- *Corresponding Author:
- C. M. Modi
Department of Veterinary Pharmacology and Toxicology,
College of Veterinary Science and Animal Husbandry,
Junagadh Agricultural University,
Junagadh,
Gujarat 362001,
India
E-mail: chiragvets@yahoo.co.in
| Date of Received | 25 August 2020 |
| Date of Revision | 21 August 2021 |
| Date of Acceptance | 24 March 2022 |
| Indian J Pharm Sci 2022;84(2):369-379 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study was carried out to evaluate the protective effects of polyherbal extract against gentamicin induced renal injury in rats. 36 male Wistar rats were divided into six groups. Gentamicin was administered (100 mg/kg, intraperitoneally for 10 d) to all groups except normal control group. Polyherbal extract was administered to three groups (gentamicin+polyherbal extract 100 mg/kg, gentamicin+polyherbal extract 200 mg/kg and gentamicin+polyherbal extract 300 mg/kg) at 100, 200 and 300 mg/kg body weight orally for 21 d, respectively. Taurine was taken as standard drug and given at the dose rate of 50 mg/ kg, orally for 21 d to positive group. The in vitro antioxidant activity was determined using 2,2-diphenyl- 1-picryl-hydrazyl-hydrate. The polyherbal extract was also subjected to phytochemical analysis using liquid chromatography quadrupole time-of-flight mass spectrometry. Renal functions were assessed by evaluating biochemical parameters and oxidative stress parameters. Histopathological examinations were also conducted to confirm the renal damage. The treatment of polyherbal extract at dose of 300 mg/kg body weight attenuated significantly (p<0.05) urea, creatinine and uric acid levels in gentamicin induced nephrotoxicity model in rats. Similarly, activity of catalase in kidney of rats was found to have significant (p<0.05) increase in polyherbal extract treatment at 300 mg/kg body weight. Insignificant altered level of glutathione and superoxide dismutase activity was observed in kidney of rats in all treatment groups. Renal histopathology also showed that polyherbal extract reduced the tubular degeneration, interstitial infiltration of inflammatory cells and tubular necrosis. Liquid chromatography quadrupole time-of-flight mass spectrometry analysis of polyherbal extract showed the presence of flavonoids, tannins, gallic acid and tricarboxylic acid. The polyherbal extract at the dose of 200 μg/ml have shown the maximum radical scavenging activity which indicated concentration dependent antioxidant activity. These results suggest that polyherbal extract at dose of 300 mg/kg body weight, orally for 21 d exhibits a potent nephroprotective effect on gentamicin-induced kidney damage in rats, which may be due to the increase in biochemical parameters, antioxidant enzymes activity in rats).
Keywords
Rats, gentamicin, polyherbal extract, renal injury
Gentamicin is commonly used against life-threatening infections caused by Gram-negative bacteria[1]. Besides the beneficial properties of gentamicin, it produces the nephrotoxic side effects[2]. A large number of studies have been reported that the treatment with gentamicin for more than 7 d can affect the proximal tubular cell morphology and causes renal damage[3]. Aminoglycoside drugs are freely filtered and quickly taken up by the epithelial cells of proximal tubule, where they are incorporated into lysosomes after interacting with phospholipids on the brush border membranes. The association of gentamicin with negativelycharged phospholipids and its accumulation in the lysosomes of tubular cells leads to phospholipidosis by inhibition of lysosomal phospholipases, which triggers the renal necrosis[4]. The specificity of gentamicininduced renal toxicity is linked to its deposition in the renal convoluted tubules and lysosomes. Moreover, gentamicin also produces the oxidative stress caused by generation of Reactive Oxygen Species (ROS)[5]. Many experiments demonstrated that superoxide anion and hydrogen peroxidases generation followed by hydroxyl radicals enhanced by gentamicin in renal cortical mitochondria[6]. Inhibition of the activity of antioxidant enzymes is associated with gentamicin-induced acute renal failure[7].
Nowadays, use of the herbal medicines or phytochemicals has been increased all over the world due to their bioactivity and fewer side effects as compared to the current synthetic medicines. Herbs are generally considered as safe and proved to be effective against various ailments and their medicinal uses are gradually increasing in developed countries. Medicinal plants are having curative properties and therapeutic values due to the presence of various complex phytochemical compounds. The various experiments in the area of hepatoprotective and nephroprotective effects of drugs of herbal origin have been carried out by many researchers[8-10]. Medicinal herbs have ability to protect cell injury induced by gentamicin through elimination of hydroxyl radicals which prevents oxidative damage. Many studies have reported that the natural antioxidants present in medicinal plants or herbs can prevent gentamicin-induced nephrotoxicity including; Aegle marmelos L. (A. marmelos)[8,9], Abutilon indicum L. (A. indicum)[10], Boerhavia diffusa L. (B. diffusa)[11], Phyllanthus emblica L. (P. emblica)[12], Ficus racemosa L. (F. racemosa)[13], Tribulus terrestris L. (T. terrestris) [14], etc.
According to the literature, number of crude herbal extracts offer a vast source of potentially useful new compounds to combat the kidney problems[15]. Polyherbal Extract (PHE) which may exert synergistic, potentiative or agonistic actions by virtue of its diverse active principles within themselves. We selected six plants upon folklore claims which were having a role in protection of kidney. Extracts of plants like, A. indicum, A. marmelos, B. diffusa, F. racemosa, P. emblica and T. terrestris were used to make PHE. Selected plants contain flavonoids and phenolic compounds with potent antioxidant properties, which may help in the inhibition of oxidative damage caused by gentamicin. We hypothesized that the PHE might produce good nephroprotective effect as well as such study with mixture containing phytoconstituents have not been evaluated for having antioxidant mediated protective role against nephrotoxicity. Thus, above all plants have been selected to prepare PHE and used to evaluate its effect on biochemical profile with other antioxidant parameters and histopathological changes in gentamicin-induced renal toxicity rodent model. By keeping this hypothesis in mind, this study has been designed in order to investigate the nephroprotective activity of PHE against gentamicin induced toxicity in male rats.
Materials and Methods
Collection and authentication of plant materials:
Fruit pulp of the P. emblica (L.), root of the A.indicum (L.), fruit of the A. marmelos (L.), fruit of the T. terrestris (L.), leaves of the B. diffusa (L.) and stem bark of the F. racemosa (L.) were collected from Junagadh district of Gujarat, India and authenticated by Mr. Punit Bhatt, Pharmacognosist, Department of Pharmacology and Toxicology, Veterinary College, Junagadh. The voucher specimens of plant materials (JVC/VPT/SP/02/2018 to JVC/VPT/SP/07/2018) were deposited in the Departmental Herbarium, College of Veterinary Science and Animal Husbandry, Junagadh Agricultural University, Junagadh, Gujarat for future reference.
