- *Corresponding Author:
- Zhifu Guo
Department of Cardiovascular Medicine, Changhai Hospital, Naval Medical University, Yangpu, Shanghai 200433, China
E-mail: 16340700005@fudan.edu.cn
| This article was originally published in a special issue, “Innovations in Biomedical Research and Drug Development” |
| Indian J Pharm Sci 2023:85(3) Spl Issue “102-109” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Acute myocardial infarction has a rapid onset, is severe and has a high mortality rate that seriously threatens the lives of the population. Coronary intervention combined with drug treatment can open the criminal blood vessel and save the patient's life, but the surviving patient is not "cured" but must go through the process of myocardial ischemia reperfusion injury. Myocardial cells are end-stage cells and death of myocardial cells due to myocardial ischemia reperfusion injury irreversibly damages the structure and function of the heart. In the past, apoptosis was thought to be the only regulated form of cell death, whereas cell necrosis was not. However, recent studies suggest that cell necrosis can also be regulated, which is grouped under the term programmed cell death. Since regulated cell death is actively mediated by molecular signaling pathways, there is a possibility to inhibit this signaling and thus attenuate programmed cell death myocardial ischemia reperfusion injury. Currently, programmed cell death mainly includes apoptosis, autophagy, pyroptosis, necroptosis, and ferroptosis. In this paper, we explore the relationship between programmed cell death and myocardial ischemic reperfusion injury, in order to support the prevention and treatment of myocardial ischemic reperfusion injury by intervening in the perspective of programmed cell death.
Keywords
Acute myocardial infarction, programmed cell death, apoptosis, autophagy, pyroptosis, necroptosis
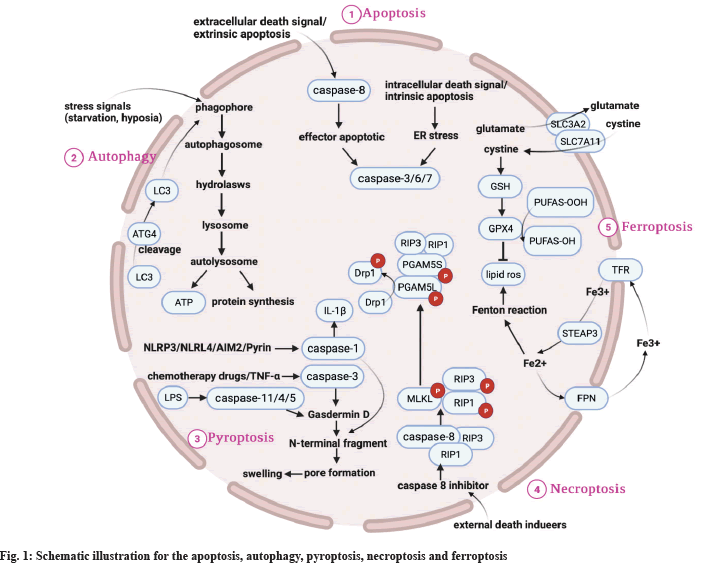
Acute Myocardial Infarction (AMI) is a serious cardiovascular disease that currently threatens human health[1]. Reperfusion therapy is the most effective core treatment for AMI, but it is a "double-edged sword"; while it saves the surviving myocardial cells, it can also cause a series of adverse cardiac events such as cardiac arrest, reperfusion arrhythmia and no-reflow phenomenon, leading to Myocardial Ischemic Reperfusion Injury (MIRI). MIRI significantly affects the prognosis of patients. The lack of effective therapeutic strategies for MIRI has made MIRI a hot topic in cardiovascular research. The mechanisms of MIRI are complex and include oxidative stress, inflammatory responses, calcium overload and mitochondrial damage. In recent years, Programmed Cell Death (PCD) has been found to play an important role in the development of MIRI by causing cardiomyocytes loss[2], which mainly includes apoptosis, autophagy, pyroptosis, necroptosis and ferroptosis. In this paper, we review the relationship between apoptosis, autophagy, pyroptosis, necroptosis and ferroptosis, and MIRI in the context of recent research advances to provide new perspectives for MIRI prevention and treatment as shown in Table 1 and fig. 1.
