- *Corresponding Author:
- C. Y. Liu
College of Animal Science and Technology, Anhui Agricultural University, West Changjiang Road 130, Shushan District, Hefei, Anhui 230036
E-mail: cyliu@anau.edu.cn
| Date of Received | 10 August 2020 |
| Date of Revised | 05 February 2021 |
| Date of Acceptance | 20 March 2021 |
| Indian J Pharm Sci 2021;83(2):269-277 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The experiment was conducted to investigate the protective effect of Smilax glabra Roxb. aqueous extract on carbon tetrachloride induced acute liver injury in rats by regulating the nucleotide binding leucine rich repeat receptors family pyrin domain containing 3 inflammasome. In this study, a rat acute liver injury model was established by a single intraperitoneal injection of 50 % carbon tetrachloride oil solution (1 ml/kg body weight). A total of 50 rats were randomly divided into control group, model group and 3 Smilax glabra Roxb. aqueous extract intervention groups (0.75, 1.5 and 3 g/kg body weight/d). Smilax glabra Roxb. aqueous extract or distilled water was orally administered by gavage once daily for seven consecutive days. Then the blood and liver samples were collected. The results showed that in the 3 intervention Smilax glabra Roxb. aqueous extract groups, the levels of serum aspartate aminotransferase and Alanine aminotransferase, the content of malondialdehyde in rat liver tissues significantly reduced and superoxide dismutase, catalase and glutathione increased significantly, the liver lobule structures were repaired and the liver cells were aligned in an orderly manner, the expressions of nucleotide binding leucine rich repeat receptors family pyrin domain containing 3 inflammasome, apoptosis associated speck like protein containing and caspase-1 proteins, Interleukin 1 beta messenger ribonucleic acid was decreased. Smilax glabra Roxb. aqueous extract exerted a protective effect against carbon tetrachloride induced acute liver injury by inhibiting the activation of nucleotide binding leucine rich repeat receptors family pyrin domain containing 3 inflammasome, which suggest a promising avenue for the exploration of Smilax glabra Roxb. aqueous extract in improving acute liver injury.
Keywords
Smilax glabra Roxb, rat, acute liver injury, nucleotide binding leucine rich repeat receptors family pyrin domain containing 3 inflammasome.
Acute liver injury (ALI) is a disease caused by drug poisoning, viral infection, immune reaction, or vascular disorders resulting in acute abnormal liver function and hepatocyte death. The clinical manifestation is acute hepatic dysfunction [1]. The pathogenesis of acute hepatic injury includes the activation of the innate immune, release of pro inflammatory cytokines and the alteration of signal transduction pathways related to hepatic inflammation, necrosis and apoptosis [2]. Its main features include the release of inflammatory cytokines, the overload of oxidative stress, an increase in the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), hepatocyte apoptosis and coagulation [3]. ALI can subsequently develop into acute liver failure, which is characterized by rapidly progressing hepatic encephalopathy and multiple organ failure with poor prognosis and high mortality rate [4].
The treatment of liver injury is based on different basic causes, there are no remarkable and effective medicine to cure it in clinic [5] and most of them are chemical synthetic drugs such as vitamins, nucleoside analogues, liver protecting and enzyme inhibiting drugs and α-interferon [6]. These drugs have some disadvantages such as significant side effects, adverse reactions and high prices, which greatly limited their clinical application [7]. Accordingly, it is an urgent need for rational drug use in clinical to develop safe and effective anti-liver injury drugs [8]. Considering the complex pathogenesis of ALI, natural herbal medication with anti-inflammatory, antioxidation and immunoregulatory function is usually recommended in China. Natural herbal medication routinely contains complex ingredients and plays a role in the multiple factors of the pathogenesis [9,10], as well as has the unique advantage in treating liver diseases due to its minor side effects. According to recent literature, Chinese Herbal medicine (CHM) has been one of the main hot points of hepatoprotective and widely used in clinical therapy [11]. Lots of single herbals or extracts, the active components of CHM and herbal formulas have been reported to exert protective effects on liver injury. In particular, Chinese patent medicine Fuzheng Huayu tablet has been proven to have good drug safety and patient tolerance by US Phase II clinical trials [12-14], which brought new opportunities and laid a good foundation for the development of CHM.
