- *Corresponding Author:
- Benjaporn Buranrat
Faculty of Medicine, Mahasarakham University, Maha Sarakham 44000, Thailand
E-mail: buranrat@gmail.com
| Date of Received | 29 June 2021 |
| Date of Revision | 24 April 2022 |
| Date of Acceptance | 12 September 2022 |
| Indian J Pharm Sci 2022;84(5):1210-1217 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Piper nigrum is broadly acted as a herbal medicine and anticancer activity as well. Previously, we reported that Piper nigrum extract caused induction of breast cancer cells death and reduction of cell migration in in vitro study. In this present study, we need to explore the anticancer effects of Piper nigrum on the human HeLa cervical cancer cells and underlying mechanism. Sulforhodamine B, colony formation, cell cycle arrest, acridine orange/ethidium bromide staining was performed to analyze cytotoxic effects on the cells. Tetraethylbenzimidazolylcarbocyanine iodide staining, dichlorodihydrofluorescein diacetate staining was used to study apoptotic effects and followed by wound healing method, investigated the cells migration. This Piper nigrum crude extract represented cytotoxic effect against HeLa cervical cancer cells by dose and time-dependent manner with the half-maximal inhibitory concentration values was 48.59±5.37, 36.49±4.34, 22.71±2.12 μg/ml for 24 h, 48 h and 72 h, respectively. Further, these extract caused reduction of cell viability by examining the acridine orange/ethidium bromide staining and decreased the colony formation by dose-dependent manner as well. Next, HeLa cervical cancer cells were significantly arrested in the resting/growth 1 phase and further reported with attenuating mitochondrial function at 24 h incubation, especially at the dose of 100 μg/ml of extract. Then, the molecular mechanisms of Piper nigrum extract were evaluated for reactive oxygen species formation, and data showed that these extracts increased reactive oxygen species production by a dose-dependent amount. On the other hand, the cancer cells migration was suppressed by Piper nigrum extract at 48 h observation time. These results indicated that Piper nigrum extract inhibited HeLa cervical cancer cells through the down-regulation of cell proliferation, induction of apoptosis and attenuation of cell migration. Piper nigrum may be used as anticancer agent for preventing/treating cervical cancer.
Keywords
Anti-cancer, apoptosis, cell proliferation, HeLa cervical cancer cells, Piper nigrum
Piper nigrum (PN) belongs to family Piperaceae and can be used as herbal medicines in many diseases including anti-bacterial, anti-fungal, anti-diarrhoeal, anti-inflammatory, anti-mutagenic, anti-metastatic, antioxidative and anti-tumor activities[1]. Several compounds have been isolated from PN are aromatic essential oils, alkaloids, amides, prophenylphenols, lignans, terpenes, flavones, phenolics, flavonoids, terpenes and steroids as well as piperine, pipernonaline and purpurogallin[1,2]. Interestingly, PN and active compounds are focusing in anticancer action in several studies such as colon, breast, bile duct, ovary, melanoma and osteosarcoma[3-8]. For example, ethanolic crude extract of PN consists of high total phenol content which shows antioxidant and anti-inflammation, as well as cytotoxic property against colorectal carcinoma cell lines[5].
Based on the data of PN and its active compounds indicated that these extract caused suppression of cancer cells proliferation, induction of apoptosis and attenuation of migration as well. PN crude extracts exhibits cytotoxicity against Michigan Cancer Foundation-7 (MCF-7) breast cancer cells with median half-maximal Inhibitory Concentration (IC50) of 14.40±3.30 μg/ml and represents tumor inhibitory effect in mammary adenocarcinoma mouse[5,9]. Consistency with our previous report reported that PN enhanced MCF-7 breast cancer cells death with low IC50 values 42.2±5.8 and 15.6±2.1 μg/ml for 24 h to 48 h[4]. PN was highly powerful against several types of cancer cells in in vitro and in vivo studies by which decreasing cyclooxygenase-2, Nuclear Factor Kappa B (NF-κB) along with inducing cyclin-dependent kinase inhibitor 1 protein[3]. Moreover, PN extract can activate cancer cells apoptosis by increasing tumor protein-p53 and B-cell lymphoma-2 associated X protein levels[3].
From migration studies found that, Piperine Free PN Extract (PFPE) strongly inhibited MCF-7 breast cancer cells migration by reducing E-cadherin, Matrix Metalloproteinase-9 (MMP-9), MMP-2, cellular Myelocytomatosis oncogene (c-Myc) and Vascular Endothelial Growth Factor (VEGF) in the in vitro and in vivo[10]. However, the effects of PN extract against HeLa cervical cancer cells are still unclear. The purpose of this research was to explore the anticancer actions of young fruit of PN extract on human HeLa cervical cancer cell proliferation, apoptosis and migration, explore the possible underlying molecular mechanisms through Reactive Oxygen Species (ROS) formation and mitochondrial function, in order to examine whether PN may be useful as a possible drug for treating cervical cancer.