| Features | Apoptosis | Autophagy | Pyroptosis | Necroptosis | Ferroptosis |
|---|---|---|---|---|---|
| Biological characteristics | DNA fragmentation | Lysosomal activity increased | Inflammatory response | ATP level decrease | Iron accumulation, lipid peroxidation |
| Related genes | Caspase-3/6/7/8, Bcl-2/Bax/P53/Fas | ATG5/ATG7/LC3/Beclin-1/DRAM3/TFEB/BNIP3/P62 | NLRP3/IL-1β/ASC/IL-18/GSDMD/caspase-1/3/4/5/11 | RIP1/RIP3/MLKL/PGAM5 | GPX4/TFR1/SLC7A11/NRF2/NCOA4/P53/HSPB1/ACSL4/FSP |
| Signaling pathways | Death receptor/mitochondrial/endoplasmic reticulum stress pathway | mTOR/Beclin-1/P53 | Classical pathway activated by caspase-1, non-classical pathway activated by caspase-4/5/11 | TNF-R1/RIP1-RIP3-MLKL/PKC-MAPK-AP-1/ROS related metabolic pathway | System-Xc/GPX4/MVA/P62-Keap1-NRF2/P53-SLC7A11/ATG5-ATG7-NCOA4/HSPB1-TRF1/FSP1-COQ10-NADPH |
Table 1: Biological Difference among Apoptosis, Autophagy, Pyroptosis, Necroptosis and Ferroptosis
Apoptosis and MIRI
Apoptosis is the orderly initiate of cell death under a specific genetic regulation by the organism to maintain the stability of its internal environment under certain factors. Apoptosis is one of the most typical forms of programmed death, and its apoptotic pathways mainly include the following three types; exogenous apoptotic pathway regulated by death receptors, endogenous apoptotic pathway regulated by cell mitochondria, and Endoplasmic Reticulum Stress (ERS). In the exogenous apoptotic pathway, cysteine-containing aspartate protein hydrolase is activated by the death receptor at the cell membrane, which allows caspases to cleave additional nuclease-inhibitory proteins to eventually induce apoptosis. In the mitochondrial-regulated endogenous apoptotic pathway, some apoptotic factors are released via the mitochondrial pathway, activating caspases, which then degrade the cell's internal proteins and eventually trigger the cell's apoptotic fate[3]. The ERS pathway is also part of the endogenous apoptotic signaling pathway. In the development of MIRI, Reactive Oxygen Species (ROS) are activated by the activation of ERS, where upon ERS further activates c-Jun Amino-Terminal Kinase (JNK), leading to a further increase of ROS in mitochondria and eventually enhancing apoptosis[4]. When ERS occurs, Calcium ions (Ca2+) is again released from the endoplasmic reticulum to increase the intra cytoplasmic Ca2+ concentration, leading to calcium overload and consequent mitochondrial dysfunction, triggering apoptosis[5]. Apoptosis is a process tightly controlled by a large number of genes with highly conserved gene species, such as the caspase family, the B Lymphocyte 2 gene family (Bcl-2) and the P53 oncogene. Disruptions in the apoptotic program are inextricably linked to the development of numerous diseases, such as cardiovascular disease, neurological disease, tumors and autoimmune disease, and are triggered by many factors, including drugs, ROS, high temperatures, radiation and carbon monoxide.
Several studies have confirmed that myocardial apoptosis plays an important role in the pathological mechanism of MIRI and that inhibition of apoptosis can significantly reduce the extent of MIRI.
Massive production of ROS:
ROS reacts with internal cell components and damages the structure and function of Deoxyribonucleic Acid (DNA) and Ribonucleic Acid (RNA) in cells and impairs the cellular signaling system, leading to apoptosis[6].
Calcium overload:
Once blood supply to the ischemic myocardium is restored, calcium overload may occur, damaging mitochondrial membrane transport proteins and leading to Na+ overload and impaired energy supply. In addition, calcium overload can activate certain protein kinases and initiate the apoptotic process.
