Grouping | Gefitinib concentrations | IC50 (μmol/l) | |||||
|---|---|---|---|---|---|---|---|
| 0.1 μmol/l | 0.5 μmol/l | 1 μmol/l | 5 μmol/l | 10 μmol/l | 20 μmol/l | ||
| Control | 3.36±0.31 | 8.69±0.83 | 12.58±1.23 | 19.68±1.94 | 27.41±2.73 | 35.14±3.52 | 76.47±7.21 |
| Pristimerin-L | 6.32±0.61* | 11.32±1.14* | 20.63±1.57* | 28.61±2.85* | 37.21±3.51* | 46.87±4.22* | 28.82±3.01* |
| Pristimerin-M | 8.36±0.72*# | 17.33±1.74*# | 29.28±2.33*# | 40.21±4.01*# | 51.66±5.21*# | 63.58±6.24*# | 8.22±1.13*# |
| Pristimerin-H | 11.98±1.32*#& | 26.32±2.41*#& | 38.41±3.41*#& | 55.32±5.58*#& | 65.14±6.84*#& | 76.21±7.29*#& | 2.95±0.28*#& |
| F | 172.995 | 203.523 | 211.819 | 144.549 | 104.957 | 96.485 | 646.888 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 compared with control group; #p<0.05 compared with the pristimerin-L group and &p<0.05 compared with the pristimerin-M group
Table 2: Effect of Pristimerin on Gefitinib Resistance of HCC827/GR Cells (X͞±s, n=9)
Compared with the control group, the expression of miR-936 was increased in the cells of the pristimerin-L group, pristimerin-M group and pristimerin-H group, and the differences of miR-936 among the expression of the pristimerin-L group, pristimerin-M group and pristimerin-H group were statistically significant (p<0.05), as shown in Table 3.
| Grouping | miR-936 |
|---|---|
| Control | 1.00±0.08 |
| Pristimerin-L | 1.45±0.14* |
| Pristimerin-M | 2.03±0.24*# |
| Pristimerin-H | 2.89±0.27*#& |
| F | 153.069 |
| p | 0.000 |
Notes: *p<0.05 compared with control group; #p<0.05 compared with the pristimerin-L group and &p<0.05 compared with the pristimerin-M group
Table 3: Effect of Pristimerin on miR-936 Expression in HCC827/GR Cells (X͞±s, n=9)
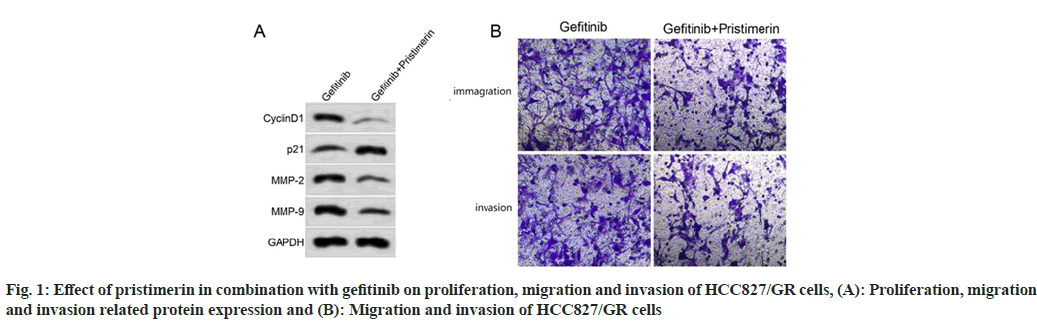
Compared with the gefitinib group, the inhibition rate of cell proliferation increased, the number of migratory and invasive cells decreased, and the expression levels of cyclinD1, MMP-2, MMP-9 decreased (p<0.05) and p21 increased (p<0.05) in the gefitinib+pristimerin group, as shown in fig. 1 and Table 4.
