- *Corresponding Author:
- Xiaoting Wu
Department of Pharmacy, Dushu Lake Hospital Affiliated to Soochow University ,Suzhou 215124, Jiangsu,China
E-mail: 18605188880@163.com
| Date of Received | 25 November 2022 |
| Date of Revision | 14 September 2023 |
| Date of Acceptance | 23 January 2024 |
| Indian J Pharm Sci 2024;86(1):346-352 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the action of procaine on ovarian cancer cell oncogenic phenotypes and its molecular mechanism. Ovarian cancer cells were divided into control group, procaine low, medium and high dose group, microRNA-204 group, microRNA-NC group, high dose group+anti-microRNA-ovarian cancer group, high dose group+anti-microRNA-204 group; 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide, clone formation and transwell assays detected cell proliferation, migration and invasion, Western blotting and quantitative reverse transcription polymerase chain reaction detected protein and ribonucleic acid expression. Procaine treatment dose-dependently impaired the proliferation, migration, invasion and epithelial mesenchymal transition progression in ovarian cancer cells. Moreover, microRNA-204 levels were observed to be dose-dependently increased by procaine treatment. MicroRNA-204 overexpression suppressed the oncogenic phenotypes mentioned above in ovarian cancer cells. Importantly, the inhibition of microRNA-204 abolished the anticancer effects of procaine on ovarian cancer cells. In conclusion, procaine up-regulated microRNA-204 to ovarian cancer cell oncogenic phenotypes.
Keywords
Procaine, microRNA-204, ovarian cancer, tumor, anesthesia
Ovarian Cancer (OC) is a highly invasive malignant tumor occurring in female reproductive system with high invasiveness, the postoperative metastasis and recurrence have been identified to be important causes of death in OC patient[1]. Anesthetics have been exhibited that can affect tumor growth, metastasis, and patient prognosis, however, whether postoperative recurrence of tumors can be reduced from the perspective of anesthesia management is one of the research hotspots in recent years[2,3]. Procaine is a local anesthetic, and research has shown that procaine has achieved certain effects in the treatment of various tumors, it can affect tumor progression by modulating tumor suppressor genes that not only serve as diagnostic markers, but also as potential therapeutic targets[4]. For example, procaine could impair Hepatocellular Carcinoma (HCC) development by attenuating cell Epithelial- Mesenchymal Transition (EMT) process via mediating the phosphorylation of c-Met[5]. Procaine has growth inhibition and apoptosis induction effects on gastric cancer cells[6]. In addition, procaine restrained the survival and induced death in colon cancer cells by modulating RhoA expression[7]. However, the effects and mechanisms of procaine on OC are still unclear. Additionally, recent researches showed that microRNA (miR)- 204 showed the anticancer action in OC by suppressing cancer cell oncogenic phenotypes[8,9]. Through the preliminary experiments, we observed that procaine could elevate miR- 204 expression in OC cells. Herein, this work probed the regulatory action of procaine and miR- 204 on OC cell proliferation and metastasis, and further investigated whether procaine affect OC progression via modulating miR-204.
Materials and Methods
Cells and main reagents:
OC cell line SKOV3 (American Type Culture Collection (ATCC)); Roswell Park Memorial Institute (RPMI)-1640 medium, 10 % Fetal Bovine Serum (FBS) (Gibco, United States of America (USA)); procaine (Shanghai Xinyu Biotechnology Co., Ltd.); transwell insert, Matrigel gel (Corning, USA); 3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) colorimetric assay (Aimijie Technology Co., Ltd.); Trizol reagent and SYBR Green Taq Mix (Beijing Tiangen Biotechnology Co., Ltd.); Lipofectamine 2000 (Invitrogen, USA); miR-204 mimic (miR-204), inhibitor (anti-miR-204) and the contrasts (miRNC or anti-miR-NC) (Gima Biol Engineering Inc., Shanghai, China), The antibodies against Matrix Metalloproteinase-2 (MMP-2) (ab37150, 1:500), Ki67 (ab16667, 1:1000), MMP-9 (ab137867, 1:500), N-cadherin (1:1000, ab18203), Vimentin (1:500, ab92547), E-cadherin (1:500, ab40772), β-actin (ab6276, 1:1000) and secondary Horseradish Peroxidase (HRP)-conjugated antibody (ab6721, 1:2000) (Abcam, United Kingdom).