Mitochondrial damage:
Calcium overload associated with MIRI leads to massive opening of the mitochondrial Permeability Transition Pore (mPTP), depolarization of mitochondria, swelling and rupture of the outer mitochondrial membrane, uncoupling of the respiratory chain and release of several pro-apoptotic proteins, including Cytochrome C (Cyt C). Some proapoptotic proteins are released, initiating the apoptotic cascade reaction of caspases[7]. At MIRI, the signaling pathways involved in apoptosis are mainly the AMPActivated Protein Kinase (AMPK) pathway, the Mitogen-Activated Protein Kinase (MAPK) pathway, the Janus Kinase (JAK) pathway, Protein kinase C (PKC) pathway, Signal Transducers and Activators of Transcription (STAT), Phosphatidylinositol 3-Kinase (PI3K) and the PI3K pathway. The PI3K signaling pathway, caspase signaling pathway, First apoptosis signal/First apoptosis signal Ligand (Fas/ FasL) signaling pathway, Nuclear factor erythroid 2-related factor 2 (Nrf2), Antioxidant Response Element (ARE) signaling pathway and Extracellular Signal Regulated Kinase (ESRK) signaling pathway were also identified. The signaling pathways such as ESRK, which lead directly or indirectly to apoptosis, are interrelated. In the process of MIRI, apoptosis directly affects the extent of MIRI, but because apoptosis is disrupted and regulated in cardiomyocytes, MIRI can be reduced by finding anti-apoptotic drugs, inhibiting apoptosis-related genes or disrupting apoptotic transmission pathways.
Autophagy and MIRI
Autophagy is one of the internal cellular selfprotection mechanisms. It can bind to lysosomes and degrade itself within the cell to maintain homeostasis of protein metabolism and stability of the overall internal environment. Recent studies have shown that there are three main forms of autophagy, namely autophagy mediated by molecular chaperones, micro autophagy and macro autophagy. Among them, macro autophagy is commonly known as autophagy, i.e. autophagy is triggered under stress conditions, such as starvation, further expanded and closed by septa to form autophagosomes, which combine with lysosomes to form autophagic lysosomes to degrade autophagosomes and some abnormal cellular components inside and finally lead to cell death. Autophagosomes formation is the most important feature of the autophagic process. Currently, the most studied autophagy-related markers are yeast Atg6 homolog (Beclin-1) and microtubule associated protein 1 Light Chain 3 (LC3), which are the major proteins involved in autophagy. The initiation factor of autophagy is Beclin 1, and the LC3 protein is essential for the formation of autophagosomes during autophagy. LC3 is divided into two isoforms, LC3-Ⅰ and LC3-Ⅱ, and LC3-Ⅰ must first be converted into LC3-Ⅱ. Therefore, the expression level of LC3-II protein or the ratio of LC3-II/LC3-Ⅰ may reflect the degree of cellular autophagy[8]. Autophagy is closely related to many diseases such as neurodegenerative diseases, tumors, cardiovascular diseases and aging, and a large number of studies are constantly updating the new role of autophagy in diseases, but the functions of some genes related to autophagy and the mechanisms of the occurrence of autophagy still need to be elucidated in detail, so further studies on the mechanisms of cellular autophagy in various diseases should provide new ideas for the prevention and treatment of diseases.
105 Indian Journal of Pharmaceutical Sciences Special Issue 3, 2023 Dysregulation of autophagy is considered one of the main factors contributing to MIRI. Although there are still some controversial studies on autophagy, it is generally accepted that autophagy plays a dual role in MIRI. During the ischemic phase, cardiomyocytes that are in a state of ischemia and hypoxia may activate autophagy to exert a protective effect on damaged cardiomyocytes. Activation of the autophagic system derived from the cellular energy sensor, AMPK, induces autophagy in cardiomyocytes via AMPK-rapamycin Target Protein Complex C1 (mTORC1)-Autophagy Associated Protein 1 (ULK1), whose degradation products, such as fatty acids and amino acids, can be converted to ATP via the tricarboxylic acid cycle. To provide energy for cardiomyocytes damaged by ischemia and hypoxia[9]. Meanwhile, functionally impaired mitochondria can be removed by autophagy, reducing the release of apoptotic factors. During the reperfusion phase, autophagy is triggered by a variety of signals that activate cellular autophagy, such as ROS and calcium overload, as ROS can upregulate the expression of the autophagy initiator Beclin-1 and in the case of calcium overload, by the mammalian Target of Rapamycin (mTOR) signaling pathway[10]. When the autophagy level increases further, autophagy is over activated, leading to massive autophagic death of cardiomyocytes, which impairs left ventricular remodeling and increases myocardial infarct size[11]. Since cellular autophagy is essentially a mechanism that serves cell survival, the regulation of autophagy will determine its dual course and the extent of autophagy may determine whether it exerts a protective or damaging effect.