| Grouping | Inhibition rate (%) | Number of migration cells (s) | Number of invasion cells (s) | CyclinD1 protein | p21 protein | MMP-2 protein | MMP-9 protein |
|---|---|---|---|---|---|---|---|
| Gefitinib | 8.11±0.83 | 95.36±9.55 | 78.59±7.25 | 0.55±0.05 | 0.29±0.03 | 0.66±0.06 | 0.78±0.07 |
| Gefitinib+pristimerin | 41.25±4.21* | 47.51±4.33* | 33.47±3.54* | 0.17±0.02* | 0.71±0.07* | 0.24±0.02* | 0.31±0.03* |
| t | 23.169 | 13.69 | 16.777 | 21.169 | 16.546 | 19.922 | 18.514 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 compared with gefitinib group
Table 4: Effect of Pristimerin in Combination with Gefitinib on Proliferation, Migration and Invasion of HCC827/GR Cells (X͞±s, n=9)
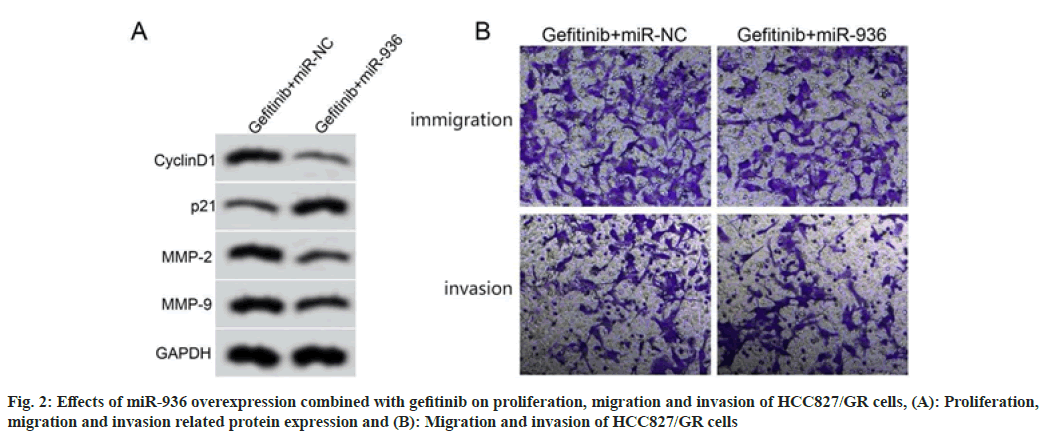
Compared with the gefitinib+miR-NC group, the gefitinib+miR-936 group showed higher cell proliferation inhibition rate, lower number of migratory and invasion cells, lower expression levels of cyclinD1, MMP-2, MMP-9 and higher expression level of p21 (p<0.05), as shown in fig. 2 and Table 5.
| Grouping | miR-936 | Inhibition rate (%) | Number of migration cells (s) | Number of invasion cells (s) | CyclinD1 protein | p21 protein | MMP-2 protein | MMP-9 protein |
|---|---|---|---|---|---|---|---|---|
| Gefitinib+miR-NC | 1.00±0.07 | 7.66±0.78 | 93.25±9.33 | 75.36±7.52 | 0.57±0.05 | 0.28±0.03 | 0.64±0.06 | 0.77±0.07 |
| Gefitinib+miR-936 | 2.58±0.24* | 36.51±3.71* | 56.24±5.84* | 42.21±4.38* | 0.25±0.02* | 0.67±0.06* | 0.29±0.03* | 0.36±0.03* |
| t | 18.96 | 22.83 | 10.087 | 11.428 | 17.827 | 17.441 | 15.652 | 16.151 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with gefitinib+miR-NC group, *p<0.05
Table 5: Effects of miR-936 Overexpression Combined with Gefitinib on Proliferation, Migration and invasion of HCC827/GR Cells (X͞±s, n=9)
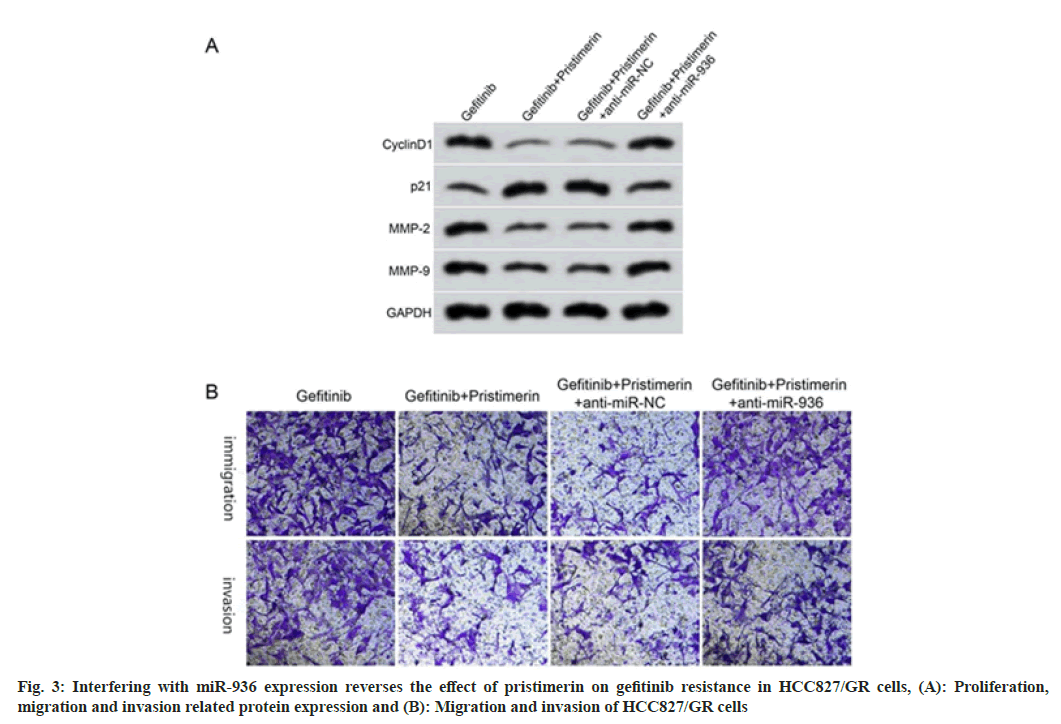