Preparation of PHE:
After collection of various parts of the selected plants, they were cleaned with tap water followed by shed drying. The powder was made using mixture grinder from dried pieces of parts. The powder (100 g) was macerated with methanol:water (60:40) solvent for three times with occasional stirring. It was allowed to stand for 7 d at room temperature then content was filtered. The extracted material was evaporated to dryness in a vacuum with the help of a rotary evaporator[16]. The crude extract of each plant material was enough air-dried to collect a solid mass which were stored at -20° for further use. PHE was made by adding equal proportion of six herbal extracts suspended in distilled water. The doses of PHE were prepared daily prior to dosing.
In vitro antioxidant assay:
The radical scavenging ability of individual plant extract was evaluated by using 2,2-diphenyl-1-picryl-hydrazylhydrate (DPPH) assay[17]. The different concentrations (10 μg/ml-200 μg/ml) of extract mixtures contained 3 ml of the extract and 1 ml of 0.1 mM DPPH. After 30 min of incubation at room temperature in dark and the absorbance was measured at 517 nm. The assay was performed in triplicate and the results were expressed as percent inhibition. Ascorbic acid was used as the standard control. The radical scavenging activity was calculated using the following equation: Percent (%) scavenging activity=[(A0-A1)/A0)]×100, Where, A0 is the absorbance of the control reaction and A1 is the absorbance of the test sample.
Liquid Chromatography Quadrupole Time-of- Flight Mass Spectrometry (LC-QTOF-MS) analysis of polyherbal hydroalcoholic extract:
Chromatographic separation was achieved with chromatographic system (Agilent Technologies, United States of America (USA), Model 6540) with C18 column Zorbax 300SB (4.6×100 mm, 3.5 μm) at 25° as the stationary phase. The gradient mobile phase comprising of solvent A (0.1 % formic acid in water) and solvent B (acetonitrile), programmed as follows: 0 min, linear change from A–B (95:5 v/v) to A–B (5:95 v/v); 12 min, isocratic A–B (5:95 v/v); 20 min, linear change to A–B (95:5 v/v) 22 min and 25 min, linear change to A–B (95:5 v/v) with constant flow rate (0.6 ml/min). The 10 μl volume of extract was injected into system. Mass Spectrometry (MS) analysis was carried out using a 6540 Agilent ultra-high definition accurate mass QTOF-MS coupled to the Liquid Chromatography (LC), equipped with an Dual Agilent Jet Stream Electrospray Ionization (Dual AJS ESI) interface in positive ionization mode at the following conditions: Drying gas flow (Nitrogen): 10.0 l/min; nebulizer pressure: 45 psi; gas drying temperature: 325°; gas vaporize temperature: 350°; capillary voltage: 0.051 μA; chamber voltage: 4.23 μA. Fixed collision energies were set to 10, 20, 30, 40, 50 V, precursors per cycle were set to 5 and precursor threshold was 400 counts, mass scan range: 100-1700 m/z and scan speed was 25 000 counts/spectrum. Integration and data interpretation were performed using Mass Hunter software (Agilent Technologies, Santa Clara, California (CA), USA). Agilent Technologies has provided the METLIN personal compound database with accurate mass MS/MS Library (Personal Compound Database and Library (PCDL)). The METLIN PCDL includes all compounds and additionally accurate mass Q-TOF MS/ MS library reference spectra.
Procurement and housing of animals:
Male Wistar rats of 6-8 w of age (weighing 220-250 g) were procured from Cadila Pharmaceutical Ltd., Ahmedabad. The rats were acclimatized for 2 w before the start of the experiment. They were housed at a controlled temperature (22°±0.5°) with a 12 h light and dark cycle. Rat pelleted feed (VRK biological system, India) was provided ad libitum to animals throughout the study period, except overnight fasting, prior to pretermination and blood collection. This experimental protocol (No.: JAU/JVC/IAEC/SA/19/2016) including 36 rats and various procedures was approved by the Institutional Animal Ethics Committee (IAEC) of College of Veterinary Science and Animal Husbandry, Junagadh (Gujarat, India), constituted under the Committee for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA), Animal Welfare Division, New Delhi.
Acute oral toxicity study:
Acute oral toxicity was conducted according to the method of Organisation for Economic Co-operation and Development (OECD)[18]. Animals were kept under fasting by providing only water, after which the PHE extract was administered by oral gavage at the single dose of 2000 mg/kg body weight and observed for any toxic symptoms and mortality for 14 d.
Experimental protocol:
2 w after acclimatization, a total number of 36 rats were grouped according to its body weight in six different treatment groups, each group comprised of six rats. Group I served as Normal Control (CON) and received distilled water, whereas group II considered as Gentamicin Intoxicated Group (GM) that received gentamicin at a dose of 100 mg/kg/d, Intraperitoneally (IP) for 10 d. Group III served as positive control (GM+Taurine (TAU)), animals were administered gentamicin with TAU (50 mg/kg/d for 21 d) by oral gavage. Group IV (GM+PHE100), group V (GM+PHE200) and group VI (GM+PHE300) were considered as treatment groups that received PHE doses at 100, 200 and 300 mg/kg body weight, respectively for 21 d by oral gavage along with gentamicin treatment.
Assessment of renal biomarkers:
At the end of treatment period, blood samples were withdrawn from retro-orbital plexus using a glass capillary collector. Samples were allowed to clot by leaving them undisturbed for 1 h at 4° and then centrifuged at 3000 rpm for 10 min to separate serum. The serum was stored at -20° until analysis. The separated serum was used for the estimation of serum creatinine, Blood Urea Nitrogen (BUN), total protein, urea and uric acid level by using commercial kits with fully automatic biochemistry analyzer (Diatek Health Care Pvt. Ltd.).
Change in body weight and wet kidney weight:
At the start and end of the experiment, the body weight of each animal was measured which was used to evaluate the change in the body weight during the study period. The both kidneys were excised, weighed using analytical balance and the result was expressed in gram.
Evaluation of oxidative stress markers:
Collection and preparation of samples: All rats were euthanized by Carbon Dioxide (CO2) asphyxiation on d 22 and the kidneys were collected, washed and weighed. Kidney samples (0.5 g) was isolated from each rats after sacrificing rats and immediately stored in ice-cold 2 ml 0.1 M Phosphate Buffer Saline (PBS, pH 7.4). A teflon homogenizer was used to prepare 25 % of homogenate in ice cold PBS, then it was centrifuged at 10 000 rpm/min at 4° for 10 min to purge cellular debris. The supernatant was collected and stored at -81° for the evaluation of antioxidant enzymes like Catalase (CAT) and reduced Glutathione (GSH). Whereas tissue samples were homogenized with ice-cold 30 mM Tris- Ethylenediaminetetraacetic Acid (Tris-EDTA) buffer (pH 8.2) to prepare 10 % homogenate (1 g of kidney was crushed in 10 ml of ice-cold 30 mM Tris-EDTA buffer (pH 8.2)) using a teflon homogenizer. Then, homogenate was centrifuged at 10 000 rpm for 10 min at 4°. The supernatant was collected and used for assay of Superoxide Dismutase (SOD)[19].