Smilax glabra Roxb. (SGR), the dried rhizome of the liliaceous plant, is used as both medicine and food all the time in China. Since 1963, SGR has been listed in the Pharmacopoeia of the People’s Republic of China. According to the theory of traditional Chinese medicine, SGR is sweet, light, mild and non-toxic and is associated with stomach and liver meridians. “Compendium of Materia Medica” of Li Shizhen in the Ming dynasty indicated that SGR can strengthen the spleen, stomach, bones and muscles, eliminate wind damp, ease joint movement, cure spasm and ostealgia, malignant sores and swollen welling abscess and is an antidote to mercury and silver poisoning. SGR has been extensively used worldwide for its marked pharmacological activities for treating syphilitic poisoned sores, limb hypertonicity, morbid leukorrhea, eczema pruritus, strangury due to heat, carbuncle toxin, headache, diarrhoea, hepatitis, cholecystitis, stranguria, tumours, gout, mercury poisoning and many other human ailments in clinical [15,16]. In the previous study, we found that SGR not only effectively cured gentamicin induced kidney injury in rats [17], but also showed a powerful protective effect on carbon tetrachloride (CCl4) induced ALI by tentative experiments. However, the protective mechanism has not been revealed yet. In this study, a pathological model of CCl4 induced ALI in rats was established and Smilax glabra Roxb. aqueous extract (SGRAE) was used as a therapeutic drug. The histological changes of the liver were observed and the biochemical indexes of liver and the level of nucleotide binding leucine rich repeat receptors family pyrin domain containing 3 (NLRP3), apoptosis associated speck like protein containing (ASC) and caspase-1 protein in liver and messenger Ribonucleic acid (mRNA) expression levels of Interleukin 1 beta (IL-1β) were detected. Through inter group comparisons, we explored the protective effect of SGR on CCl4 induced ALI in rats and its related molecular mechanisms, which will provide an experimental basis for the clinical use of SGR in the control of liver injury diseases
Materials and Methods
Reagents
CCl4 was purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. (Beijing, China). Cell lysis buffer for western blotting and immunoprecipitation (IP) was purchased from Beyotime Biotechnology Co., Ltd. (Shanghai, China). Phenylmethanesulfonyl fluoride (PMSF) was purchased from Sigma Aldrich Co., Ltd. (St. Louis, USA). EasySee Western Marker was purchased from TransGen Biotech Co., Ltd. (Beijing, China). Pro light horseradish peroxidase (HRP) was purchased from Tiangen Biotech Co., Ltd. (Beijing, China). Bovine serum albumin or Fraction V (BSA-V) was purchased from Roche Co., Ltd. (Basilea, Switzerland). Ultrasensitive two step immunohistochemical detection reagent was purchased from ZSGB-Bio Co., Ltd. (Beijing, China). All antibodies were purchased from Cell Signaling Technology, Inc. (Lexington, KY, USA). Total RNA extraction reagent (RNAiso Plus) was purchased from Takara Bio Inc (Europe). HiScript Q Reverse Transcriptase SuperMix for quantitative polymerase chain reaction (qPCR) and AceQ qPCR Synergy Brands (SYBR) Green Master Mix was purchased from Vazyme Biotech Co., Ltd (Nanjing, China). Primers were synthesized by Sangon Biotech Co., Ltd., Shanghai, China. All detection kits were purchased from Jiancheng Bioengineering Institute production, Nanjing, Jiangsu Province, China. Tris (hydroxymethyl) aminomethane (THAM) hydrochloride (Tris-HCl), Polysorbate 20 (Tween 20) and other reagents were purchased from Solarbio Life Sciences Co., Ltd. (Beijing, China).
Animals
In total, 55 w old male Sprague Dawley (SD) rats (weight, 180-200 g) were obtained from Aier Matt Technology Co., Jiangsu Province, China (animal license: SCXK (Su), 2014-0007). The animals were maintained in standard housing facilities (temperature: 24±1º, humidity: 45±5 % and a 12 h light/dark cycle) and fed a standard laboratory diet, with ad libitum access to water. All animals were given a week to acclimatize before the experiment. All of the animals involved in this protocol were bred and managed in strict accordance with the Chinese regulations on the use and breeding of experimental animals and the guidelines developed by the Experimental Animal Research Institute of Anhui Agricultural University. This research was approved by the Animal Feeding and Use Committee (approval no. AHAU2018028) of Anhui Agricultural University.
Preparation and quality evaluation of SGRAE
SGR was purchased from Hefei Lejia Herbal Pieces Co. Ltd. (Anhui, China) and authenticated/identified by Prof. Weijing Shi, which geographical origin was Guangxi Province of China and deposited at the herbarium of Hefei Lejia Herbal Pieces Co. Ltd. (HFLJHP) (Voucher No. 20181208), Hefei, Anhui, P. R. China. After super micro grinding, the powder of SGR was extracted twice using an SJC-II-10L Ultrasonic extraction apparatus (Wuxi Shangjia Biological Technology Co., Ltd., China) and aqueous extract of SGR (SGRAE) were obtained by filtering. Then, the filtrate was concentrated to desired concentrations by RE-52CS rotary evaporation (Shanghai Yarong Biochemical Instrument Factory, China) and stored at 4º after sterilization. For more details on the method and result of HPLC analysis of SGRAE, please see the reference [17].
CCl4 induced ALI rat model
A total of 50 SD rats were randomly divided into 5 groups (10 rats in each group) after a week acclimatization: the control group, the model group and 3 SGRAE intervention groups (0.75, 1.5 and 3 g/kg body weight/d). Rats in 3 intervention groups were given different dosages of SGRAE and the rats in the model group and control group were given distilled water by intragastric administration for 7 d consecutively. 6 h after the last administration, rats in the intervention groups and model group were given 50 % CCl4 (1 ml/kg) by intraperitoneal injection and rats in the control group were given normal saline and then they were observed in a 24 h period for gross behavioural change and mortality. No mortality was noted. 24 h after the injection, the rats were anesthetized with an intraperitoneal injection of 1.0 % pentobarbital sodium (40 mg/body weight) and then weighed and the blood samples were taken. The liver samples were collected after euthanasia by cervical dislocation. Considering the toxicity of CCl4 when inhaled through respiratory tract or absorbed through skin, causing serious damage to heart, liver and kidney, we operated in a ventilated place and wore gloves, work clothes, masks and other protective facilities in the process of experimental operation and parameters were evaluated (loss of weight, possible changes in the social interactions.