Compared with the gefitinib+pristimerin+anti-miR-NC group, the cell proliferation inhibition rate decreased, the migration and invasion cell numbers increased, and the expression levels of cyclinD1, MMP-2, MMP-9 increased, and p21 decreased in the gefitinib+pristimerin+anti-miR-936 group (p<0.05), as shown in fig. 3 and Table 6.
| Grouping | miR-936 | Inhibition rate (%) | Number of migration cells (s) | Number of invasion cells (s) | CyclinD1 protein | p21 protein | MMP-2 protein | MMP-9 protein |
|---|---|---|---|---|---|---|---|---|
| Gefitinib | 1.00±0.07 | 7.93±0.78 | 97.25±9.36 | 77.21±7.36 | 0.56±0.05 | 0.27±0.03 | 0.67±0.06 | 0.79±0.07 |
| Gefitinib+pristimerin | 2.68±0.27* | 45.32±4.55* | 49.25±4.53* | 36.58±3.66* | 0.19±0.02* | 0.72±0.07* | 0.27±0.02* | 0.34±0.03* |
| Gefitinib+pristimerin+anti-miR-NC | 2.71±0.26 | 46.98±4.71 | 48.21±4.22 | 34.87±3.69 | 0.18±0.02 | 0.70±0.06 | 0.25±0.03 | 0.32±0.03 |
| Gefitinib+pristimerin+anti-miR-936 | 1.32±0.13# | 20.65±2.33# | 79.65±7.33# | 61.25±6.47# | 0.45±0.04# | 0.38±0.03# | 0.56±0.05# | 0.68±0.06# |
| F | 178.031 | 269.154 | 115.754 | 122.047 | 265.714 | 179.971 | 214.5 | 198.379 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with gefitinib group, *p<0.05 and compared with gefitinib+pristimerin+anti-miR-NC group, #p<0.05
Table 6: Interfering with miR-936 Expression Reverses the Effect of Pristimerin on Gefitinib Resistance in HCC827/GR Cells (X͞±s, n=9)
The occurrence of gefitinib resistance in lung cancer cells has severely affected the treatment efficacy and even reduced the quality of life of patients, and studies have shown that noncoding RNAs such as miRNAs may decrease lung cancer cell gefitinib resistance by regulating the expression of related genes[9,10]. But the effect of some drugs on gefitinib resistance of lung cancer cells is still unknown.