Pyroptosis and MIRI
Pyroptosis is a new PCD modality that has recently been discovered. Pyroptosis, also known as inflammatory necrosis, is the body's main nonspecific defense mechanism, capable of resisting pathogen invasion as well as sensing endogenous danger signals. Pyroptosis, as opposed to apoptosis, occurs more quickly and is accompanied by the release of large amounts of pro-inflammatory factors. The pyroptosis signaling pathway is found to be primarily composed of a classical pathway that is dependent on caspase-1 activation and a non-classical pathway that is dependent on caspase-4/5/11 activation. Different inflammatory bodies detect their respective microbial signals (viruses, pore-forming toxins, etc.) and activate caspase-1 by binding to pro-caspase-1 via the downstream junction protein-death Apoptosis associated Spot-Like Protein (ASC) to form a multi-protein complex in the classical pathway. Gasdermin D (GSDMD) protein causes cell membrane perforation and the release of cellular contents, resulting in an inflammatory response; however, it activates Interleukin (IL)-1 and IL-18 activity via precursor cleavage of IL-1 and IL- 18, thereby recruiting more inflammatory factors and amplifying the inflammatory response[12]. However, in the non-classical pathway, Lipopolysaccharide (LPS), a component of Gramnegative bacteria’s cell wall, can directly activate caspase-11 (caspase-4/5 in humans) in activated murine macrophages, cleaving GSDMD and causing scorching. Recently, it was discovered that caspase-3, a key protein in apoptosis, also cleaves Gasdermin E (GSDME) and forms cell membrane pore structures, releasing inflammatory factors such as IL-18 and IL-1, which ultimately leads to pyroptosis[13,14]. Pyroptosis is a type of pro-inflammatory cell death that is linked to a variety of diseases, including autoimmune disorders, neurodegenerative disorders, metabolic disorders and cardiovascular diseases. The effective regulation of pyroptosis will lead to new directions in disease prevention and treatment, and thus pyroptosis holds great promise as a new target for many diseases.
Pyroptosis plays a significant role in the progression of MIRI. The expression levels of NLRP3, caspase-1, IL-1 and ASC proteins in myocardial tissue were significantly higher in the rat MIRI model compared to the sham-operated group. NLR Family Pyrin Domain Containing 3 (NLRP3) and ASC formed inflammatory vesicles, which activated caspase-1, increased IL-1 and IL- 18 secretion, triggered the inflammatory response and infiltrated macrophages. The inflammatory response amplifies the effect and myocardial cells appear scorched, exacerbating the degree of MIRI. Using a mouse MIRI model, an intraperitoneal injection of NLRP3 inhibitor immediately after MIRI significantly reduced the area of myocardial infarction after 24 h. Delayed administration of an NLRP3 inhibitor for 1 h after reperfusion significantly reduced caspase-1 activity and the area of myocardial infarction after 24 h[15,16]. Because cellular scorching requires caspase-1 activation, researchers created a caspase-1
Necroptosis and MIRI
Necroptosis is a type of cell death that is distinct from apoptosis and necrosis. Degterev et al.[18] discovered in 2005 that, in the absence of intracellular caspase signaling factors, a new type of cell death can be triggered by the binding of death receptors to ligands, which has both the morphological characteristics of necrotic cells and a signaling mechanism similar to that of apoptotic cells, and dubbed it necroptosis. Zhou et al.[19] conducted a thorough review. When caspase-8 activity is inhibited, the receptor proteins form necroptosis vesicles by interacting with Receptor-Interacting Protein Kinase 1 (RIP1) and RIP3, which affects the downstream Mixed Lineage Kinase Domain-like Proteins (MLDs). The phosphorylated MLKL is oligomerized and its N-terminal helical bundle is associated with Plasma Membrane Intrinsic Proteins (PIPs) and mitochondria-specific Cardiolipin (CL). CL, which translocates MLKL from the cytoplasm to the plasma membrane enriched with PIPs or CL, disrupts membrane integrity, opens MPTP, and is involved in inducing mitochondrial dysfunction, while Damage-Associated Molecular Patterns (DAMP) is released. DAMP are released, resulting in a cascade of immune-inflammatory responses and necroptosis[20].