Materials and Methods
Plant extract:
Young fruit of PN was used in our previous study (MSUT-72344)[4]. Percentage yield of the extracts are 5.27 % per dry weight of PN, standardized by phenolic and flavonoid contents with gallic acid and rutin in crude extract and the gallic acid was 154.96±22.66 mg/g and rutin was 62.39±11.21 µg/g.
High-Performance Liquid Chromatography (HPLC) method:
HPLC assay was used to measure the piperine of the PN extracts. The 100 µg/ml PN extract was loaded onto a InertSustain® C18 column (250 mm×4.6 mm i.d., 5 µm, GL Sciences Inc., Tokyo, Japan). Mobile phase consisted of purified water with acetic acid (pH 2.74) (solvent A) and acetonitrile (solvent B) at a flow rate of 1.5 ml/min. Gradient elution was performed as follows: from 0 to 5 min, linear gradient from 5 % to 9 % solvent B; from 5 to 15 min, 9 % solvent B; from 15 to 22 min, linear gradient from 9 % to 11 % solvent B; from 22 to 38 min, linear gradient from 11 % to 18 % solvent B; from 38 to 43 min, gradient from 18 % to 23 % solvent B; from 43 to 44 min, gradient from 23 % to 90 % solvent B; from 44 to 45 min, linear gradient from 90 % to 80 % solvent B; from 45 to 55 min, isocratic at 80 % solvent B; from 55 to 60 min, linear gradient from 80 % to 5 % solvent B and a re-equilibration period of 5 min with 5 % solvent B used between individual runs. Operating conditions were as; column temperature 38°, injection volume 20 μl and Ultraviolet (UV)-diode array detection at 280 nm for piperine. Spectra were recorded from 200 to 600 nm. Piperine was identified by comparing their relative retention times and UV spectra with those of authentic compounds and were detected using an external standard method.
Cell culture and cytotoxicity methods:
HeLa, Human cervical cancer cell line was obtained from American Type Culture Collection (ATCC) and cells were grown in 10 % Fetal Bovine Serum in Dulbecco's Modified Eagle Medium (DMEM) with antibiotic solution (penicillin/streptomycin). For testing the PN effect on Human cervical cancer HeLa cells viability and Sulforhodamin B (SRB) method was performed. Cells were seeded onto 96-well culture plates (1×104 cells/well) for overnight and then the different PN concentrations (0-250 µg/ml) were added into each well for three time points, 24 h to 72 h. After discarding the medium, the cells were treated with 10 % trichloroacetic acid, incubated for 1 h at room temperature with 0.4 % SRB and pipetted up and down with 10 mM Tris-base buffer. Read the optical density at 540 nm by spectrophotometer and calculated the cell viability compared with control groups.
Colony formation method:
For testing the PN effect on colony forming activity and colony formation method was performed. Cells were seeded onto 6-well culture plates (500 cells/well) for overnight and then the different PN concentrations (0-250 µg/ml) were added into each well for 24 h. Next, medium was discarded and the cells were changed to new complete DMEM medium, every 2 d for further 10 d. Afterwards, cells were stained with 0.5 % crystal violets, counted and calculated the colonies compared with control groups.
Cell cycle arrest method:
For testing the PN effect on cell cycle arrest and Propidium Iodide (PI) staining by flow cytometric method was performed. HeLa cells (2×105 cells/well) were exposed to different doses of PN extract (0-100 µg/ml) for 24 h. After incubation, the cells were trypsinized, centrifuged and also exposed to 70 % cold ethanol at 20° for 24 h. Consequently, the cells were washed two times with cold Phosphate-Buffered Saline (PBS) buffer and added PI solution (Cat No. 550825, BD Biosciences, California, United States of America) at 4° in the dark for 30 min. After that, the analysis of cell cycle distribution was determined using flow cytometric method and then calculated the percentages of arresting of cells cycle at Resting/Growth 1 (G0/G1), Synthesis (S) and Growth 2/Mitotic (G2/M) phases by BD AccuriTM C6 Plus flow cytometer.
Acridine Orange/Ethidium Bromide (AO/EB) staining:
For testing the PN effect on cell proliferation, AO/EB staining method was performed. HeLa cells (1×104 cells/well) were exposed to different doses of PN extract (0-100 µg/ml) for 24 h. Next, the cancer cells were gently washed with PBS buffer for two times and also added a dye solution composing AO and EB (final concentration 1 µg/ml of each dye) stained cells were directly captured under a fluorescence microscope (20x magnification, CKX53 Olympus).