Pristimerin belongs to a compound extracted from the plant Celastrus orbiculatus Thunb. of Celastraceae which has anti-cancer and other effects and also inhibits tumor cell activity such as gastric, cervical and nasopharyngeal cancer[11]. Pristimerin can inhibit breast cancer cell proliferation by regulating apoptosis protein expression[12]. Pristimerin may also inhibit asthma development by regulating body immune balance[13]. Consistent with the above findings, our results showed that the inhibition of proliferation of HCC827/GR cells treated with different concentrations of gefitinib was significantly decreased, and the IC50 value of gefitinib was significantly increased, whereas the inhibition of proliferation of HCC827/GR cells treated with different concentrations of pristimerin was significantly increased, it was significantly increased with the increase of the dosage of pristimerin, and the IC50 value was significantly decreased, significantly decreased by increasing the dosage of pristimerin used, suggested that pristimerin may contribute to HCC827/GR cell sensitivity by inhibiting HCC827/ GR cell proliferation. But further exploration is still needed regarding its specific mechanism of action. The study demonstrated that pristimerin could exert anticancer effects by regulating miR expression, as in breast cancer; in breast cancer cells treated with pristimerin, miR-524-5p was significantly upregulated and DUB3 was significantly downregulated, thereby inhibiting breast cancer progression[14]. In glioblastoma multiforme cells, pristimerin inhibits glioma progression via miR-524-5p/Argonaute 2/ PTPN1[15]. Alternatively, pristimerin may reduce multidrug resistance by regulating miRNA expression, as in lung cancer, where co-treatment of pristimerin with cisplatin was through inhibition of miRNA-23a/ Protein kinase B (Akt)/glycogen synthase kinase 3 beta (β) signaling pathway, inhibiting autophagy to enhance cisplatin sensitivity in lung cancer cells[16]. In gastric cancer tissues and cells, miR-936 expression was down regulated and overexpression miR-936 inhibited cell proliferation and invasion and promoted cell apoptosis through ERBB4 and Akt related pathways[17]. MiR- 936 inhibited the invasiveness of epithelial ovarian cancer by inactivating phoshatidylinositol-3 kinase/Akt pathway[18]. Similar to the above findings, the present results showed that the expression of miR-936 was elevated after treatment with different concentrations of pristimerin and increased with the increasing dosage of pachymeninx used, suggesting that pristimerin may affect lung cancer cell gefitinib resistance by up regulating the expression of miR-936.
In this study, co-treatment of lung cancer cells with gefitinib and pristimerin resulted in a significant increase in cell proliferation inhibition; whereas co-treatment of lung cancer cells with miR-936 overexpression and gefitinib resulted in a significant increase in cell proliferation inhibition. Our results showed that cyclinD1 was down regulated and p21 was up regulated after treatment of lung cancer cells with gefitinib together with pachymeninx, whereas overexpression of miR-936 together with gefitinib was down regulated and p21 was up regulated, suggesting that pristimerin may inhibit lung cancer cell proliferation by regulating the cell cycle. Our results showed that HCC827/GR cells were significantly less able to migrate and invade after co-treatment of gefitinib with pristimerin or miR-936 overexpression with gefitinib, suggesting that pristimerin overexpression with miR- 936 may enhance cell sensitivity by inhibiting migration and invasion of GR cells.
In conclusion, pristimerin inhibits proliferation, migration and invasion of GR lung cancer cells and then enhances cell gefitinib sensitivity and the mechanism may be related to the up regulation of miR-936, which may provide an experimental foundation for further unraveling the molecular mechanism of gefitinib resistance in lung cancer and may provide a new direction for pristimerin to reduce gefitinib resistance in lung cancer.
Conflict of interests:
The authors declared no conflict of interests.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61(2):69-90.
- Chen H, Yao X, Li T, Lam CW, Zhang R, Zhang H, et al. Compound Kushen injection combined with platinum-based chemotherapy for stage III/IV non-small cell lung cancer: A meta-analysis of 37 RCTs following the PRISMA guidelines. J Cancer 2020;11(7):1883-98.