Cell culture and treatment:
SKOV3 cells were cultured in 10 % FBS-contained RPMI-1640 medium and incubated at 37° with 5 % Carbon dioxide (CO2). For procaine treatment, SKOV3 cells were incubated with completed medium plus 3, 6, and 12 mmol/l procaine. Untreated cells were used as the control.
Cell transfection:
SKOV3 cells were placed onto a 6-well plate (1×105 cells/ml per well). After washing with Opti-MEM I medium without serum, each well was added with 100 nM Opti-MEM containing 0.4 ml miR-204, anti-miR-204 or the contrasts (miRNC or anti-miR-NC) and 10 μg/ml Lipofectamine 2000, and incubated with cells for 48 h. Then cells were treated with 12 mmol/l procaine for further analysis.
MTT assay:
SKOV3 cells were reacted with 20 μl of MTT for 4 h in 96-well plates (5×103 cells/well). Then each well was incubated with Dimethyl Sulfoxide (DMSO) to dissolved MTT crystals, and spectrophotometric absorbance at 490 nm was examined to assess cell viability.
Colony formation assay:
SKOV3 cells were incubated in 12-well plates at 500 cells per well for (10-14) d. Then colonies (≥50 cells) were visualized and counted with crystal violet staining.
Transwell assay:
For migration analysis, 100 μl cell suspension (in serum-free medium) were transferred into the upper chamber of transwell inserts. 24 h later, migrating cells in bottom surface was dyed with crystal violet for 30 min, then an invert microscope was used to capture and count cells. For invasion analysis, the upper chamber of Transwell inserts were pre-coated with Matrigel gel and the other steps are the same as described above.
Western blotting:
The total protein of cells in each group was extracted, quantified, and then subjected to polyacrylamide gel electrophoresis (100 V, 2 h), and then transferred to the Polyvinylidene Difluoride (PVDF) membranes, followed by incubating at 4° overnight with primary antibodies, and secondary antibody at 37° for 2 h. The gray value of bands was analyzed.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR):
TRIzol® reagent was applied for the isolation of total Ribonuclic Acid (RNAs), which were then reverse-transcribed into complementary Deoxyribonucleic Acid (cDNA). Thereafter, qRT-PCR was performed with primers. miR-204, forward 5’-AACCTGATCCCGTGCTGAGATTG-3’, reverse 5’-GCGGATCAAGATTGAGTTCGGTT-3’ and U6, forward 5’-CGCTTCGGCAGCACATATACTA-3’, reverse 3’-CGCTTCACGAATTTGCGTGTCA-3’. The fold changes were normalized to U6 Threshold Cycle (CT) values adopting the 2-ΔΔCT method.
Statistical analysis:
Data are presented as the x±s. The t-test and analysis of variance with Least Significant Difference (LSD)-t were used for comparison between two and multiple groups. p<0.05 were considered significant.
Results and Discussion
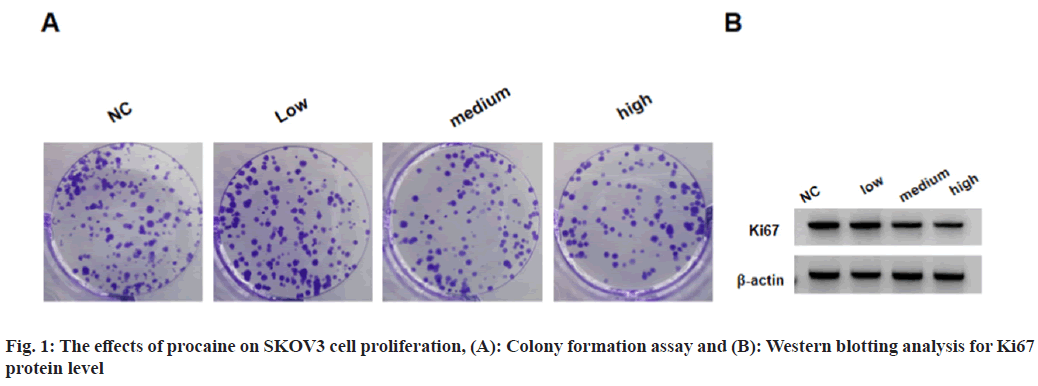
SKOV3 cell viability and the number of colonies formed by SKOV3 cells, and Ki67 protein levels in SKOV3 cells were suppressed in low, medium and high procaine treatment groups as shown in fig. 1 and Table 1.