Tetraethylbenzimidazolylcarbocyanine Iodide (JC-1) staining method:
For testing the PN effect on mitochondrial function and JC-1 staining by flow cytometry was performed. HeLa cells (2.5×105 cells/well) were exposed to different doses of PN extract (0-100 µg/ml) for 24 h. After treatment, the cancer cells were washed with PBS buffer and then incubated with 5 μl JC-1 dye (Cat. No. 1-800-346-9897, Cayman Chemical, Michigan, United States of America) at 37° in dark for 30 min. Finally, the samples were mixed and ready for flow cytometric analysis and percentage of mitochondrial function with depolarized (unhealthy cells) or non-depolarized cells (healthy cells) were examined by BD AccuriTM C6 Plus flow cytometer with an excitation wavelength at 488 nm (FL1 channels) and emission wavelength at 525-530 nm (FL2 channels).
ROS formation method:
For testing the PN effect on ROS formation and Dichlorodihydrofluorescein Diacetate (DCF-DA) staining by flow cytometry was performed. HeLa cells (2×105 cells/well) were exposed to different doses of PN extract (0-100 µg/ml) for 24 h. After treatment, cells were resuspended in PBS and mixed with DCF-DA (Cat.no. D6883, Sigma Merck KGaA, Darmstadt, Germany) at a final concentration of 25 μM for 30 min at 37° in dark. The ROS formation was performed by BD Accuri™ C6 Plus flow cytometer. The data was represented as the percentage of the mean fluorescence intensity.
Wound healing scratch method:
For testing the PN effect on cell migration activity and in vitro wound healing scratch method was performed. HeLa cells (2×105 cells/well) were grown to confluence on 24-well culture plates, then cells were made a wound using 0.2 ml sterile pipette tip and added to the new medium containing the PN extract (0-100 µg/ml) for 48 h. After that, images of the wounds were captured and measured the length of nude area for comparison between 0 h and 48 h of each groups. The results were calculated between the control and treatment groups.
Statistical analysis:
The difference between control and PN treatment groups was statistically compared by one-way Analysis of Variance (ANOVA), followed by Tukey's post-hoc test and p<0.05.
Results and Discussion
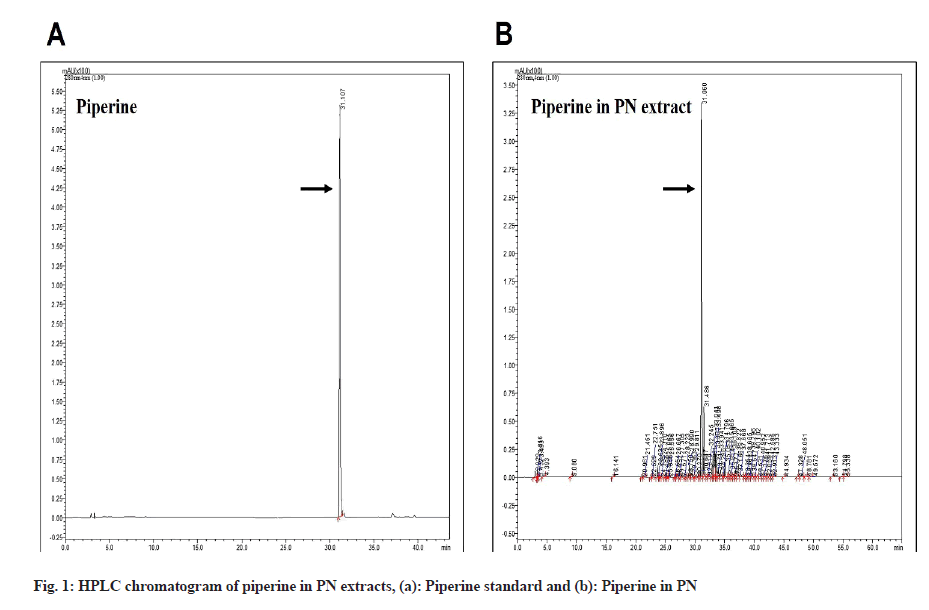
Piperine is the most abundant alkaloid in PN and it is the active anti-cancer compound. To examine the piperine content in the PN extract, we used the HPLC method. The data revealed the presence of piperine in the PN extract, as shown in fig. 1, to be 63.68±0.13 µg/ ml of piperine/dry weight extract.