Numerous studies have demonstrated that necrotic apoptosis is important in MIRI[21]. The generation of large amounts of oxygen radicals during MIRI, as well as the accompanying calcium overload phenomenon, can cause oxidative stress and the opening of mPTP, which can cause cardiomyocytes damage. It has been reported that ROS production can be reduced by targeting Nox2 (a key component of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase) with small interfering RNA (siRNA) or by using Tiron (ROS scavenger). ROS inhibition was found to reduce RIP3-induced necrotic apoptosis and thus protect cardiomyocytes. Similarly, blocking mPTP opening during the reperfusion phase reduced necrotic apoptosis in the later stages of injury[22]. Furthermore, RIP3 activation can promote ERS, resulting in increased Ca2+ and ROS levels during MIRI and eventual MPTP opening, which aggravates MIRI[23]. MLKL was also discovered to be capable of continuing phosphorylation of Phosphoglycerate Metastable Enzyme Family Member (PGAM) 5 after activation. The Kinesin-related protein (Drp) 1, which regulates mitochondrial cleavage, is dephosphorylated in the presence of PGAM5, activating Drp1 and forming the RIP1-RIP3- MLKL-PGAM5-Drp1 signaling pathway, in which mitochondria are excessively cleaved, affecting ATP production and eventually necroptosis of cardiomyocytes due to a lack of sufficient energy[24,25]. The inhibitor necrostatin-1 was found to significantly reduce myocardial infarct size and improve cardiac function in a rat MIRI model, reducing the extent of MIRI[26]. This finding was seen not only in the rat MIRI model, but also in a porcine MIRI model prepared by administering necrostatin-1 inhibitor 10 min before reperfusion, which demonstrated that necrostatin-1 reduced myocardial infarct size and improved cardiac function, reducing MIRI[27,28]. Furthermore, RIP3 deficiency improved MIRI-induced myocardial necrosis and cardiac dysfunction[29]. The findings suggest that actively seeking drugs or treatments that inhibit necroptosis may be an effective way to mitigate MIRI and may be a new target for anti- MIRI.
Ferroptosis and MIRI
Ferroptosis is a new type of cell death caused by an increase in lipid peroxidation caused by iron ions. Cell death cannot be regulated by apoptosis, pyroptosis, or autophagy inhibitors during the onset of ferroptosis, but can be effectively inhibited by lipophilic antioxidants and iron chelators, while there is significant iron accumulation, which is not observed in any other form of death and is a unique phenomenon that distinguishes it from oxidative stress and is thus defined as ferroptosis. The mechanism of action of ferroptosis is unknown. Excess iron ions in cells, according to current research, can catalyze the production of hydroxyl radicals via the Fenton reaction, while unsaturated fatty acids are further hydroxylated, causing severe damage to the structure and function of cell membranes and causing intracellular oxidative imbalance, leading to iron-dependent lipid peroxidative death[30]. However, in the presence of Glutathione (GSH) and Glutathione Peroxidase 4 (GPX4), these toxic lipid peroxides can be reduced to non-toxic alcohols, avoiding oxidative damage to cells[31,32]. Ferroptosis is a newly discovered form of regulated death that has been linked to a number of human diseases, including tumors, cardiovascular disease and neurological disorders. At the moment, ferroptosis research is still in its early stages and research results are dispersed, particularly because the detection mode and related pathways of ferroptosis have not been thoroughly clarified. As a result, expanding the study of ferroptosis and understanding the mechanism of ferroptosis and its relationship with related diseases will undoubtedly aid in the prevention and treatment of a variety of diseases.