Liver coefficient analysis
The liver tissues of the rats were weighed to calculate the kidney coefficient (liver coefficient=kidney weight/ body weight).
Serum analysis
After fasting for 12 h, 3-5 ml blood was drawn from each rat’s caudal vein. Samples were centrifuged at 2500 rpm for 5 min to separate the serum. Finally, serum levels of AST and ALT were detected using a Vet Test™ Automatic Biochemistry Analyzer (IDEXX Laboratories, Inc., USA).
Liver tissue biochemical analysis
A portion of the liver was taken and made into a 10 % tissue homogenate using a tissue homogenizer. It was then centrifuged and the supernatant was collected. Levels of superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA) and reduced glutathione (GSH) in the supernatant were detected by a spectrophotometer.
Histopathological examinations
The liver tissues of rats were fixed in 4 % buffered formalin solution. The tissues were subjected to standard alcohol xylol processes and embedded in paraffin. The samples were then cut into 5 μm sections using a LS-2055+Paraffin Semiautomatic Machine (Shenyang Longshou Electronic Instrument Co., China) and stained with hematoxylin eosin (H&E). Liver sections were examined by light microscopy using a CX21FS1 Biological Microscope (OLYMPUS Co., Japan) and assessed at 20x magnification by randomly selecting areas in the microscopic field at 10x in order to determine changes in degeneration and necrosis.
Western blot analysis
Total proteins were extracted after cell lysis for Western and IP with PMSF. The bicinchoninic acid assay (BCA) was used for quantification of proteins. Equal amounts of protein were loaded onto a 12 % sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) gel and subjected to electrophoresis. The proteins were transferred onto a nitrocellulose (NC) membrane and blocked with 5 % skim milk. Primary antibodies against anti-NLRP3, ASC, caspase-1 and α/β Tubulin were used. The membrane was incubated overnight at 4º and then washed with Tris buffered saline with 0.1 % Tween® 20 detergent (TBST), followed by incubation with peroxidase labeled secondary antibody. Protein visualization was achieved using enhanced chemiluminescence Western blot reagents and a Versa Doc™ 4000 MP multi spectral imaging system (Bio Red Co., USA).
Immunohistochemistry analysis:
Before immunostaining, 5 μm thick liver tissue sections were dewaxed in xylene, rehydrated through decreasing concentrations of ethanol and washed in phosphate buffered saline (PBS). Tissue sections were blocked with BSA after antigen retrieval. Tissue sections were then incubated with primary antibodies against anti- NLRP3, ASC, caspase-1 followed by peroxidase labeled secondary antibody. After treatment with 3,3’-diaminobenzidine (DAB) condensed chromogen and staining with hematoxylin, tissue sections were examined under light microscopy.
Real time quantitative polymerase chain reaction (RT-qPCR) analysis:
Liver tissues were stored at -80º. Liver samples were ground with liquid nitrogen and total liver RNA was extracted with RNAiso Plus and then reverse transcribed to Complementary DNA (cDNA) according to the specifications. Samples were then amplified using qPCR according to the instructions of the AceQ qPCR SYBR Green Master Mix. The PCR conditions were as follows: 95º for 10 min, 40 cycles of 95º for 15 s and 60º for 1 min and 95º for 15 s. Oligonucleotide sequences of PCR are listed in Table 1.
| Gene | Sequence(5’-3’) | Product length |
|---|---|---|
| β-actin | F: CCCATCTATGAGGGTTACGC R: TTTAATTGTCACGCACGATTTC |
150 bp |
| IL-1β | F: GCCTGACAGACCCCAAAAGA R: CTCCACGGGCAAGACATAGG |
177 bp |
Table 1: Oligonucleotide Sequence of Quantitative Rt-Q Pcr
Statistical analysis:
Data were analyzed by conducting analysis of variance (ANOVA) and Dunnett’s test using SPSS (v 19.0; IBM, USA). Results of all groups are shown as mean±standard deviation (SD). Association between two groups was determined by Dunnett’s multiple comparison tests. p<0.05 was considered statistically significant.
Results and Discussion
Organ coefficient, also known as visceral body ratio, is the ratio of the weight of an organ to its body weight. In normal condition, the ratio of organs to body weight was relatively constant. After being poisoned, the weight of damaged organs can be changed, so the organ coefficient will also change. Organ coefficient increased, indicating organ congestion, edema or hypertrophy, etc. In this study, rat liver coefficient in the model groups was significantly higher when compared with the control group (p<0.05), the coefficient in the 3 SGRAE intervention groups was significantly lower than that in the model group (p<0.05) and there was no significant difference between the high dose SGRAE group and the control group (p>0.05), as shown in Table 2.