| Group | A value | Colonies | Ki-67 |
|---|---|---|---|
| NC | 1.055±0.09 | 128±10.53 | 0.91±0.09 |
| Low | 0.816±0.08* | 98±8.46* | 0.78±0.07* |
| Medium | 0.643±0.06* | 71±6.46* | 0.61±0.06* |
| High | 0.517±0.05* | 53±5.08* | 0.49±0.04* |
| F | 94.881 | 154.23 | 67.698 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. NC group
Table 1: The Effects of Procaine on SKOV3 Cell Proliferation
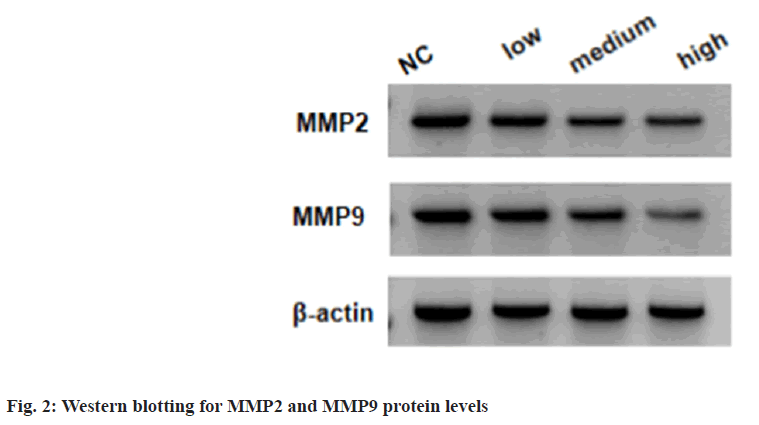
Relative to the NC group, the migratory and invasive SKOV3 cells were markedly reduced in low, medium and high procaine treated SKOV3 cells; moreover, the related markers were measured, the low, medium and high procaine treatment suppressed the expression of MMP2 and MMP9 relative to untreated cells as shown in fig. 2 and Table 2.
| Group | MMP2 | MMP9 | Migratory cells | Invasive cells |
|---|---|---|---|---|
| NC | 0.88±0.07 | 0.75±0.07 | 231±18.91 | 181±16.03 |
| Low | 0.72±0.07* | 0.62±0.06* | 197±15.18* | 144±12.68* |
| Medium | 0.57±0.05* | 0.51±0.04* | 153±12.18* | 105±9.76* |
| High | 0.45±0.04* | 0.40±0.03* | 119±9.03* | 81±6.91* |
| F | 89.871 | 73.527 | 106.221 | 124.179 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. NC group
Table 2: Procaine Treatment Suppresses SKOV3 Migration and Invasion
The low, medium and high procaine treatment, relative to NC group, notably elevated miR-204 levels in SKOV3 cells as shown in Table 3.
| Group | miR-204 |
|---|---|
| NC | 1.00±0.11 |
| Low | 1.57±0.13* |
| Medium | 1.94±0.16* |
| High | 2.31±0.19* |
| F | 123.903 |
| p | 0.000 |
Note: *p<0.05 vs. NC group
Table 3: Procaine Promotes miR-204 Expression in SKOV3 Cells
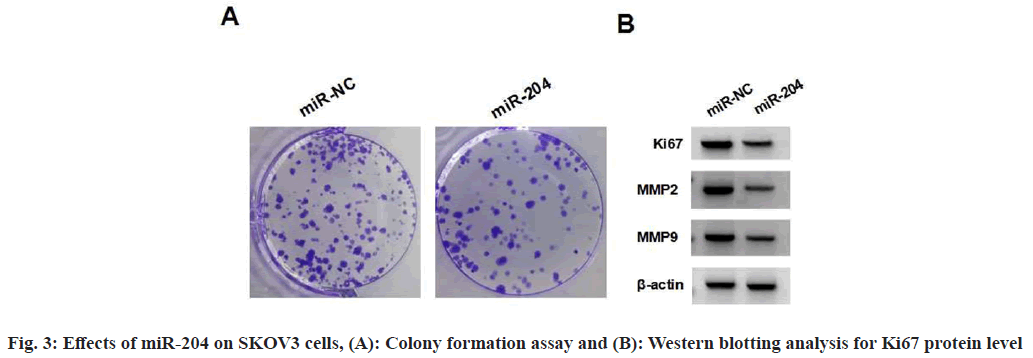
miR-204 mimic introduction markedly elevated miR-204 expression relative to miR-NC as shown in Table 4. Moreover, miR-204 overexpression suppressed the viability and colonies formation in SKOV3 cells, reduced the levels of Ki67, MMP2 and MMP9, as well as the number of migratory and invasive SKOV3 cells as shown in fig. 3 and Table 4.