Ferroptosis is a novel mode of cardiomyocytes death in MIRI. Reperfusion-induced cell injury can be effectively repaired with ferroptosis inhibitors. It was discovered that when creating a rat MIRI model, intraperitoneal injections of the iron chelator’s desferrioxamine were started 3 d before modeling and finished 2 h before modeling. Deferoxamine reduced the size of the infarct from 55 % to 22 %, reducing the extent of MIRI[33]. MIRI was also discovered to be reduced by inhibiting glutamine catabolism[34]. To determine whether iron death is mediated by the mTOR signaling pathway, two cardiac-specific transgenic mouse models were created, one overexpressing the mTOR gene and the other knocking out the mTOR gene. Cardiomyocytes were isolated from both models and treated with erastin (class I iron).
Cardiomyocytes were isolated from two models and treated with erastin (class I iron death inducer) and RSL3 (class II iron death inducer) to induce iron death in cardiomyocytes in the other model. The findings revealed that mTOR overexpression prevented cardiomyocytes death while mTOR deficiency exacerbated cell death. Furthermore, when mTOR overexpression transgenic mice cardiomyocytes were compared to controls, ROS levels were significantly lower. Co-treatment with Ferrostatin-1 (Fer-1), a ferroptosis inhibitor, also significantly reduced cell death. The findings suggest that ferroptosis is a common cause of cardiomyocytes death and that mTOR protects cardiomyocytes from ferroptosis, possibly by regulating ROS and iron metabolism levels. These findings imply that drugs may reduce ferroptosis via the mTOR pathway, protecting cardiomyocytes and improving MIRI. As a result, ferroptosis may be a new anti-MIRI target. Wang Fulong's team from Zhejiang University detailed the potential role of ferroptosis in MIRI prevention and treatment in a paper published in PNAS. A MIRI mouse model was created, ischemic for 30 min and reperfused for 24 h. Fer-1 was administered intraperitoneally twice, 24 h and 2 h before surgery. When compared to the model group, the Fer-1 pretreatment group significantly reduced non-heme iron content, inhibited the expression of ferroptosis-related protein Prostaglandin-endoperoxide synthase 2 (Ptgs2) and reduced ferroptosis, thereby reducing the area of myocardial necrosis, improving cardiac function, and ultimately reducing the extent of MIRI. The article concludes by investigating whether inhibiting ferroptosis can provide longterm protection against MIRI. Fer-1 was given to mice before 30 min of myocardial ischemia and reperfusion for 4 w, with Fer-1 injected every 2 d. MIRI induced cardiac remodeling and fibrosis were discovered to be harmful. MIRI induced cardiac remodeling and fibrosis were found to be significantly reduced in the treated group. Furthermore, Fer-1 reduced the expression of Cytb and Atp6 messenger RNA (mRNAs), which are specifically encoded by mitochondrial DNA, implying that inhibiting ferroptosis may provide significant anti-cardio protective effects, possibly through improved mitochondrial function. The data presented above suggest that inhibiting ferroptosis can reduce the severity of MIRI, and its inhibitor Fer-1 has a wide range of clinical applications.
This significant discovery opens up a new avenue for the prevention and treatment of myocardial infarction and other cardiac diseases[35].
Association of Apoptosis, Autophagy, Pyroptosis, Necroptosis and Ferroptosis
Although the transduction pathways for apoptosis, autophagy, pyroptosis, necroptosis and ferroptosis are distinct, numerous studies have revealed "cross-talk" between them, such as cells stimulated by Tumor Necrosis Factor (TNF) and oxidative factors can induce the release of apoptotic factors from mitochondria. However, inhibiting apoptosis can also cause autophagy, as some damaged mitochondria in apoptotic cells can be removed by autophagy, reducing apoptosis; however, inhibiting caspase-8 can lead to necroptosis[36], and if GSDME protein is present in the cell, it will cause the cell to rapidly transition from apoptosis to pyroptosis[36]. The presence of GSDME protein in the cell causes it to rapidly transition from apoptosis to scorch death[37]. Similarly, autophagy can inhibit apoptosis by reducing inflammatory vesicle production and the autophagy-initiating kinase ULK1 is also involved in controlling RIP1-mediated necrotic apoptosis[38]. Apoptosis can also result in ferroptosis, which makes cells more susceptible to apoptosis. According to ferroptosis and autophagy correlation studies, oxidative stress damage in ferroptosis induces lysosome rupture, while autophagic death also binds to and degrades lysosomes and its Kelchlike epichlorohydrin-associated protein 1 (Keap1)/ Nrf2 signaling pathway may be an important point of contact between ferroptosis and autophagy[39]. Currently, researchers have proposed the concept of ferritinophagy[40]. As a result, it is hypothesized that apoptosis, autophagy, pyroptosis, necroptosis and ferroptosis are in a mutually governing and dynamic balance. To fully exploit the therapeutic potential of inhibiting cardiomyocytes death during MIRI, the common junctions between these cell death pathways must be investigated further in order to interfere with their key targets, which could lead to a significant breakthrough in MIRI therapy. Similarly, future research is needed to determine how many MIRI patients are affected by each of the various forms of cell death and to determine the extent to which the various forms of cell death contribute directly to MIRI. Because cell death during MIRI is a multifactorial and multilinked signaling process, more understanding of the conditions of activation of various factor-mediated signaling pathways and their relationships is required to provide new directions for the prevention and treatment of MIRI.