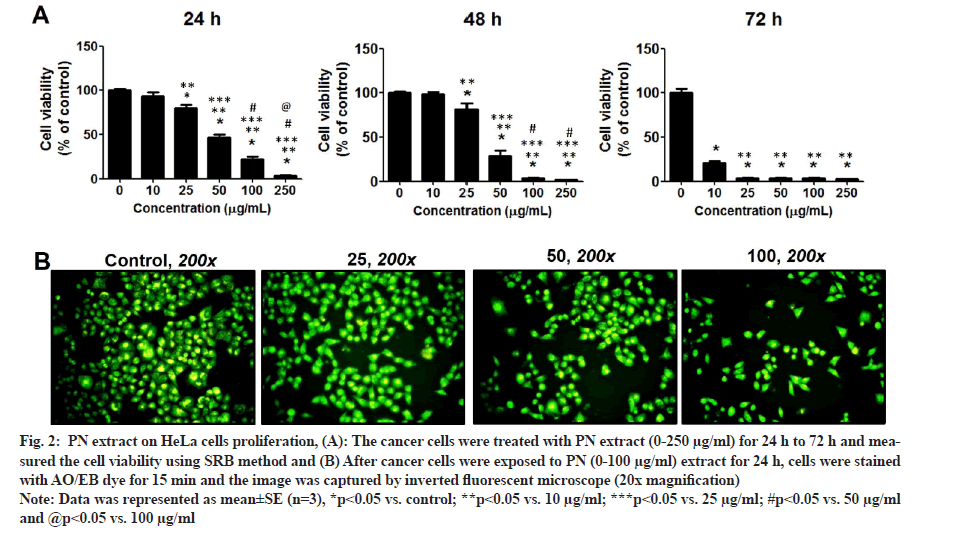
The data of the SRB method indicated that the cancer cell growth of HeLa cervical cells in the PN treatment was significantly different when compared with the untreated control group (fig. 2A). Moreover, the inhibitory effect was increased progressively with increasing PN in a dose and time-dependent manner (p<0.05). The inhibitory rates of cell proliferation were high in PN treatment group and the IC50 values were 48.59±5.37, 36.49±4.34, 22.71±2.12 µg/ml for 24 h, 48 h and 72 h, respectively. PN extract significantly suppressed the growth of HeLa cells.
Fig. 2: PN extract on HeLa cells proliferation, (A): The cancer cells were treated with PN extract (0-250 µg/ml) for 24 h to 72 h and measured the cell viability using SRB method and (B) After cancer cells were exposed to PN (0-100 µg/ml) extract for 24 h, cells were stained with AO/EB dye for 15 min and the image was captured by inverted fluorescent microscope (20x magnification).
Note: Data was represented as mean±SE (n=3), *p<0.05 vs. control; **p<0.05 vs. 10 µg/ml; ***p<0.05 vs. 25 µg/ml; #p<0.05 vs. 50 µg/ml and @p<0.05 vs. 100 µg/ml.
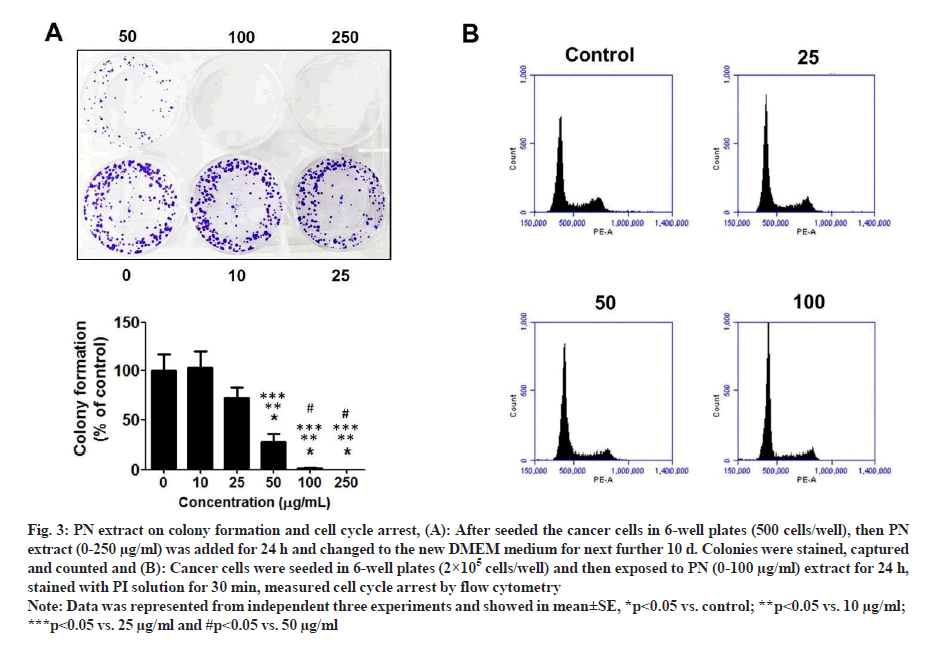
Following staining with AO/EB, the morphology of treated-cells in each group was slightly altered; however, the viable cell number was suppressed by PN extract in a dose-dependent manner. At the dose of 100 mg/ml extract showed that HeLa cells became smaller and more rounded when compared with cells in the control group (fig. 2B). These data were consistent with the SRB results, in that PN extract inhibited growth of HeLa cells and its effect was comparable to that of the control group. Consequently, the colony formation assay was performed to examine the long-term anti-growth activity of PN extract in HeLa cells. The results showed that the colony formation in the case of cells treated with various concentrations of PN extract was significantly suppressed compared to untreated control cells (fig. 3A).