| Group | miR-204 | A value | Colonies | Ki67 | MMP2 | MMP9 | Migratory cells | Invasive cells |
|---|---|---|---|---|---|---|---|---|
| miR-NC | 1.00±0.10 | 1.059±0.09 | 125±9.83 | 0.93±0.09 | 0.86±0.07 | 0.76±0.07 | 228±19.17 | 185±14.91 |
| miR-204 | 3.15±0.25* | 0.527±0.05* | 62±5.34* | 0.46±0.04* | 0.41±0.03* | 0.35±0.03* | 108±8.36* | 73±6.19* |
| t | 23.955 | 15.502 | 16.895 | 14.316 | 17.726 | 16.151 | 17.214 | 20.813 |
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. miR-NC group
Table 4: Effects of miR-204 Overexpression on SKOV3 Cells
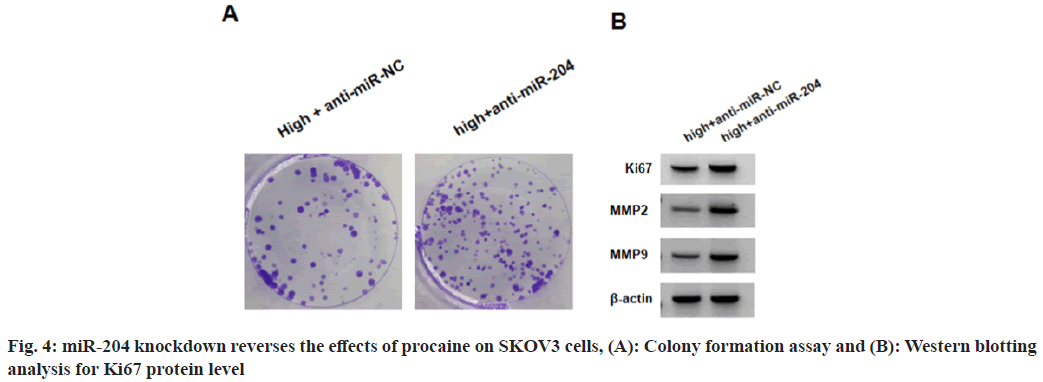
SKOV3 cells in high+anti-miR-204 group relative to high+anti-miR-NC group showed decreased miR-204 levels as shown in Table 5. Functionally, high+anti-miR-204 group exhibited increased viability, colonies, protein levels of Ki67, MMP2 and MMP9, as well as the number of migratory and invasive SKOV3 cells relative to high+antimiR-204 group (Table 5 and fig. 4).
| Group | miR-204 | A value | Colonies | Ki-67 | MMP2 | MMP9 | Migratory cells | Invasive cells |
|---|---|---|---|---|---|---|---|---|
| High+anti-miR-NC | 1.00±0.09 | 0.513±0.05 | 55±5.27 | 0.48±0.04 | 0.44±0.04 | 0.39±0.03 | 121±10.19 | 83±7.68 |
| High+anti-miR-204 | 0.43±0.03* | 1.089±0.09* | 119±9.73* | 0.97±0.09* | 0.84±0.08* | 0.71±0.06* | 247±21.61* | 194±18.04* |
| t | 18.025 | 16.784 | 17.351 | 14.926 | 13.416 | 14.311 | 15.821 | 16.984 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs. high+anti-miR-NC group
Table 5: miR-204 Knockdown Reverses the Effects of Procaine on SKOV3 Cells
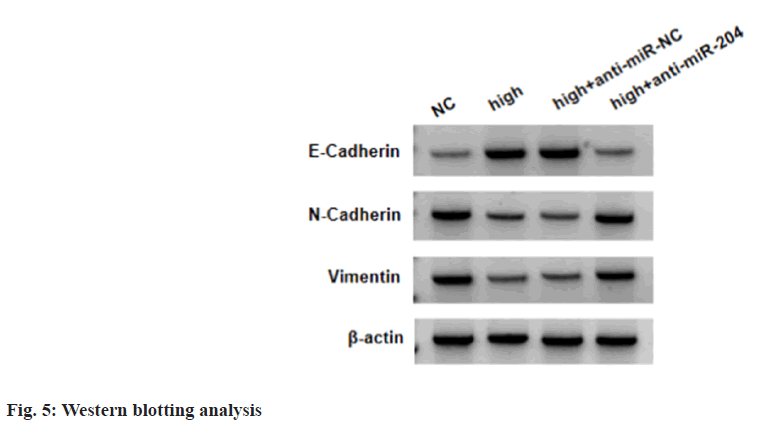
Relative to NC group, E-cadherin levels were boosted, and N-cadherin and vimentin levels were declined in high procaine-treated SKOV3 cells, while these effects mediated by high procaine were abolished after anti-miR-204 introduction (Table 6 and fig. 5).