Determination of protein content: The protein content of each kidney homogenate sample was estimated according the method of Bradford[20]. These data were used to calculate CAT activity in kidney tissues.
Determination of GSH level: GSH level in each renal tissue homogenate was measured using the method described by Ellman[21] with slight modifications. Tissue homogenate (0.5 ml) was mixed with equal volume of trichloroacetic acid (20 %) containing 1 mM EDTA to precipitate the tissue protein. This reaction mixture was allowed to stand for 5 min prior to centrifugation for 10 min at 10 000 rpm. The supernatant (200 μl) was collected and transferred to a new set of test tube and added with 1.8 ml of the Ellman’s reagent (5,50-dithiobis-2-nitrobenzoic acid, (0.1 mM) prepared in 0.3 M phosphate buffer with 1 % of sodium citrate solution). After completion of the total reaction, absorbance was measured at 412 nm using a spectrophotometer (Fusiontek-Ultraviolet (UV) 2900) against blank having mixture of PBS and supernatant. Absorbance values were compared with a standard curve generated from known concentrations of GSH. Reduced GSH was expressed as μM/mg tissue.
Determination of SOD activity: The enzymatic activity of SOD in renal tissues was determined by the method of Marklund, et al.[22]. 10 μl of supernatant from tissues homogenate was mixed with 2.989 ml Tris-EDTA buffer (pH-8.2). The reaction was initiated after adding 100 μl pyrogallol in a cuvette and then change in the absorbance was recorded at 420 nm at 1 min interval for 3 min using spectrophotometer against reagent blank containing 2.99 ml Tris-EDTA and 100 μl pyrogallol (2 mM). 1 unit of SOD activity is the amount of the enzyme that inhibits the rate of auto oxidation of pyrogallol by 50 % and was expressed as units/mg protein.
Rate (R)=Final Optical density (OD)-Initial OD/3 min
Percentage (%) of inhibition= Blank OD-R×100/ Blank OD
Enzyme unit (U)=(% of inhibition/50)×common dilution factor
[50 % inhibition is similar to 1 U]
Determination of CAT activity: The CAT activity in renal tissues was measured according to the method of Aebi[23]. The reaction mixture consisted of 20 μl of supernatant from tissue homogenate and mixed with 1.8 ml PBS (0.1 M, pH 7.4) which was added with Hydrogen Peroxide (H2O2) (30 mM) to start the reaction. Absorbance was observed by continuous recording at 240 nm at 20 s interval up to 1 min against blank having mixture of PBS and tissue homogenate. The results were expressed as micromole H2O2 decomposed per milligram of protein per minute.
CAT activity (U/mg protein)=ΔA/min×1000×3/43.6×mg protein in sample
ΔA/min is mean absorbance change per minute.
Histopathological assessment:
After sacrifice of each animal, kidney was excised and fixed in 10 % neutral formalin for a period of at least 24 h. The tissues were dehydrated in graded (50 %-100 %) alcohol series, cleaned in xylene and embedded in paraffin. About 5 μ thick sections were taken using semi-automated rotary microtome (Leica Biosystems, Germany). The slides were then deparaffinated and stained with hematoxylin and eosin and mounted with a cover slip using Dibutylphthalate Polystyrene Xylene (DPX)[24]. The stained slides were observed for microscopic pathological changes in the renal tissue using an optical microscope (Zeiss primo star) attached with a microscopic camera (ZEISS Axiocam ERc 5) and digital histological photographs were captured with the help of Carl Zeiss ZEN 2 (Blue edition) software.
Statistical analysis:
Results were expressed as mean±Standard Error of Mean (SEM) and have been subjected to statistical analysis. The difference between groups were analysed through one-way analysis of variance, followed by Duncan’s Multiple Range Test (DMRT)[25].
Results and Discussion
Gentamicin is used for Gram-negative bacterial infections, because of its potent bactericidal action and less bacterial resistance properties. However, it causes 10 %-15% acute renal failure and 30 % nephrotoxicity in patients who received the gentamicin for more than 7 d[26]. There is selective accumulation of gentamicin in renal cortex and it interrupts various intracellular renal functions leading to renal injury. The several times higher concentration of aminoglycosides achieved in the renal proximal tubule cells was associated with their nephrotoxicity[27].
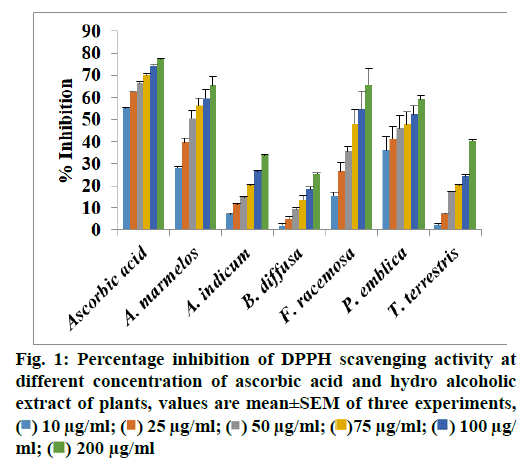
The hydroalcoholic extract of each plant material was tested for in vitro antioxidant activity using DPPH radical scavenging assay and results are presented in fig. 1. The radical scavenging activity of the A. marrmelos and F. racemosa showed higher (65.23 % and 65.45 %, respectively) at concentrations 200 μg/ ml. Results of in vitro antioxidant assay indicate that all extracts exhibited increase inhibition of DPPH free radicals with increasing in concentrations. They protect the radical scavenging activity such as peroxide, hydroperoxide or lipid peroxyl and thus inhibit the oxidative stress and prevent of disease[28,29]. Natural active constituents such as flavonoids and gallic acid have been detected in extracts. Hence, the probable mechanism of nephroprotection by PHE may be attributed to its antioxidant and free radical scavenging property.