| Group | Liver coefficient |
|---|---|
| Control group | 0.307±0.035a |
| Model group | 0.429±0.055b |
| Low dose SGRAE group | 0.379±0.030c |
| Middle dose SGRAE group | 0.371±0.027c |
| High dose SGRAE group | 0.307±0.024a |
Values are expressed as mean±SD for ten rats in each group. The same letter in the same line indicates no significant difference (p>0.05) and different letters indicate significant difference (p<0.05)
Table 2: Liver Coefficient Of Rats in Different Groups (N=10)
| Group | AST (U/L) | ALT (U/L) |
|---|---|---|
| Control group | 23.859±1.234a | 26.738±1.044a |
| Model group | 56.197±1.111b | 77.659±2.297b |
| Low dose SGRAE group | 39.093±1.922c | 54.142±1.594c |
| Middle dose SGRAE group | 34.718±1.461d | 38.505±1.349d |
| High dose SGRAE group | 24.791±1.257a | 27.437±2.303a |
Values are expressed as mean±SD for ten rats in each group. The same letter in the same line indicates no significant difference (p>0.05) and different letters indicate significant difference (p<0.05)
Table 3: Serum Levels of Ast And Alt in Rats of Different Groups (N=10)
Serum aminotransferases ALT and AST are the most effective indicators for early liver injury [18] and 1 % liver cell necrosis will double the ALT level [19]. When hepatocytes are damaged, ALT and AST are released in large amounts into the blood and serum ALT and AST levels are increased. In our current study, the levels of serum ALT and AST in the model group were significantly increased, indicating that CCl4 caused severe liver damage in rats. Compared with the model group, serum AST and ALT levels of the 3 SGRAE intervention groups were significantly lowered (p<0.05) and no significant difference was showed (p>0.05) between the high dose SGRAE group and the control group, as shown in Table 3. This indicated that SGR can alleviate CCl4 induced ALI to a certain extent, consistent with the histopathological results.
SOD and GSH are the important antioxidant enzymes in the body, which can effectively eliminate free radicals and inhibit free radical induced lipid peroxidation [20]. MDA is the final metabolite of lipid peroxidation and its level reflects the degree of oxidative stress caused by toxophores [21]. Our study team found that SGRAE can protect gentamicin induced renal injury by regulating the mechanism of apoptosis induced by oxidative stress activated caspase-3 protein [17]. In the current research, compared to the model group, SOD, CAT and GSH of the 3 SGRAE intervention groups increased significantly (p<0.05) and