Fig. 3: PN extract on colony formation and cell cycle arrest, (A): After seeded the cancer cells in 6-well plates (500 cells/well), then PN extract (0-250 µg/ml) was added for 24 h and changed to the new DMEM medium for next further 10 d. Colonies were stained, captured and counted and (B): Cancer cells were seeded in 6-well plates (2×105 cells/well) and then exposed to PN (0-100 µg/ml) extract for 24 h, stained with PI solution for 30 min, measured cell cycle arrest by flow cytometry.
Note: Data was represented from independent three experiments and showed in mean±SE, *p<0.05 vs. control; **p<0.05 vs. 10 µg/ml; ***p<0.05 vs. 25 µg/ml and #p<0.05 vs. 50 µg/ml
To understand the mechanism through which PN extract attenuated cancer cell growth, we explored the action of these extract on the distribution of cancer cell cycle in HeLa cells. The data indicated that the extract showed a significant arrest in the cell cycle and stopped at the G0/ G1 phase and started at the dose of 25 µg/ml extract. The highest level of cell cycle arrest indicated in the dose of 100 mg/ml of extract was approximately 69.10 % of G0/G1 phase when compared with the control group, which was 62.33 % (fig. 3B).
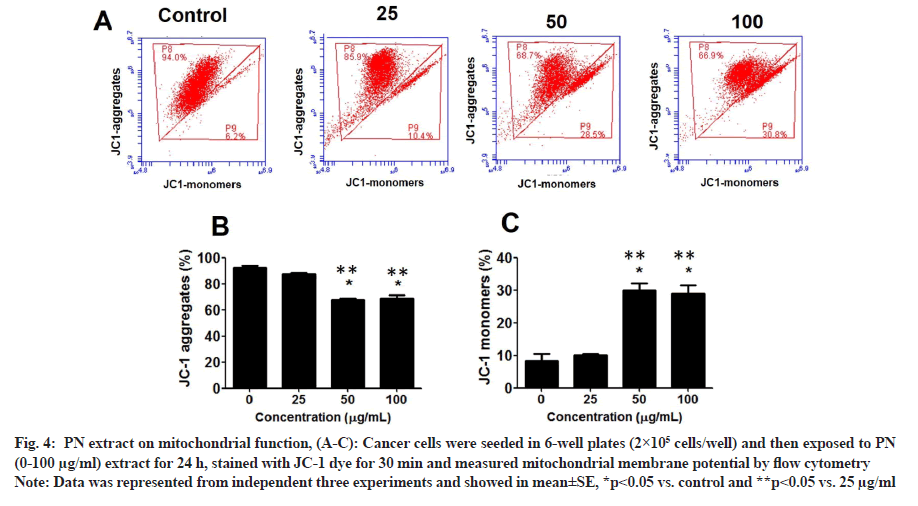
Since PN extract inhibited the viability of HeLa cells, the effect of PN on mitochondrial function and ROS levels in cancer cells was further examined. At 25 µg/ ml, PN extract increased the number of HeLa cells with depolarized MMP (JC1-monomers, unhealthy cells) by approximately 10.4%, and then gradually increased MMP in a dose-dependent manner, by approximately 28.5 % and 30.8 % at 50 and 100 µg/ml, respectively (p<0.05) (fig. 4A-fig. 4C). Statistical analysis also revealed that healthy cells (JC-1 aggregates) were decreased in a dose-dependent manner as well.
Fig. 4: PN extract on mitochondrial function, (A-C): Cancer cells were seeded in 6-well plates (2×105 cells/well) and then exposed to PN (0-100 µg/ml) extract for 24 h, stained with JC-1 dye for 30 min and measured mitochondrial membrane potential by flow cytometry.