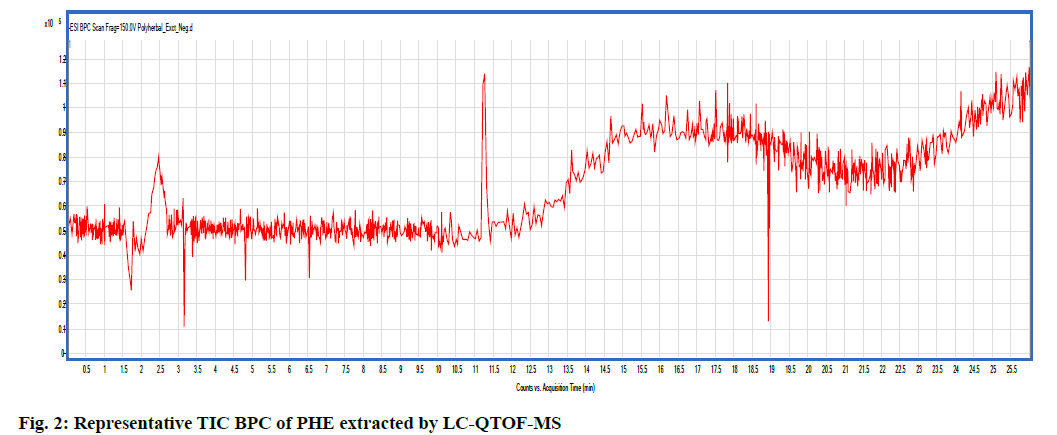
Metabolite profile of PHE was assessed by applying non-targeted LC-QTOF-MS using ESI in negative ionization mode. Details of compound name, Retention time (Rt), experimental mass (m/z), height of the peak and area covered by the individual peak of the identified compounds are given in Table 1. The Total Ion Current (TIC) Base Peak Chromatogram (BPC) of PHE obtained in extracted ion chromatogram in negative ionization mode is shown in fig. 2. Total 9 metabolites detected in PHE by LC-QTOF-MS are responsible for antioxidant activity. Natural antioxidants from herbs, vegetables and fruits are claimed to prevent gentamicin nephrotoxicity[30]. In the present investigation, preliminary phytochemical screening of PHE determined the presence of flavonoids, gallic acid and tannins. Similarly, LC-QTOF-MS analysis revealed the presence of quercetin, 1,3,4-trigalloyl-beta-Dglucopyranose, benzyl oxyphenyl isoserine methylester, pteridine, gallic acid, 2,4,6-trihydroxybenzoic acid, xanthine and carboxymethyl oxysuccinate. Quercetin is a flavonoid found in fruits, vegetables and in plants commonly used in traditional medicine[31]. Flavonoids are polyphenolics compounds which possess antioxidant properties due to their reducing and chelating capabilities. They can prevent injury caused by free radicals by their significant free radical scavenging activity[32]. Quercetin has antioxidant and anti-inflammatory effects which may help to prevent the renal disease. Xanthine oxidase plays an important role in various forms of ischemic and other types of tissue and vascular injuries, inflammatory diseases and chronic heart failure[33]. In evidence of this, the polyphenolic compounds of PHE could contribute to nephron protection by its antioxidant activity. Yet, the precise mechanism is not known, its protective effect can be linked with the presence of phytoconstituents.
| S. No. | Class | Compound name (Cpd#) | Molecular formula | m/z value (mass) | Rt (min) | Peak height | Area fraction (%) | Peak area |
|---|---|---|---|---|---|---|---|---|
| 1 | Flavonoids | Quercetin 3-galactoside-7-rhamnoside | C27H30O16 | 610.1541 | 11.334 | 31375 | 2.93 | 265403 |
| 2 | Tannins | 1,3,4-Trigalloyl-beta-D-glucopyranose | C27H24O18 | 636.0952 | 10.163 | 7516 | 2.3 | 208890 |
| 3 | Ester derivatives | Benzyl oxyphenyl isoserine methyl ester | C17H17NO4 | 299.1171 | 11.282 | 118624 | 11.84 | 1073767 |
| 4 | Benzenesulfonic acids and derivatives | N-Undecylbenzenesulfonic acid | C17H28O3S | 312.1772 | 15.871 | 70861 | 37.66 | 3414143 |
| 5 | Flavonoids | Floribundoside | C21H22O10 | 434.1216 | 10.876 | 12190 | 1.27 | 115359 |
| 6 | Pterins and flavins | Pteridine | C6H4N4 | 132.0436 | 1.79 | 54523 | 9.71 | 880051 |
| 7 | Tricarboxylic acid | Carboxymethyloxysuccinate | C6H8O7 | 192.0276 | 2.106 | 11120 | 3.75 | 340018 |
| 8 | Endogenous Metabolite | Xanthosine | C10H12N4O6 | 284.0764 | 2.803 | 5768 | 1.39 | 125866 |
| 9 | Gallic acid | 2,4,6-Trihydroxybenzoic acid | C7H6O5 | 170.0223 | 2.478 | 79820 | 29.15 | 2643169 |
Note: Cpd#: Compound name as matched with the library of the Agilent mass hunter software of the model QTOF/LCMS 6540
Table 1: Metabolite Profiling Of PHE by LC-QTOF-MS
The oral acute toxicity test showed no lethality or signs of toxicity at 2000 mg/kg and the PHE was considered as safe. Therefore, 1/10th dose was selected as therapeutic dose (200 mg/kg) from maximum tolerance dose, one and a half of it as highest dose (300 mg/kg/d) and then a half of it as lowest dose (100 mg/kg/d) for evaluation of nephroprotective and antioxidant activity in vivo.
The changes of body and kidney weight of the animals of different treatments are shown in Table 2. Renal injury induced by gentamicin caused significant (p<0.05) decrease in body weight and increase in kidney weight in GM group when compared with the CON group. There was significant difference in body weights in all experimental groups when compared with the gentamicin-treated group at the end of experiments.