| Group | E-cadherin | N-cadherin | Vimentin |
|---|---|---|---|
| NC | 0.31±0.03 | 0.82±0.08 | 0.73±0.08 |
| High | 0.69±0.06* | 0.43±0.04* | 0.35±0.03* |
| High+anti-miR-NC | 0.68±0.07 | 0.41±0.04 | 0.36±0.02 |
| High+anti-miR-204 | 0.33±0.03# | 0.85±0.08# | 0.76±0.07# |
| F | 155.505 | 129.656 | 145.333 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 vs.NC group, and #p<0.05 vs. high+anti-miR-NC,
Table 6: Procaine Suppresses EMT Process In SKOV3 Cells via Regulating miR-204
The high incidence and mortality rate of malignant tumors are major challenges that threaten human life and health. In recent years, the anti-tumor effects and mechanisms of local anesthetics are constantly being revealed[10,11]. For instance, sevoflurane impeded the growth of OC cells by decreasing the content of stanniocalcin 1[12]. Sevoflurane hindered the growth in vivo, and impaired cell survival and mobility in vitro in breast cancer[13]. Propofol restrained the growth and mobility of colon cancer cells[14]. Xia et al.[15] showed that lidocaine suppressed Epidermal Growth Factor Receptor (EGFR) expression by elevating miR-520a-3p, and then suppressed the tumorigenesis of retinoblastoma. Procaine is a local anesthetic with less toxicity, and has been reported to possess anticancer activity[4]. Herein, whether procaine performed antitumor effects in OC was explored. We found that procaine treatment suppressed the viability and colony formation ability, and reduced the invasive and migratory number in OC cells. Ki67 is a nuclear proliferation-associated protein involving in cell cycle progression[16]. MMP2 and MMP9 are proteolytic enzymes involving in extracellular matrix degradation, the deregulation of them is associated with invasion and migration[17]. Here, we also observed that procaine treatment declined the Ki67, MMP2 and MMP9 protein levels. The increase of E-cadherin, and decrease of vimentin and N-cadherin are the hallmark of EMT[18]. Then we further confirmed that procaine treatment repressed EMT progression in OC cells.
It has been identified that anesthetics are able to affect tumor cell biology by a variety of cellular pathways regulated through the modification of miRNAs[2]. Through the preliminary experiments, we observed that procaine could elevate miR- 204 content in OC cells. As a functional miRNA, miR-204 promoted drug sensitivity and hampered metastasis in HCC cells[19]. It also evoked gastric cancer cell apoptosis and restrained cell migration[20]. In our study, as previously reported, we also discovered that miR-204 exhibited the anticancer action in OC cells by repressing cancer cell growth, invasion, migration and EMT process. More importantly, we found inhibition of miR-204 could abolish the suppressing activity of procaine on above oncogenic behaviors; implying procaine might modulate OC tumorigenesis via miR-204.
In conclusion, this work confirmed that procaine restrained OC cell proliferative, invasive and migratory abilities and EMT process via elevating miR-204 expression, suggesting a new therapeutic strategy for OC patients.
Conflict of interests:
The authors declared no conflict of interests.
References
- Roett MA, Evans P. Ovarian cancer: An overview. Am Family Phys 2009;80(6):609-16.
[Google Scholar] [PubMed]
- Ishikawa M, Iwasaki M, Sakamoto A, Ma D. Anesthetics may modulate cancer surgical outcome: A possible role of miRNAs regulation. BMC Anesthesiol 2021;21(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Jin Z, Zhang W, Liu H, Ding A, Lin Y, Wu SX, et al. Potential therapeutic application of local anesthetics in cancer treatment. Recent Patents Anticancer Drug Discov 2022;17(4):326-42.
[Crossref] [Google Scholar] [PubMed]
- Villar-Garea A, Fraga MF, Espada J, Esteller M. Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells. Cancer Res 2003;63(16):4984-9.