Note: Data was represented from independent three experiments and showed in mean±SE, *p<0.05 vs. control and **p<0.05 vs. 25 µg/ml.
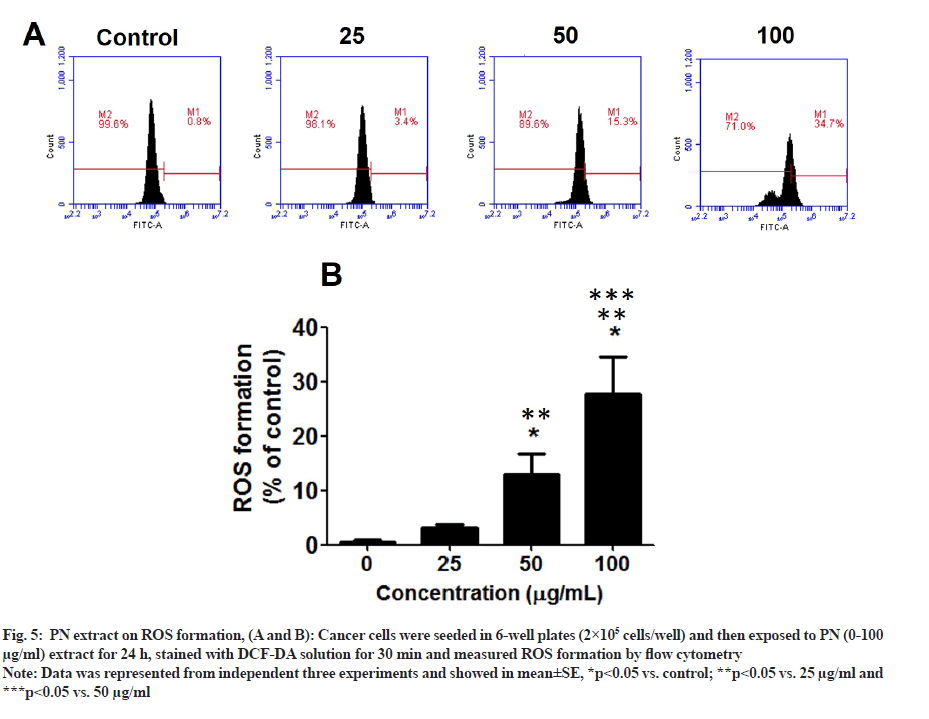
Since PN inhibited the viability and mitochondrial function of HeLa cells, the effect of PN on ROS levels was determined. The results are represented in fig. 5A and fig. 5B. In the following PN treatment groups, the ROS level in HeLa cells was significantly induced, especially at the dose of 50-100 µg/ml extract, reaching 30 % of that of the control group (p<0.05). PN extract induces HeLa cell death through ROS production and decreased mitochondrial function.
Fig. 5: PN extract on ROS formation, (A and B): Cancer cells were seeded in 6-well plates (2×105 cells/well) and then exposed to PN (0-100 µg/ml) extract for 24 h, stained with DCF-DA solution for 30 min and measured ROS formation by flow cytometry.
Note: Data was represented from independent three experiments and showed in mean±SE, *p<0.05 vs. control; **p<0.05 vs. 25 µg/ml and ***p<0.05 vs. 50 µg/ml
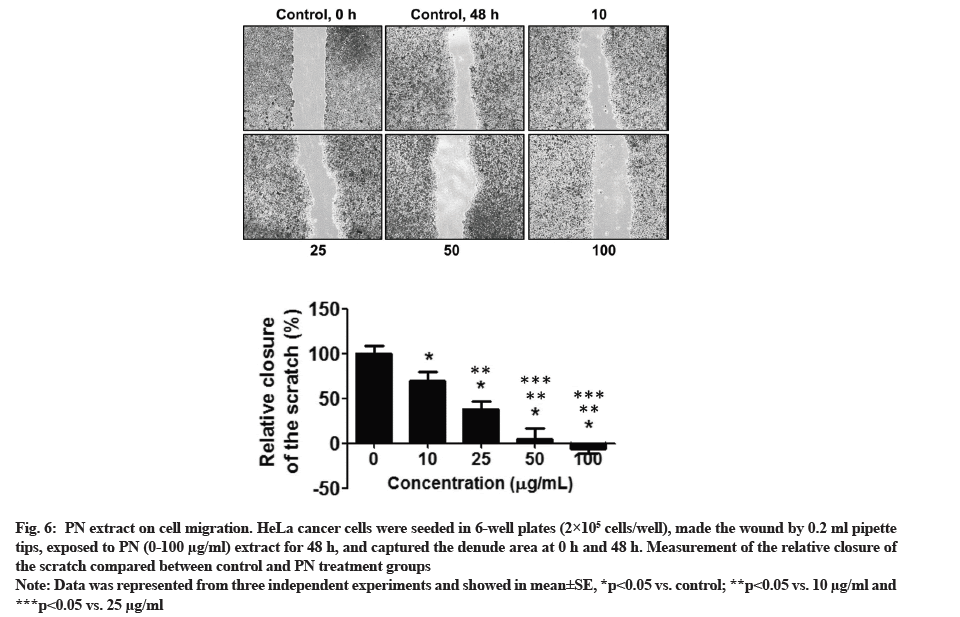
The cancer cell migration was performed using a wound migration assay. HeLa cells were incubated with 10-100 µg/ml PN extract, with purpose to identify whether the migratory activity of HeLa cells were significantly reduced at all doses of PN extract. The data from the wound healing method demonstrated that cancer cells incubated with PN extract showed decreased wound closure (fig. 6). The data showed that PN extract may stimulate significant inhibition on the migratory activity of HeLa cells in a dose-dependent manner.