MDA decreased significantly (p<0.05) in liver tissues. None of the oxidative stress indexes of liver in the high dose SGRAE group showed significant difference when compared with the control group (p>0.05), as shown in Table 4, which is consistent with previous findings [19,22]. The results suggested that SGR ameliorated CCl4 induced liver injury by increasing SOD and GSH levels and reducing MDA and the hepatoprotective effects of SGR may be related to antioxidative stress.
| Group | SOD (U/mg Pro) | CAT (U/mg Pro) | MDA (nmol/mg Pro) | GSH (mg/g Pro) |
|---|---|---|---|---|
| Control group | 141.894±16.947a | 16.861±1.07 a | 0.721±0.130a | 20.993±1.170a |
| Model group | 55.901±8.100b | 6.772±0.973b | 3.416±0.459b | 7.718±1.318b |
| Low dose SGRAE group | 88.676±10.949c | 9.156±1.232c | 2.243±0.330c | 12.254±1.224c |
| Middle dose SGRAE group | 127.321±10.395d | 11.959±1.215d | 1.322±0.333d | 14.890±1.490d |
| High dose SGRAE group | 161.292±10.426a | 16.867±1.327a | 0.796±0.111a | 20.094±0.889a |
Values are expressed as mean±SD for ten rats in each group. The same letter in the same line indicates no significant difference (p>0.05) and different letters indicate significant difference (p<0.05)
Table 4: Levels of Liver Oxidative Stress Indexes in Rats of Different Groups (N=10)
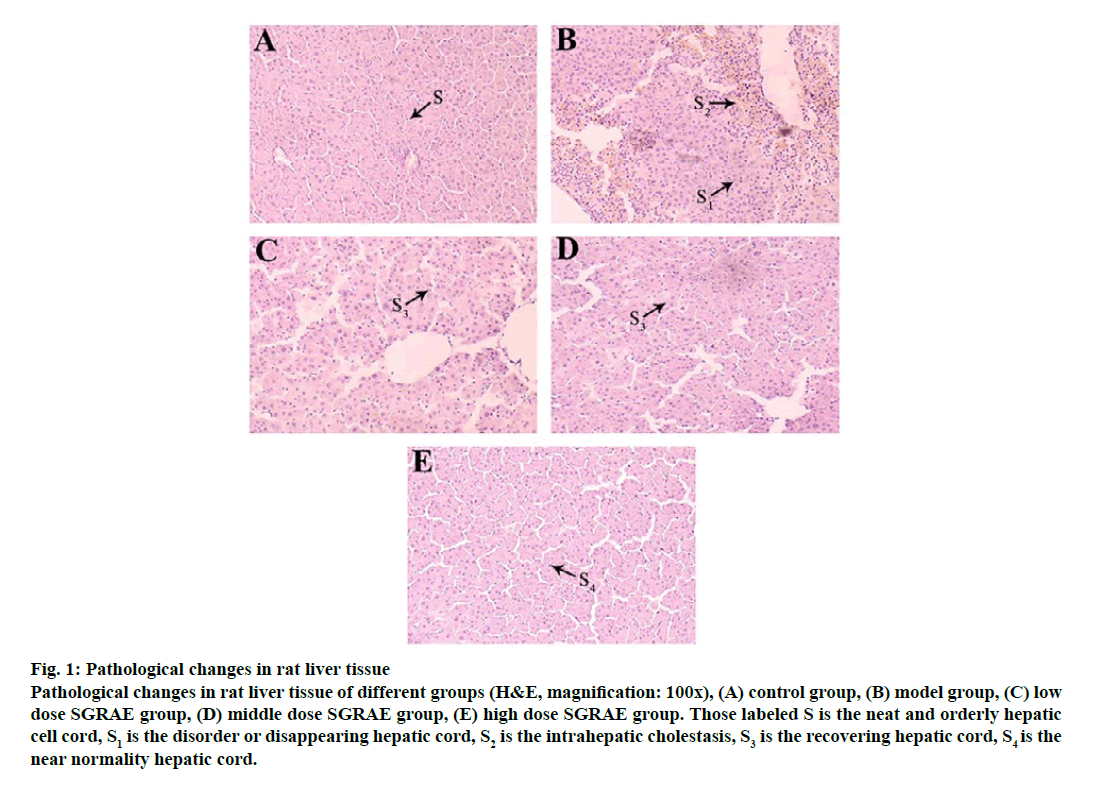
Pathological changes in liver tissue were observed by H&E staining. In the control group, the structure of rat hepatic lobules was complete and the liver cells arranged regularly, as shown in fig. 1A. Obvious liver structure of rats in model group, hepatic lobule structure was not clear, liver cells arranged in disorder and showed intrahepatic cholestasis, as shown in fig. 1B. These findings are consistent with the previous findings [20]. In the 3 SGRAE intervention groups, the normal liver lobule structures began to recover and the liver cells were aligned in an orderly manner. Cell morphology in the high dose SGRAE group was similar to the control group as shown in fig. 1C, 1D and 1E.
Figure 1: Pathological changes in rat liver tissue
Pathological changes in rat liver tissue of different groups (H&E, magnification: 100x), (A) control group, (B) model group, (C) low dose SGRAE group, (D) middle dose SGRAE group, (E) high dose SGRAE group. Those labeled S is the neat and orderly hepatic cell cord, S1 is the disorder or disappearing hepatic cord, S2 is the intrahepatic cholestasis, S3 is the recovering hepatic cord, S4 is the near normality hepatic cord.