Funding:
This work was supported by the grant from the National Natural Science Foundation of China (Grant No. 2022009604).
Acknowledgement:
Changjin Li, Xiaolong Li and Xianbu Gao were considered as the co-first authors.
Conflict of interests:
The authors declared no conflict of interests.
References
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation 2019;139(10):e56-28.
[Crossref] [Google Scholar] [PubMed]
- Bai Y, Lam HC, Lei X. Dissecting programmed cell death with small molecules. Acc Chem Res 2020;53(5):1034-45.
[Crossref] [Google Scholar] [PubMed]
- Ramirez ML, Salvesen GS. A primer on caspase mechanisms. Semin Cell Dev Biol 2018;82:79-85.
[Crossref] [Google Scholar] [PubMed]
- Chaudhari N, Talwar P, Parimisetty A, Lefebvre d’Hellencourt C, Ravanan P. A molecular web: Endoplasmic reticulum stress, inflammation and oxidative stress. Front Cell Neurosci 2014;8:213.
[Crossref] [Google Scholar] [PubMed]
- Son SM, Byun J, Roh SE, Kim SJ, Mook-Jung I. Reduced IRE1α mediates apoptotic cell death by disrupting calcium homeostasis via the InsP3 receptor. Cell Death Dis 2014;5(4):e1188.
[Crossref] [Google Scholar] [PubMed]
- Münzel T, Camici GG, Maack C, Bonetti NR, Fuster V, Kovacic JC. Impact of oxidative stress on the heart and vasculature: Part 2 of a 3-part series. J Am Coll Cardiol 2017;70(2):212-29.
[Crossref] [Google Scholar] [PubMed]
- Walters AM, Porter Jr GA, Brookes PS. Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circ Res 2012;111(9):1222-36.
[Crossref] [Google Scholar] [PubMed]
- Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett 2010;584(7):1374-8.
[Crossref] [Google Scholar] [PubMed]
- Gallo S, Sala V, Gatti S, Crepaldi T. Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clin Sci 2015;129(12):1173-93.
[Crossref] [Google Scholar] [PubMed]
- Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-β and Bcl-2. Mol Cell 2007;25(2):193-205.
[Crossref] [Google Scholar] [PubMed]
- Dong Y, Undyala VV, Gottlieb RA, MentzerJr RM, Przyklenk K. Autophagy: Definition, molecular machinery and potential role in myocardial ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther 2010;15(3):220-30.
[Crossref] [Google Scholar] [PubMed]
- Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 2017;42(4):245-54.
[Crossref] [Google Scholar] [PubMed]
- Feng S, Fox D, Man SM. Mechanisms of gasdermin family members in inflammasome signaling and cell death. J Mol Biol 2018;430(18):3068-80.
[Crossref] [Google Scholar] [PubMed]
- Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun 2017;8(1):14128.
- Toldo S, Marchetti C, Mauro AG, Chojnacki J, Mezzaroma E, Carbone S, et al. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia–reperfusion in the mouse. Int J Cardiol 2016;209:215-20.
[Crossref] [Google Scholar] [PubMed]
- Jong WM, Leemans JC, Weber NC, Juffermans NP, Schultz MJ, Hollmann MW, et al. Nlrp3 plays no role in acute cardiac infarction due to low cardiac expression. Int J Cardiol 2014;177(1):41-3.