| Groups | Body weight | Kidney weight (g) | |
|---|---|---|---|
| 0 d (g) | 22 d (g) | ||
| CON | 339.83±12.33a | 353.67±15.64a | 2.20±0.10a |
| GM | 323.33±16.49a | 326.50±12.62b | 2.89±0.32b |
| GM+TAU | 322.67±15.31a | 348.17±15.88a | 2.74±0.13ab |
| GM+PHE100 | 320.33±12.99a | 343.67±10.30a | 2.72±0.16ab |
| GM+PHE200 | 320.50±12.87a | 343.50±8.19a | 2.62±0.09ab |
| GM+PHE300 | 319.67±13.91a | 351.00±10.91a | 2.78±0.19ab |
Note: Data are expressed as mean±SEM (n=6). (a, b)Values in a column differ significantly (p<0.05). CON: Normal control group; GM: Gentamicinintoxicated group; GM+TAU: Gentamicin+taurine group; GM+PHE100: Gentamicin+polyherbal extract group (100 mg/kg); GM+PHE200: Gentamicin+polyherbal extract group (200 mg/kg); GM+PHE300: Gentamicin+polyherbal extract group (300 mg/kg)
Table 2: Effect of PHE on Animal Body Weight and Kidney Weight in Gentamicin-Induced Nephrotoxicity in Rats
Furthermore, control animals did not change the body and the kidney weight throughout the study period. The significant and progressive weight loss in gentamicintreated rats might be due to injury of renal tubules and the subsequent loss of the tubular cells to reabsorb water, leading to dehydration and loss of body weight. Loss of body weight in the gentamicin-treated group provides a condition of negative nitrogen balance, which provides a statement for potassium loss[34]. Oral administration of graded doses of the PHE has a protective role against weight loss induced by gentamicin. It is reasonable to conclude that the antioxidant effect was responsible for the prevention of weight loss recorded in this study. Similar to our observation, gentamicin-treated animals shown significant loss in body weight in a dose-dependent manner[35]. The weight of kidney was found to be significantly increased in rats treated with gentamicin which may be due to edema induced by acute tubular necrosis[36] and administration of PHE was failed to decrease normalized kidney weight induced by gentamicin. Several studies supports the present finding that the increase in kidney weight after gentamicin treatment[9,13,37,38]. All the treatment groups treated with PHE resulted to prevent change in body and kidney weight during the treatment period.
The effects of treatment of PHE at various doses were studied on renal biomarkers against gentamicininduced toxicity are presented in Table 3. GM group had shown significant (p<0.05) increase in the level of urea, creatinine, uric acid and BUN in serum as compared to CON group which indicates renal dysfunction. On the other hand, the treatment of GM+PHE300 decreased insignificantly (p>0.05) the level of creatinine and decrease significantly (p<0.05) the level of BUN, urea and uric acid in serum compared to GM group. However, the values of total protein in all the treatment groups were insignificantly (p>0.05) altered in gentamicin induced nephrotoxicity modal in rats.
| Groups | Creatinine (mg/dl) | BUN (mg/dl) | Total protein (g/dl) | Urea (mg/dl) | Uric acid (mg/dl) |
|---|---|---|---|---|---|
| CON | 0.36±0.03a | 16.44±1.27c | 6.47±0.12a | 35.19±2.72c | 1.18±0.10b |
| GM | 0.77±0.20ab | 30.12±1.64a | 6.45±0.14a | 64.47±3.51a | 2.25±0.17a |
| GM+TAU | 0.50±0.09ab | 28.89±2.07ab | 6.38±0.12a | 61.82±4.42ab | 1.23±0.35b |
| GM+PHE100 | 0.62±0.15ab | 24.98±1.58ab | 6.35±0.06a | 53.45±3.38ab | 1.03±0.25b |
| GM+PHE200 | 0.42±0.05ab | 26.05±2.00ab | 6.55±0.06a | 55.75±4.29ab | 0.98±0.24b |
| GM+PHE300 | 0.40±0.07b | 24.75±1.51b | 6.41±0.17a | 52.97±3.23b | 1.05±0.12b |
Note: Data are expressed as mean±SEM (n=6). (a, b, c)Values in a column differ significantly (p<0.05). CON: Normal control group; GM: Gentamicin-intoxicated group; GM+TAU: Gentamicin+taurine group; GM+PHE100: Gentamicin+polyherbal extract group (100 mg/kg); GM+PHE200: Gentamicin+polyherbal extract group (200 mg/kg), GM+PHE300: Gentamicin+poly herbal extract group (300 mg/kg)
Table 3: Effect of PHE on Serum Renal Biomarkers in Various Treatment Groups
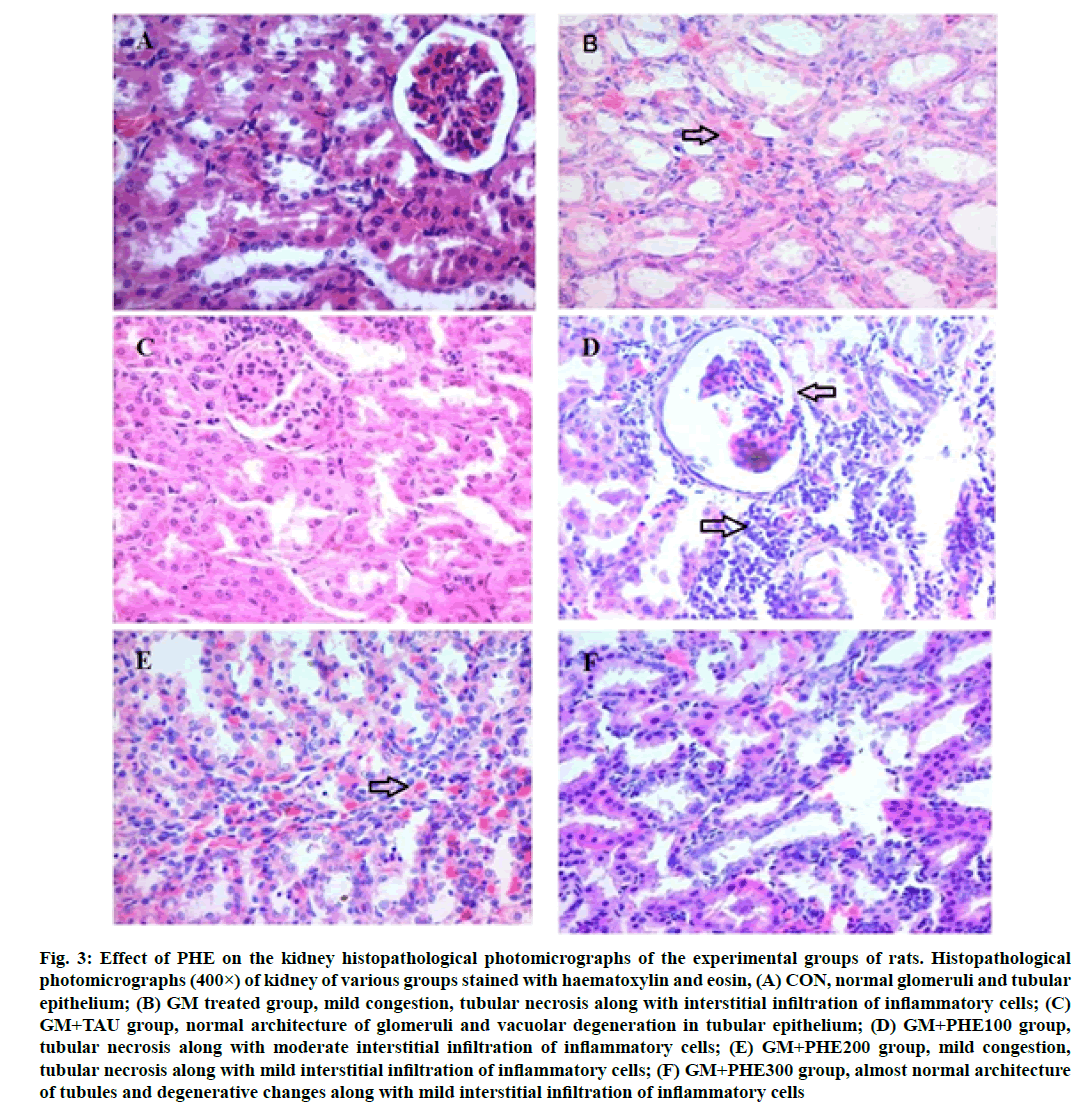
Fig. 3:Effect of PHE on the kidney histopathological photomicrographs of the experimental groups of rats. Histopathological photomicrographs (400×) of kidney of various groups stained with haematoxylin and eosin, (A) CON, normal glomeruli and tubular epithelium; (B) GM treated group, mild congestion, tubular necrosis along with interstitial infiltration of inflammatory cells; (C) GM+TAU group, normal architecture of glomeruli and vacuolar degeneration in tubular epithelium; (D) GM+PHE100 group, tubular necrosis along with moderate interstitial infiltration of inflammatory cells; (E) GM+PHE200 group, mild congestion, tubular necrosis along with mild interstitial infiltration of inflammatory cells; (F) GM+PHE300 group, almost normal architecture of tubules and degenerative changes along with mild interstitial infiltration of inflammatory cells
The treatment of GM+PHE300 reversed elevation of gentamicin-induced serum creatinine and uric acid levels to almost normal values. These observations indicate improved renal function in the form of effective clearance of urea and creatinine. This showed the protective effect of PHE against toxic effects of gentamicin on kidney. A similar findings by the administration of A. marmelos[9], T. terrestris[14] and B. diffusa[11] to rats have also been reported.