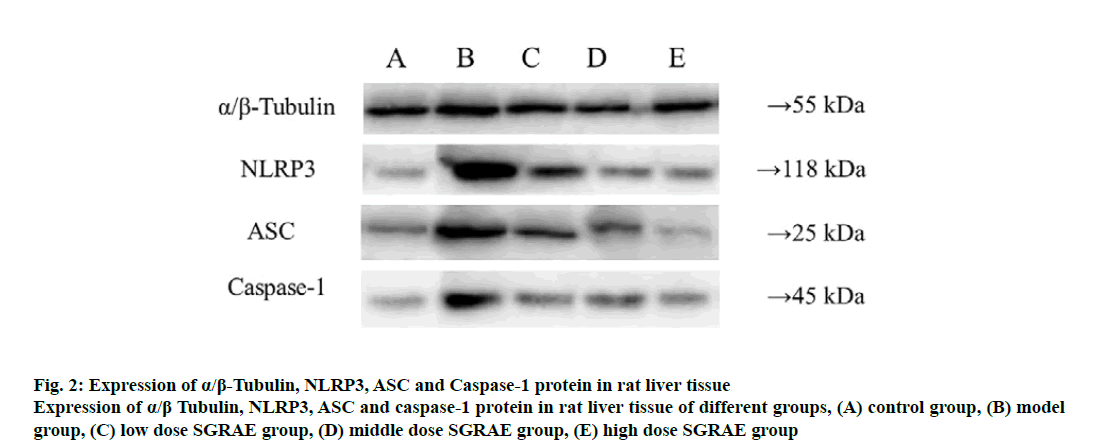
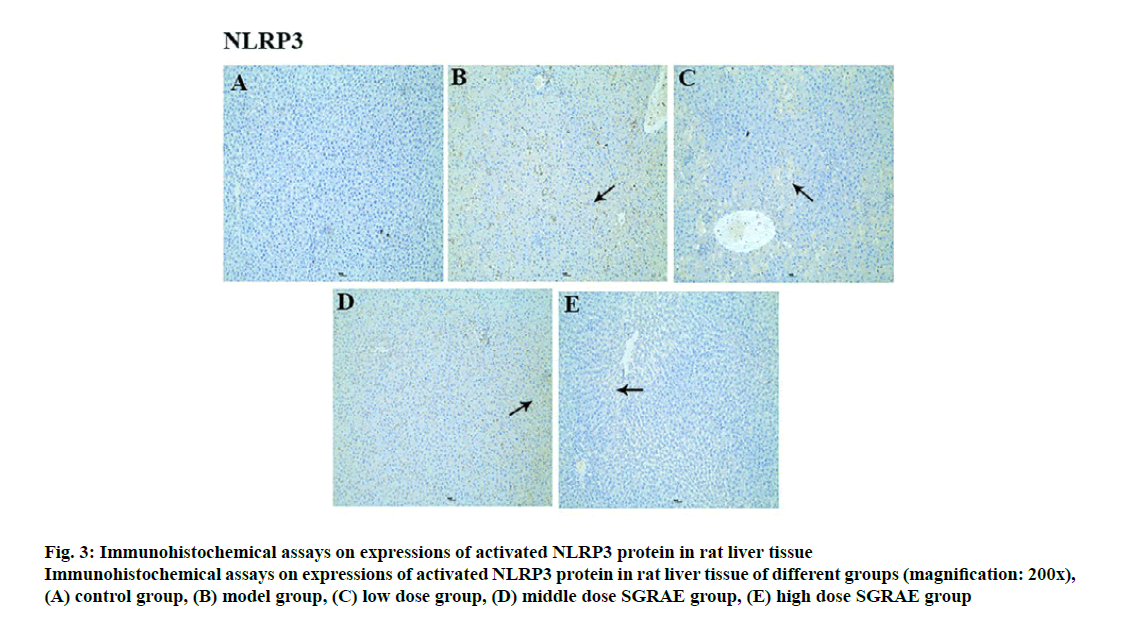
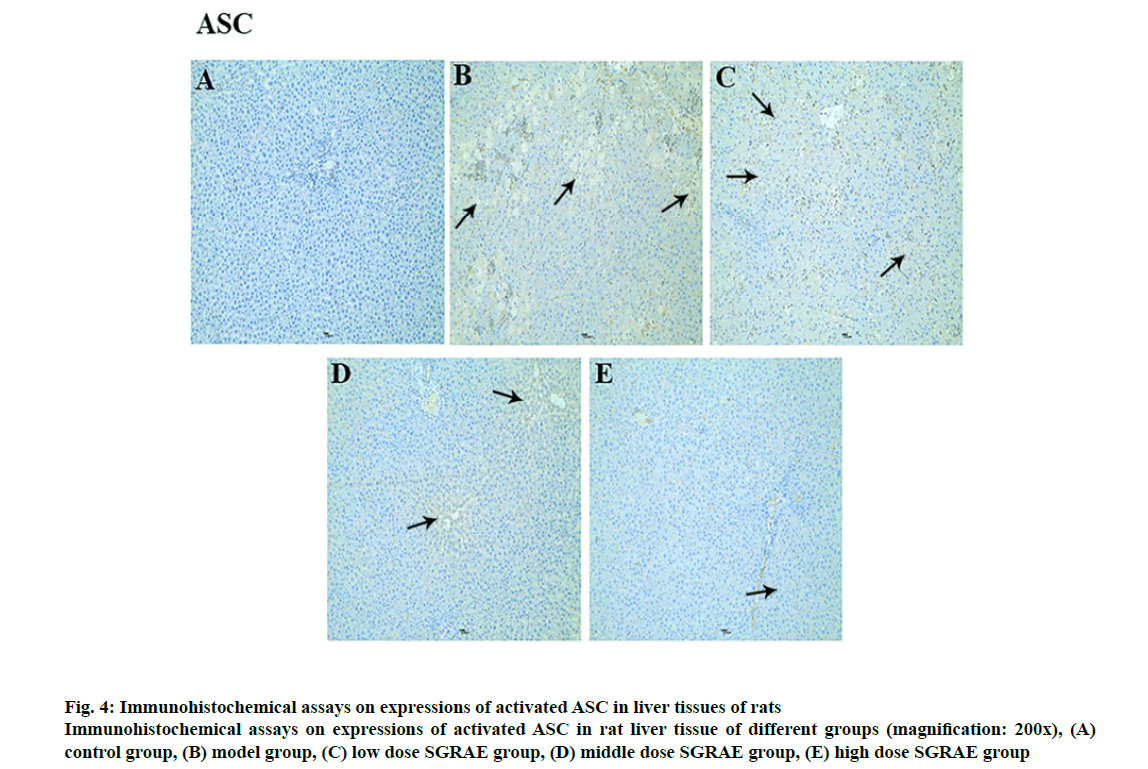
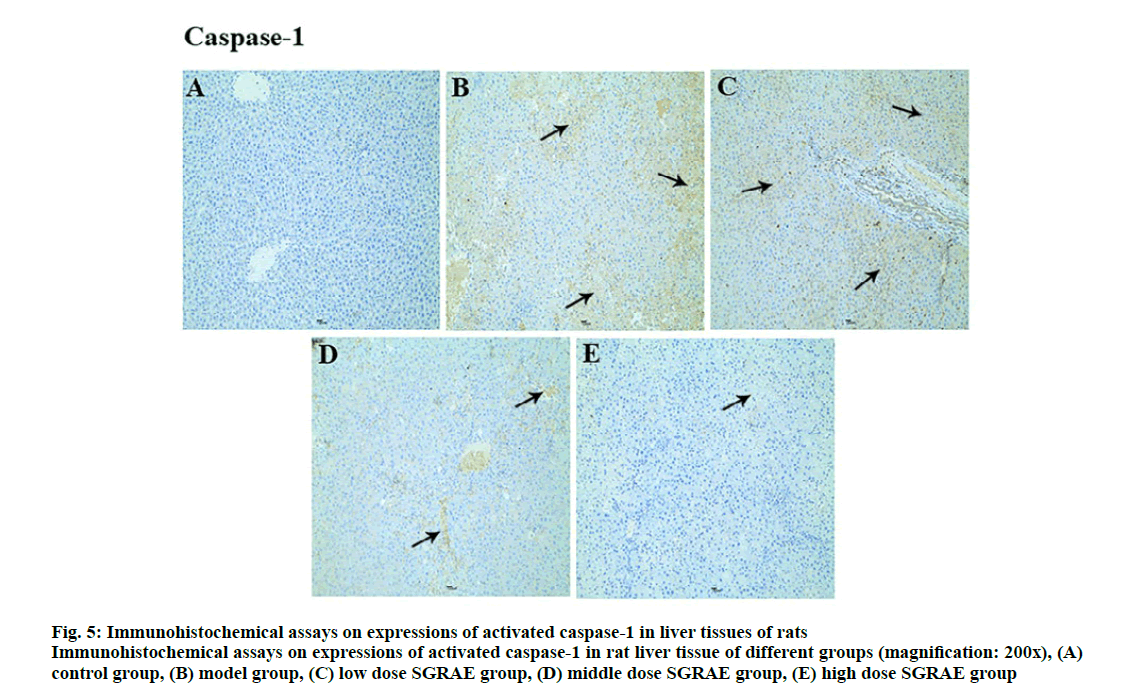
Studies in recent years have shown that the inflammasome plays a huge role in the onset and development processes of a variety of liver diseases [23-25]. The inflammasome is a poly protein complex present in the cytoplasm and is one of the important components of innate immunity. The inflammasome can recognize the molecular patterns of different pathogens and cause the release of various cytokines and inflammatory mediators through signal transduction and cell activation, ultimately triggering an inflammatory response [26]. Currently, various inflammasomes, including NLRP1, NLRP3, NLRC4, absent in melanoma-2 (AIM2) and retinoic acid inducible gene I (RIG-Ia), have been identified [26]. The most important of these is the NLRP3 inflammasome, which is formed mainly through the aggregation of NLRP3, ASC and caspase-1 [27]. The NLRP3 inflammasome is expressed in a variety of cells in liver tissues and can be activated. Its activation requires the binding of the locally recurrent domain specific structure and the factor activated or released by the stressed cells, as well as the sequential opening of the protein structures to expose the pyrimidine (PYD) so that the ASC can bind. Through the PYD-PYD interaction, the inactive pro caspase-1 is recruited by ASC caspase recruitment domain (CARD) to enable self-cleavage by pro-caspase-1 to complete the selfactivation process. The activated caspase-1 cleaves the inflammatory factor substrates (e.g., pro-IL-1β) to facilitate inflammatory responses [28-30]. In this study, compared with the control group, protein expression of NLRP3, ASC and Caspase-1 were increased in the model group; compared with the model group, three proteins were lowered in the 3 SGRAE intervention groups, as shown in fig. 2. Results of immunohistochemical staining showed no evidence of NLRP3, ASC and Caspase-1 in the control group, as shown in fig. 3A, 4A and 5A. In the model group, NLRP3, ASC and Caspase-1 was seen in liver cells, as shown in fig. 3B, 4B and 5B, while in the 3 SGRAE intervention groups, NLRP3, ASC and Caspase-1 decreased and was rarely seen in the high dose SGRAE group, as shown in fig. 3C-3E, 4C-4E, 5C-5E.
Figure 2: Expression of α/β-Tubulin, NLRP3, ASC and Caspase-1 protein in rat liver tissue
Expression of α/β Tubulin, NLRP3, ASC and caspase-1 protein in rat liver tissue of different groups, (A) control group, (B) model group, (C) low dose SGRAE group, (D) middle dose SGRAE group, (E) high dose SGRAE group