Fig. 6: PN extract on cell migration. HeLa cancer cells were seeded in 6-well plates (2×105 cells/well), made the wound by 0.2 ml pipette tips, exposed to PN (0-100 µg/ml) extract for 48 h, and captured the denude area at 0 h and 48 h. Measurement of the relative closure of the scratch compared between control and PN treatment groups.
Note: Data was represented from three independent experiments and showed in mean±SE, *p<0.05 vs. control; **p<0.05 vs. 10 µg/ml and ***p<0.05 vs. 25 µg/ml.
Numerous natural sources have been used to discover for anticancer agents. Previous studies have discovered that young fruit of PN crude extract was extracted in 95 % ethanol had more efficacies to suppress MCF-7 breast cancer cells proliferation and migration through reducing mevalonate pathway, both in Ras-related C3 botulinum toxin substrate 1 (Rac1) and Ras homolog family member A (RhoA) levels[4]. However, the actions of the PN extract on human cervical cancer HeLa cells are still understood in their underlying mechanism of action. Based on the results obtained, we estimated the active compound of PN, piperine, using the HPLC method and then, we found that the PN extract had the highest levels of piperine. The results illustrated that PN extract had the greatest effects on cancer cells via reducing cell proliferation, arresting the cell cycle at G0/G1 phase, inhibiting mitochondrial function, increasing ROS formation and suppressing migration of HeLa cells. The data indicated that PN extract could be an anticancer agent for cervical cancer.
Presently, cervical cancer is the fourth most common cancer in women worldwide and Asian country. From diagnostic data, it was found that the incidence rates about 500 000 women each year are diagnosed with cervical cancer and developing countries was reported more prevalent[11]. Presently, therapeutic treatments have been established for cure of cervical cancer. Nevertheless, its management remains a key challenge. Suppression of cancer cells via the stimulation of cancer cells death and apoptosis are main therapeutic methods in the treatment of various cancers[12]. Presently, the natural compounds are still focusing on cancer treatment because it has high efficacy and low toxicity. Interestingly, PN is the best choice for studying with anticancer effects on several cancer cell types. Our previous study showed that PN extract had high levels of piperine and then inhibited breast cancer cells via inhibiting protein-related cell growth such as cyclin D1 and NF-κB as well as inducing protein-associated cell apoptosis such as caspase-3 expression[4]. Similarly with Tedasen et al., demonstrated that PN extract may induce cholangiocarcinoma cell death by reduction of NF-κB and further induction of p21[3].
Importantly, ROS plays a major role in apoptosis regulation and the aim of anticancer agent approaches is to modulate the oxidative species and antioxidant status imbalance by stimulating ROS generation in tumour cells, which may be activated to tumour cell apoptosis[13]. The overproduction of ROS level reduces the mitochondrial function[14]. Mitochondrion is the key of intracellular organelles that generate Adenosine Triphosphate (ATP) for producing human body. Normal function of mitochondrial key was oxidative phosphorylation and ATP production, and a decreased mitochondrial function is a hallmark of various early apoptotic pathways[15]. In this study, HeLa cells were exposed to PN and generated more ROS than control cells, which was dose-dependent and experienced more apoptosis as well as a mitochondrial function decrease. Our results suggest that the mechanism of actions of PN extract on the growth inhibitory effect may be through producing ROS generation, suppressing mitochondrial function and also trigger apoptosis induction in cervical cancer cells.