The increased serum creatinine, urea and BUN level in the gentamicin treated rats were observed which decreased the glomerular filtration rate[10]. The mechanism behind elevated serum urea and creatinine might be that the gentamicin increases the entry of calcium ions (Ca+2) in the mesangial cells leading to reduced glomerular filtration rate[39]. On the other hand, rise in serum creatinine depends upon the extent of tubular necrosis which may be due to the blocking of tubules by gentamicin-induced nephrotoxicity[36]. The obstruction of tubules by necrotic debris and filtrate leaking through the damaged tubules might be responsible for elevated serum creatinine levels[40].
Effect of gentamicin alone, gentamicin plus PHE on activity of renal SOD, CAT and GHS level in different treatment groups are shown in Table 4. Gentamicin intoxication caused a significant (p<0.05) decrease in renal CAT activity. In the toxicity condition, the tissue CAT is utilized more due to production of more ROS and H2O2. In the present study, the levels of GSH and SOD in rat kidney tissues were slightly increased after GM injection to PHE compared with control group. It may be due to more production of superoxide ion and H2O2 leads to elevation in SOD and GSH activity to overcome the auto oxidation and oxidative stress. If the oxidative stress is not very strong for long duration, the SOD and GSH activity increases. If oxidative stress is persisting or its level is very high, the proteins damage became profound and a decreased SOD and GSH activity may occur either via direct oxidative damage of the SOD molecules or via oxidative stress-altered SOD and GSH gene expression or both. Treatment with chlorfenvinphos at low dose could result in an increase in the superoxide anion level and simultaneous increase in SOD activity[41]. Simultaneous administration of PHE with gentamicin resulted in dose-dependent significant improvement in CAT activity. This finding demonstrates that PHE has shown significantly antioxidant effect against gentamicin-induced oxidative damage in renal tissue.
| Groups | SOD (U/mg protein) | CAT (U/mg protein) | GSH (µg/mg tissue) |
|---|---|---|---|
| CON | 30.98±6.44a | 1.70±0.11a | 0.20±0.07a |
| GM | 40.69±4.13a | 0.72±0.07c | 0.26±0.08a |
| GM+TAU | 42.44±1.00a | 0.92±0.01c | 0.32±0.05a |
| GM+PHE100 | 32.20±1.88a | 0.86±0.06c | 0.11±0.01a |
| GM+PHE200 | 33.04±3.57a | 0.90±0.05c | 0.35±0.14a |
| GM+PHE300 | 33.05±2.04a | 1.28±0.11b | 0.22±0.04a |
Note: Data are expressed as mean±SEM (n=6). (a, b, c)Values in a column differ significantly (p<0.05). CON: Normal control group; GM: Gentamicin-intoxicated group; GM+TAU: Gentamicin+taurine group; GM+PHE100: Gentamicin+polyherbal extract group (100 mg/kg); GM+PHE200: Gentamicin+polyherbal extract group (200 mg/kg); GM+PHE300: Gentamicin+polyherbal extract group (300 mg/kg); CAT:
Catalase; GSH: Reduced glutathione; SOD: Superoxide dismutase
Table 4: Effect of PHE on Oxidative Stress Markers of Renal in Various Treatment Groups
Normal cellular metabolic activities generate ROS, which are eliminated by intrinsic antioxidant enzyme systems[42]. Gentamicin induces renal mitochondrial dysfunctions with generation of ROS[43]. The main mechanisms of acute and chronic renal pathologies are aggregation of ROS[44]. Therefore, antioxidant and ROS scavenger properties may have the capacity to eliminate the deleterious effects caused by gentamicin. This observations support the evidence of gentamicininduced inhibition of enzymes is reversible and this inhibition of enzyme was attenuated by the administration of PHE extracts in this study. The oxidative stress plays an important role for unbalance between the production of ROS and antioxidant defence. Many studies mentioned that gentamicin causes oxidative stress and nephrotoxicity, characterised by exposure to ROS[6,7]. Hence, natural antioxidants are claimed to provide nephroprotection in gentamicin renal injury. These alterations were also reversed with simultaneous administration of TAU suggesting the beneficial role of the drug with respect to renal functions. Administration of TAU significantly reduced the nephrotoxic symptoms produced by gentamicin[35].