Figure 3: Immunohistochemical assays on expressions of activated NLRP3 protein in rat liver tissue
Immunohistochemical assays on expressions of activated NLRP3 protein in rat liver tissue of different groups (magnification: 200x), (A) control group, (B) model group, (C) low dose group, (D) middle dose SGRAE group, (E) high dose SGRAE group
Figure 4: Immunohistochemical assays on expressions of activated ASC in liver tissues of rats
Immunohistochemical assays on expressions of activated ASC in rat liver tissue of different groups (magnification: 200x), (A) control group, (B) model group, (C) low dose SGRAE group, (D) middle dose SGRAE group, (E) high dose SGRAE group
Figure 5: Immunohistochemical assays on expressions of activated caspase-1 in liver tissues of rats
Immunohistochemical assays on expressions of activated caspase-1 in rat liver tissue of different groups (magnification: 200x), (A) control group, (B) model group, (C) low dose SGRAE group, (D) middle dose SGRAE group, (E) high dose SGRAE group
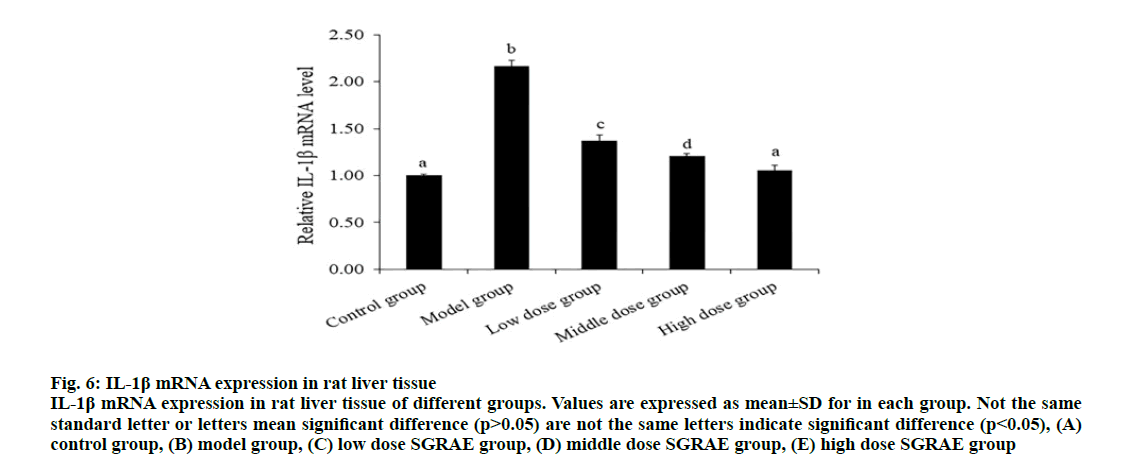
IL-1β is a pro inflammatory cytokine, which can widely participate in various pathological injuries such as tissue destruction and edema formation. As mentioned before, the aggregation of NLRP3, ASC and caspase-1 proteins forms the NLRP3 inflammasome and then induces maturation and release of IL-1β [27]. Studies have shown that SGR has anti-inflammatory effect [31]. SGRAE could inhibit Prostaglandin E2 (PGE2) synthesis and release, which significantly down regulates the functional activity of macrophages, inhibited IL-1β, Tumor necrosis factor alpha (TNF-α) and nitric oxide (NO) production [32]. In this study, the expression of IL- 1β mRNA in the model group was significantly higher than the control group and 3 SGRAE intervention groups (p<0.05) and there was no significant difference between the high dose SGRAE group and the control group (p>0.05), as shown in Table 1. The lower expression of IL-1β mRNA may be one of reasons for SGR inhibiting the activation of NLRP3 inflammatory in CCl4 induced ALI in rats (fig. 6).
Figure 6: IL-1β mRNA expression in rat liver tissue
IL-1β mRNA expression in rat liver tissue of different groups. Values are expressed as mean±SD for in each group. Not the same standard letter or letters mean significant difference (p>0.05) are not the same letters indicate significant difference (p<0.05), (A) control group, (B) model group, (C) low dose SGRAE group, (D) middle dose SGRAE group, (E) high dose SGRAE group