[Crossref] [Google Scholar] [PubMed]
- Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Jänne PA, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non–small-cell lung cancer. J Clin Oncol 2010;28(2):357-60.
[Crossref] [Google Scholar] [PubMed]
- Deeb D, Gao X, Liu YB, Pindolia K, Gautam SC. Pristimerin, a quinonemethide triterpenoid, induces apoptosis in pancreatic cancer cells through the inhibition of pro-survival Akt/NF-κB/mTOR signaling proteins and anti-apoptotic Bcl-2. Int J Oncol 2014;44(5):1707-15.
[Crossref] [Google Scholar] [PubMed]
- Chen L, Zhu Q, Lu L, Liu Y. MiR-132 inhibits migration and invasion and increases chemosensitivity of cisplatin-resistant oral squamous cell carcinoma cells via targeting TGF-β1. Bioengineered 2020;11(1):91-102.
[Crossref] [Google Scholar] [PubMed]
- Lin XJ, Liu H, Li P, Wang HF, Yang AK, Di JM, et al. miR-936 suppresses cell proliferation, invasion and drug resistance of laryngeal squamous cell carcinoma and targets GPR78. Front Oncol 2020;10:60.
[Crossref] [Google Scholar] [PubMed]
- Sun Lin. MiR-625-3p reverses gefitinib resistance in non-small cell lung cancer cells by regulating the AXL/AKT pathway. Soochow Univ J 2018:1-52.
- Xie G, Du J, Dong G. Inhibition of taxol resistant breast cancer cell growth by pristimerin. J Guiyang Med Coll 2016;41(9):1029-32.
- Lei Y, Guo W, Chen B, Chen L, Gong J, Li W. Tumor-released lncRNA H19 promotes gefitinib resistance via packaging into exosomes in non-small cell lung cancer. Oncol Rep 2018;40(6):3438-46.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Li M, Hu C. Exosomal transfer of miR-214 mediates gefitinib resistance in non-small cell lung cancer. Biochem Biophy Res Commun 2018;507(1-4):457-64.
[Crossref] [Google Scholar] [PubMed]
- Li W, Guo J, Yao Z. Inhibitory effect of pristimerin on the proliferation of HNE2 cells derived from human nasopharyngeal carcinoma. Progress Mod Biomed 2015;15(35):6826-30.
- Xie G, Du J, Xu X. Effect and mechanism of pristimerin on growth and apoptosis of human breast cancer MCF-7 cells. J Dalian Med Univ 2016;38(3):215-8.
- Chen N, Han J, Gao C. Effect and significance of pristimerin on Th17/Treg imbalance in children with bronchial asthma. Chin J Immunol 2017;33(1):58-61.
- Cheng S, Zhang Z, Hu C, Xing N, Xia Y, Pang B. Pristimerin suppressed breast cancer progression via miR-542-5p/DUB3 axis. Onco Targets Ther 2020;13:6651-60.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Hu C, Zhen Y, Pang B, Yi H, Chen X. Pristimerin inhibits glioma progression by targeting AGO2 and PTPN1 expression via miR-542-5p. Biosci Rep 2019;39(5):BSR20182389.
- Zhang Y, Wang J, Hui B, Sun W, Li B, Shi F, et al. Pristimerin enhances the effect of cisplatin by inhibiting the miR-23a/Akt/GSK3β signaling pathway and suppressing autophagy in lung cancer cells. Int J Mol Med 2019;43(3):1382-94.
[Crossref] [Google Scholar] [PubMed]
- Liu S, Gong Y, Xu XD, Shen H, Gao S, Bao HD, et al. MicroRNA-936/ERBB4/Akt axis exhibits anticancer properties of gastric cancer through inhibition of cell proliferation, migration and invasion. Kaohsiung J Med Sci 2021;37(2):111-20.
[Crossref] [Google Scholar] [PubMed]
- Li C, Yu S, Wu S, Ni Y, Pan Z. MicroRNA-936 targets FGF2 to inhibit epithelial ovarian cancer aggressiveness by deactivating the PI3K/Akt pathway. Onco Targets Ther 2019;12(1):5311-22.
[Crossref] [Google Scholar] [PubMed]