The metastasis of cancer cells is a basic mechanism of cancer cells to migrate to distant organs[16]. Truly, a herbal medicine with the capability to inhibit the metastasis could be a potential candidate for preventing and treating cancer. These studies indicated that PN extracts suppressed the basal activity of migration of HeLa cells through inhibiting wound healing, as our previous work indicated that PN extract caused migratory inhibition on breast MCF-7 cancer cells with down-regulation of metastasis-associated gene expression, including MMP 2, MMP 9, VEGF and Intracellular Adhesion Molecule-1 (ICAM-1). It should be noted that the anti-metastatic effect of PN extract was discovered at lower concentrations than growth inhibition, which minimally decreased cell migration. Our results indicate that the PN extract may be used as anti-cervical cancer and further study will be examined in deep with mechanism of actions. The data demonstrated that young fruit of PN extract suppressed growth, accelerated the apoptosis and suppressed migration of human HeLa cervical cells, which may be related to the production of ROS levels and activated the abnormal mitochondrial membrane potential. Moreover, PN extract may exert its antitumor effects through ROS production pathway.
Funding:
This research project was financially supported by Thailand Science Research and Innovation (TSRI).
Conflict of interest:
The authors declared no conflict of interests.
References
- Ahmad N, Fazal H, Abbasi BH, Farooq S, Ali M, Khan MA. Biological role of Piper nigrum L.(Black pepper): A review. Asian Pac J Trop Biomed 2012;2(3):S1945-53.
- Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, et al.Phytochemistry of the genus Piper. Phytochemistry 1997;46(4):597-673.
- Tedasen A, Khoka A, Madla S, Sriwiriyajan S, Graidist P. Anticancer effects of piperine-free Piper nigrum extract on cholangiocarcinoma cell lines. Pharmacogn Mag 2020;16(68):28-38.
- Buranrat B, Boontha S. Effect of Piper nigrum ethanolic extract on human breast cancer cell growth and cell migration. Pharmacogn Mag 2019;15(65):538-46.
- Prashant A, Rangaswamy C, Yadav AK, Reddy V, Sowmya MN, Madhunapantula S. In vitro anticancer activity of ethanolic extracts of Piper nigrum against colorectal carcinoma cell lines. Int J Appl Basic Med Res 2017;7(1):67-72.
[Crossref] [Google Scholar] [PubMed]
- Si L, Yang R, Lin R, Yang S. Piperine functions as a tumor suppressor for human ovarian tumor growth via activation of JNK/p38 MAPK-mediated intrinsic apoptotic pathway. Biosci Rep 2018;38(3):BSR20180503.
[Crossref] [Google Scholar] [PubMed]
- Yoo ES, Choo GS, Kim SH, Woo JS, Kim HJ, Park YS, et al.Antitumor and apoptosis-inducing effects of piperine on human melanoma cells. Anticancer Res 2019;39(4):1883-92.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Zhu X, Li H, Li B, Sun L, Xie T, et al.Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int Immunopharmacol 2015;24(1):50-8.
[Crossref] [Google Scholar] [PubMed]
- Grinevicius VM, Andrade KS, Ourique F, Micke GA, Ferreira SR, Pedrosa RC. Antitumor activity of conventional and supercritical extracts from Piper nigrum L. cultivar Bragantina through cell cycle arrest and apoptosis induction. J Supercrit Fluids 2017;128:94-101.
- Deng Y, Sriwiriyajan S, Tedasen A, Hiransai P, Graidist P. Anti-cancer effects of Piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J Ethnopharmacol 2016;188:87-95.
[Crossref] [Google Scholar] [PubMed]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61(2):69-90.
[Crossref] [Google Scholar] [PubMed]
- Soheilyfar S, Velashjerdi Z, Hajizadeh YS, Maroufi NF, Amini Z, Khorrami A, et al.In vivo and in vitro impact of miR-31 and miR-143 on the suppression of metastasis and invasion in breast cancer. J BUON 2018;23(5):1290-6.
[Google Scholar] [PubMed]
- Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res 2010;44(5):479-96.
[Crossref] [Google Scholar] [PubMed]
- Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, et al.Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 1995;182(2):367-77.
[Crossref] [Google Scholar] [PubMed]
- Kalpage HA, Bazylianska V, Recanati MA, Fite A, Liu J, Wan J, et al.Tissue-specific regulation of cytochrome c by post-translational modifications: Respiration, the mitochondrial membrane potential, ROS and apoptosis. FASEB J 2019;33(2):1540.
[Crossref] [Google Scholar] [PubMed]
- Morrissey MA, Hagedorn EJ, Sherwood DR. Cell invasion through basement membrane: The netrin receptor DCC guides the way. Worm 2013;2(3):903-13.
[Crossref] [Google Scholar] [PubMed]