[Google Scholar] [PubMed]
- Yang MH, Mohan CD, Deivasigamani A, Chinnathambi A, Alharbi SA, Rangappa KS, et al. Procaine abrogates the epithelial-mesenchymal transition process through modulating c-Met phosphorylation in hepatocellular carcinoma. Cancers 2022;14(20):4978.
[Crossref] [Google Scholar] [PubMed]
- Li YC, Wang Y, Li DD, Zhang Y, Zhao TC, Li CF. Procaine is a specific DNA methylation inhibitor with anti-tumor effect for human gastric cancer. J Cell Biochem 2018;119(2):2440-9.
[Crossref] [Google Scholar] [PubMed]
- Li C, Gao S, Li X, Ma L. Procaine inhibits the proliferation and migration of colon cancer cells through inactivation of the ERK/MAPK/FAK pathways by regulation of RhoA. Oncol Res 2018;26(2):209.
[Crossref] [Google Scholar] [PubMed]
- Yuan D, Qian H, Guo T, Ye J, Jin C, Liu X, et al. LncRNA-ATB promotes the tumorigenesis of ovarian cancer via targeting miR-204-3p. Oncotargets Ther 2020;13:573-83.
[Crossref] [Google Scholar] [PubMed]
- Yuan D, Guo T, Zhu D, Ge H, Zhao Y, Huang A, et al. Exosomal lncRNA ATB derived from ovarian cancer cells promotes angiogenesis via regulating miR-204-3p/TGFβR2 axis. Cancer Manag Res 2022;14:327-37.
[Crossref] [Google Scholar] [PubMed]
- Wang WT, Xu ZX, Li MS. Research progress of antitumor mechanism of local anesthetics. J Clin Anesthesiol 2019;35(2):189-92.
- Zhang XB, Zheng Y, Wang KY. Research progress on the effect of perioperative anesthesia on the prognosis of cancer patients. Chin Oncol Clin 2020;47(19):24-8.
- Zhang C, Wang B, Wang X, Sheng X, Cui Y. Sevoflurane inhibits the progression of ovarian cancer through down-regulating stanniocalcin 1 (STC1). Cancer Cell Int 2019;19:339.
[Crossref] [Google Scholar] [PubMed]
- Zeng X, Zheng Q, Wang L, Chen T, Lin W, Lin Q. Sevoflurane suppresses the malignant progression of breast cancer via the hsa_circ_0000129/miR-578/EPSTI1 axis. Thorac Cancer 2023;14(26):2665-77.
[Crossref] [Google Scholar] [PubMed]
- Liang B, Dong T. Effects of propofol on invasion and migration of colon cancer cells and JAK2/STAT3 signaling pathway. Zhong Nan Da Xue Xue Bao 2020;45(3):290-6.
[Crossref] [Google Scholar] [PubMed]
- Xia W, Wang L, Yu D, Mu X, Zhou X. Lidocaine inhibits the progression of retinoblastoma in vitro and in vivo by modulating the miR-520a-3p/EGFR axis. Mol Med Rep 2019;20(2):1333-42.
[Crossref] [Google Scholar] [PubMed]
- Uxa S, Castillo-Binder P, Kohler R, Stangner K, Müller GA, Engeland K. Ki-67 gene expression. Cell Death Differ 2021;28(12):3357-70.
[Crossref] [Google Scholar] [PubMed]
- Nikolov A, Popovski N. Role of gelatinases MMP-2 and MMP-9 in healthy and complicated pregnancy and their future potential as preeclampsia biomarkers. Diagnostics 2021;11(3):480.
[Crossref] [Google Scholar] [PubMed]
- Yu C, Zheng H, Liu X, Xie G. The analysis of E-cadherin, N-cadherin, vimentin, HER-2, CEA, CA15-3 and SF expression in the diagnosis of canine mammary tumors. Animals 2022;12(21):3050.
[Crossref] [Google Scholar] [PubMed]
- Yu Y, Wang Y, Xiao X, Cheng W, Hu L, Yao W, et al. miR-204 inhibits hepatocellular cancer drug resistance and metastasis through targeting NUAK1. Biochem Cell Biol 2019;97(5):563-70.
[Crossref] [Google Scholar] [PubMed]
- Yang S, Chen B, Zhang B, Li C, Qiu Y, Yang H, et al. miR-204-5p promotes apoptosis and inhibits migration of gastric cancer cells by targeting HER-2. Mol Med Rep 2020;22(4):2645-54.
[Crossref] [Google Scholar] [PubMed]