The histological changes in kidney sections from various treatment groups are shown in fig. 3 and Table 5. Histology of kidney sections from animals of the control group showed normal glomeruli and tubular architecture with no evidence of necrosis (fig. 3A). In contrast, rats treated with gentamicin produced congestion, interstitial infiltration of inflammatory cells, tubular necrosis, proliferation of fibrous connective tissue and cystic dilatation of tubules (fig. 3B). Simultaneous administration of gentamicin plus PHE (100 mg/ kg and 200 mg/kg) showed mild congestion, tubule necrosis alone with moderate interstitial infiltration of inflammatory cells compared to the gentamicintreated group (fig. 3D and fig. 3E). The rats treated with gentamicin along with PHE at 300 mg/kg had shown, noticeably preserved architecture of glomeruli and tubular epithelium (fig. 3F) which indicates that PHE has ability to ameliorate the gentamicin-induced renal tubular damage.
| Groups | Histopathological features | |||
|---|---|---|---|---|
| Vacuolar degeneration | Interstitial infiltration of inflammatory cells | Tubular necrosis | Congestion | |
| CON | Nil | Nil | Nil | Nil |
| GM | Nil | Severe | Moderate | Mild |
| GM+TAU | Mild | Nil | Nil | Nil |
| GM+PHE100 | Nil | Moderate | Mild | Nil |
| GM+PHE200 | Nil | Mild | Mild | Mild |
| GM+PHE300 | Nil | Mild | Nil | Nil |
Table 5: Histopathological Features of The Kidneys of Rats of Different Treatment Group
Free radicals released from mitochondria of renal tubular cells were found to be the main factor in induction of gentamicin-induced nephrotoxicity[45,46]. Severe and extensive histological alterations induced by gentamicin as seen in the present work suggest the potential of gentamicin to cause oxidative damage to macromolecules and cellular organelles. Leaking of proteins may be due to tubular cell degeneration caused by hyaline and granular casts in proximal and collecting tubules. The obstruction associated with casts may cause depression of glomerular filtration rate, which leads to kidney damage[40]. Scavenging of free oxygen radicals prevents irreversible renal cell injury and necrosis[47]. The PHE treatment at 300 mg/ kg showed near to normal architecture as observed in control group.
The present study showed that PHE protected against gentamicin-induced nephrotoxicity by improving renal biomarkers and antioxidant enzyme activities which are further supported by histological examinations. The plants are important source of various phytochemicals and antioxidant compounds also. Polyphenolic compounds possess nephroprotective property by promoting antioxidant enzyme system, thereby attenuating ROS generation. Additionally, further studies are essential to find out the mechanism of action of active compounds from PHE which are responsible for its nephroprotective role.
Acknowledgements:
Authors are thankful to Junagadh Agricultural University, Junagadh, India for providing grant and facility for research work. All authors are highly thankful to Dr. D. T. Fefar, Department of Veterinary Pathology, College of Veterinary Science and Animal Husbandry, Junagadh, India for his help during the study.
Conflict of interests:
The authors declared no conflict of interest.
References
- Krause KM, Serio AW, Kane TR, Connolly LE. Aminoglycosides: An overview. Cold Spring Harb Perspect Med 2016;6(6):a027029.
[Crossref] [Google Scholar] [PubMed]
- Le Prell CG, Ojano-Dirain C, Rudnick EW, Nelson MA, DeRemer SJ, Prieskorn DM, et al. Assessment of nutrient supplement to reduce gentamicin-induced ototoxicity. J Assoc Res Otolaryngol 2014;15(3):375-93.
[Crossref] [Google Scholar] [PubMed]
- LeBrun M, Grenier L, Gourde P, Bergeron MG, Labrecque G, Beauchamp D. Effectiveness and toxicity of gentamicin in an experimental model of pyelonephritis: Effect of the time of administration. Antimicrob Agents Chemother 1999;43(5):1020-6.
[Crossref] [Google Scholar] [PubMed]
- Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int 2011;79(1):33-45.
[Crossref] [Google Scholar] [PubMed]
- Patil CR, Jadhav RB, Singh PK, Mundada S, Patil PR. Protective effect of oleanolic acid on gentamicin induced nephrotoxicity in rats. Phytother Res 2010;24(1):33-7.
[Crossref] [Google Scholar] [PubMed]
- Negrette-Guzmán M, Huerta-Yepez S, Medina-Campos ON, Zatarain-Barrón ZL, Hernández-Pando R, Torres I, et al. Sulforaphane attenuates gentamicin-induced nephrotoxicity: Role of mitochondrial protection. Evid Based Complement Alternat Med 2013;2013.
[Crossref] [Google Scholar] [PubMed]
- Ekor M, Farombi EO, Emerole GO. Modulation of gentamicin?induced renal dysfunction and injury by the phenolic extract of soybean (Glycine max). Fundam Clin Pharmacol 2006;20(3):263-71.
[Crossref] [Google Scholar] [PubMed]
- Maity P, Hansda D, Bandyopadhyay U, Mishra DK. Biological activities of crude extracts and chemical constituents of Bael, Aegle marmelos (L.) Corr. Indian J Exp Biol 2009;47:849-61.
[Crossref] [Google Scholar] [PubMed]
- Kore KJ, Shete RV and Jadhav PJ. RP-HPLC method of simultaneous nephroprotective role of A. marmelos extract. Int J Pharm Chem Res 2011;1(3):617-23.
- Jacob Jesurun RS, Lavakumar S. Nephroprotective effect of ethanolic extract of Abutilon indicum root in gentamicin induced acute renal failure. Int J Basic Clin Pharmacol 2016;5:841-5.
- Olaleye MT, Akinmoladun AC, Ogunboye AA, Akindahunsi AA. Antioxidant activity and hepatoprotective property of leaf extracts of Boerhaavia diffusa Linn against acetaminophen-induced liver damage in rats. Food Chem Toxicol 2010;48(8):2200-5.
[Crossref] [Google Scholar] [PubMed]
- Kalra P, Karwasra R, Nag TC, Gupta YK, Singh S. Protective effect of Emblica officinalis fruit extract on cisplatin-induced nephrotoxicity in female rats. Bull Fac Pharm Cairo Univ 2017;4:1-9.
- Gowda KS, Swamy BV. Histopathological and nephroprotective study of aqueous stem bark extract of Ficus racemosa in drug induced nephrotoxic rats. IOSR J Pharm 2012;2(2):265-70.
- Abdel-Kader MS, Al-Qutaym A, Saeedan AS, Hamad AM, Alkharfy KM. Nephroprotective and hepatoprotective effects of Tribulus terrestris L. growing in Saudi Arabia. J Pharm Pharmacogn Res 2016;4(4):144-52.
- Liwa CA, Jaka HM. Renal diseases and use of medicinal herbal extracts: A concise update of reported literature in Africa. J Nephrol Renal Ther 2016;2(8):2-5.
- Singh G, Passsari AK, Leo VV, Mishra VK, Subbarayan S, Singh BP, et al. Evaluation of phenolic content variability along with antioxidant, antimicrobial, and cytotoxic potential of selected traditional medicinal plants from India. Front Plant Sci 2016;7:407.
[Crossref] [Google Scholar] [PubMed]
- Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 1995;28(1):25-30.