[Crossref] [Google Scholar] [PubMed]
- Takahashi M. NLRP3 inflammasome as a novel player in myocardial infarction. Int Heart J 2014;55(2):101-5.
[Crossref] [Google Scholar] [PubMed]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005;1(2):112-9.
[Crossref] [Google Scholar] [PubMed]
- Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol 2014;35:14-23.
[Crossref] [Google Scholar] [PubMed]
- Tummers B, Green DR. Caspase‐8: Regulating life and death. Immunol Rev 2017;277(1):76-89.
[Crossref] [Google Scholar] [PubMed]
- Adameova A, Goncalvesova E, Szobi A, Dhalla NS. Necroptotic cell death in failing heart: Relevance and proposed mechanisms. Heart Fail Rev 2016;21:213-21.
[Crossref] [Google Scholar] [PubMed]
- Zhu H, Sun A. Programmed necrosis in heart disease: Molecular mechanisms and clinical implications. J Mol Cell Cardiol 2018;116:125-34.
[Crossref] [Google Scholar] [PubMed]
- Zhu P, Hu S, Jin Q, Li D, Tian F, Toan S, et al. Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: A mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol 2018;16:157-68.
[Crossref] [Google Scholar] [PubMed]
- Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012;148(1-2):213-27.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 2012;148(1-2):228-43.
[Crossref] [Google Scholar] [PubMed]
- Garvin AM, Jackson MA, Korzick DH. Inhibition of programmed necrosis limits infarct size through altered mitochondrial and immune responses in the aged female rat heart. Am J Physiol Heart Circ Physiol 2018;315(5):H1434-42.
[Crossref] [Google Scholar] [PubMed]
- Carbone F, Oliveira PJ, Montecucco F. Protective role of necrostatin-1 in acute myocardial infarction. Eur J Clin Invest 2016;46(1):99-100.
[Crossref] [Google Scholar] [PubMed]
- Koudstaal S, Oerlemans MI, van der Spoel TI, Janssen AW, Hoefer IE, Doevendans PA, et al. Necrostatin‐1 alleviates reperfusion injury following acute myocardial infarction in pigs. Eur J Clin Invest 2015;45(2):150-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang T, Zhang Y, Cui M, Jin LI, Wang Y, Lv F, et al. CaMKII is a RIP3 substrate mediating ischemia-and oxidative stress–induced myocardial necroptosis. Nat Med 2016;22(2):175-82.
[Crossref] [Google Scholar] [PubMed]
- Stockwell BR, Jiang X, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol 2020;30(6):478-90.
[Crossref] [Google Scholar] [PubMed]
- Agmon E, Solon J, Bassereau P, Stockwell BR. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci Rep 2018;8(1):5155.
[Crossref] [Google Scholar] [PubMed]
- Magtanong L, Ko PJ, Dixon SJ. Emerging roles for lipids in non-apoptotic cell death. Cell Death 2016;23(7):1099-109.
[Crossref] [Google Scholar] [PubMed]
- Dendorfer A, Heidbreder M, Hellwig-Bürgel T, Jöhren O, Qadri F, Dominiak P. Deferoxamine induces prolonged cardiac preconditioning via accumulation of oxygen radicals. Free Radical Biol Med 2005;38(1):117-24.
[Crossref] [Google Scholar] [PubMed]
- Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 2015;59(2):298-308.
[Crossref] [Google Scholar] [PubMed]
- Fang X, Wang H, Han D, Xie E, Yang X, Wei J, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci 2019;116(7):2672-80.
- Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, et al. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol 2017;13:498-507.
- Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017;547(7661):99-103.
- Wu W, Wang X, Berleth N, Deitersen J, Wallot-Hieke N, Böhler P, et al. The autophagy-initiating kinase ULK1 controls RIPK1-mediated cell death. Cell Rep 2020;31(3):107547.
[Crossref] [Google Scholar] [PubMed]
- Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 2014;136(12):4551-6.
[Crossref] [Google Scholar] [PubMed]
- Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014;509(7498):105-9.
[Crossref] [Google Scholar] [PubMed]