- Organization for Economic Co-operation and Development (OECD). Guidance document for the development of OECD guidelines for testing of chemicals. OECD Environment, Health and Safety Publications; 1995.
- Bashir S, Gilani AH. Antiurolithic effect of Bergenia ligulata rhizome: An explanation of the underlying mechanisms. J Ethnopharmacol 2009;122(1):106-16.
[Crossref] [Google Scholar] [PubMed]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72(1-2):248-54.
[Crossref] [Google Scholar] [PubMed]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82(1):70-7.
[Crossref] [Google Scholar] [PubMed]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974;47(3):469-74.
[Crossref] [Google Scholar] [PubMed]
- Aebi H. Catalase in vitro. Methods in Enzymol 1984;105:121-6.
[Crossref] [Google Scholar] [PubMed]
- Luna LG. Routine staining procedures. In: Manual of histologic staining methods of the Armed Forces Institute of Pathology. 3rd ed. New York : Blakiston Division, McGraw-Hill; 1968;32-39.
- Snedecor GW, Cochran WG. Statistical methods, 8th ed. Ames: Iowa State University Press; 1989;54:71-82.
- Walker PD, Barri Y, Shah SV. Oxidant mechanisms in gentamicin nephrotoxicity. Ren Fail 1999;21(3):433-42.
[Crossref] [Google Scholar] [PubMed]
- Alarifi S, Al-Doaiss A, Alkahtani S, Al-Farraj SA, Al-Eissa MS, Al-Dahmash B, et al. Blood chemical changes and renal histological alterations induced by gentamicin in rats. Saudi J Biol Sci 2012;19(1):103-10.
[Crossref] [Google Scholar] [PubMed]
- Jadeja R, Thounaojam M, Ramachandran AV, Devkar R. Phytochemical constituents and free radical scavenging activity of Clerodendron glandulosum. Coleb methanolic extract. J Complement Integr Med 2009;6(1):1-23.
- Mishra N, Dubey A, Mishra R, Barik N. Study on antioxidant activity of common dry fruits. Food Chem Toxicol 2010;48(12):3316-20.
[Crossref] [Google Scholar] [PubMed]
- Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem 2003;51(22):6509-15.
[Crossref] [Google Scholar] [PubMed]
- Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem 2013;138(4):2099-107.
[Google Scholar] [PubMed]
- Nijveldt RJ, Van Nood EL, Van Hoorn DE, Boelens PG, Van Norren K, Van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr 2001;74(4):418-25.
[Crossref] [Google Scholar] [PubMed]
- Pal M, Ghosh M. Studies on comparative efficacy of α-linolenic acid and α-eleostearic acid on prevention of organic mercury-induced oxidative stress in kidney and liver of rat. Food Chem Toxicol 2012;50(3):1066-72.
[Crossref] [Google Scholar] [PubMed]
- Brinker KR, Bulger RE, Dobyan DC, Stacey TR, Southern PM, Henrich WL, et al. Effect of potassium depletion on gentamicin nephrotoxicity. J Lab Clin Med 1981;98(2):292-301.
[Crossref] [Google Scholar] [PubMed]
- Tavafi M, Ahmadvand H, Toolabi P. Inhibitory effect of olive leaf extract on gentamicin-induced nephrotoxicity in rats. Iranian J Kidney Dis 2012;6:25-32.
[Google Scholar] [PubMed]
- Erdem A, Gündogan NÜ, Usubütün A, K?l?nç K, Erdem ?R, Kara A, et al. The protective effect of taurine against gentamicin?induced acute tubular necrosis in rats. Nephrol Dial Transplant 2000;15(8):1175-82.
[Crossref] [Google Scholar] [PubMed]
- Kulkarni YR, Keshav AB, Hari KP, Rajan PR. Evaluation of nephro?protective and anti nephro toxic properties of raktapunarnava roots (Boerhaavia diffusa, L.) gokshur fruits (Tribulus terrestris, L.) in drug induced nephrotoxicity. Int Res J Pharm 2012;3:329-4.
- Jaikumkao K, Pongchaidecha A, Thongnak LO, Wanchai K, Arjinajarn P, Chatsudthipong V, et al. Amelioration of renal inflammation, endoplasmic reticulum stress and apoptosis underlies the protective effect of low dosage of atorvastatin in gentamicin-induced nephrotoxicity. PLoS One 2016;11(10):e0164528.
[Crossref] [Google Scholar] [PubMed]
- Stojiljkovi? N, Veljkovi? S, Mihailovi? D, Stoiljkovi? M, Radovanovi? D, Ran?elovi? P. The effect of calcium channel blocker verapamil on gentamicin nephrotoxicity in rats. Bosn J Basic Med Sci 2008;8(2):170-6.
[Crossref] [Google Scholar] [PubMed]
- Solez K, Pathogenesis of acute renal failure. Int Rev Exp Pathol 1983; 24: 277-333.
- Toussaint O, Houbion A, Remacle J. Relationship between the critical level of oxidative stresses and the glutathione peroxidase activity. Toxicology 1993;81(2):89-101.
[Crossref] [Google Scholar] [PubMed]
- Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 2014;94(2):329-54.
[Crossref] [Google Scholar] [PubMed]
- Yang CL, Du XH, Han YX. Renal cortical mitochondria are the source of oxygen free radicals enhanced by gentamicin. Ren Fail 1995;17(1):21-6.
[Crossref] [Google Scholar] [PubMed]
- Mullins LJ, Conway BR, Menzies RI, Denby L, Mullins JJ. Renal disease pathophysiology and treatment: Contributions from the rat. Dis Model Mech 2016;9(12):1419-33.
[Crossref] [Google Scholar] [PubMed]
- Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci 2013;16(5):571-9.
[Crossref] [Google Scholar] [PubMed]
- Silan C, Uzun Ö, Çomunoglu NÜ, Gokçen S, Bedirhan S, Cengiz M. Gentamicin-induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biol Pharm Bull 2007;30(1):79-83.
[Crossref] [Google Scholar] [PubMed]
- Al-Majed AA, Mostafa AM, Al-Rikabi AC, Al-Shabanah OA. Protective effects of oral arabic gum administration on gentamicin-induced nephrotoxicity in rats. Pharmacol Res 2002;46(5):445-51. Crossref]
[Google Scholar] [PubMed]
 ) 10 μg/ml; (
) 10 μg/ml; (  ) 25 μg/ml; (
) 25 μg/ml; (  ) 50 μg/ml; (
) 50 μg/ml; (  )75 μg/ml; (
)75 μg/ml; (  ) 100 μg/ ml; (
) 100 μg/ ml; (  ) 200 μg/ml
) 200 μg/ml