References
- Zhang CP, Zhang D. The mechanism of intrahepatic T lymphocytes in acute liver injury. Clin Exp Med 2017;16:2278-81.
- Shahidi F, Varatharajan V, Oh WY, Peng H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J Food Bioact 2019;5:57-119.
- Bernal W, Wendon J. Acute liver failure. N Engl J Med 2014;370(12):1170-1.
- Lu W, Pan M, Fang YK, Chen L, Ye F. Research progress on the mechanism of traditional Chinese medicine in treating acute liver injury. J Zhongnan Pharm 2019;17:1504-7.
- Yan LW, Li HL, Ji XN. Protective effects of Chinese traditional medicine against liver injury and liver fibrosis and mechanisms involved. World Chin J Dig 2016;24:4144-50.
- Chen M, Suzuki A, Thakkar S, Yu K, Hu C, Tong W. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016;21(4):648-53.
- Li N, Yuan HD, Li D, Zhao Q, Pu GC. Research progress on anti-liver injury mechanism of traditional Chinese medicine and its preparations. J Shandong Med 2014;54:103-5.
- Tan HY, San-Marina S, Wang N, Hong M, Li S, Li L, et al. Preclinical models for investigation of herbal medicines in liver diseases: Update and perspective. Evid Based Complement Alternat Med 2016:1-26.
- Du Guanhua WY, Ran Z, Chubing T, Xiaoli H, Juanjuan H, Li Z, et al. Multi-target and multi-component pattern, a superficial understanding of the action mechanism of Traditional Chinese Medicine [J]. World J Sci Technol 2009;4:480-4.
- Zhao X, Li Q, Bian L, Zheng X, Zheng J, Zhang Y, et al. Using immobilized G-protein coupled receptors to screen bioactive traditional Chinese medicine compounds with multiple targets. J Pharm Biomed Anal 2012;70:549-52.
- Ali M, Khan T, Fatima K, Ali QU, Ovais M, Khalil AT, et al. Selected hepatoprotective herbal medicines: Evidence from ethnomedicinal applications, animal models and possible mechanism of actions. Phytother Res 2018;32(2):199-215.
- Lv XM, Ma LJ. Advances in Chinese medicine treatment of acute hepatic injury. Chin J New Drugs2016;2:170-4.
- Wu YL, Lian LH, Nan JX. Protective effects of Chinese traditional medicine against liver injury and liver fibrosis and mechanisms involved. World Chin J Dig 2016;24(30):4144-50.
- Liu L, Tao X, Han X, Lina XU. Research Progress in the Inhibitory Effect of Traditional Chinese Medicine on Carbon Tetrachloride Induced Acute Liver Injury. Chin Pharm 2017;20(9):1638-42.
- Pu SH. Smilax glabra Roxb: a legendary healing medicine. Cent South Pharm2016;5:90-91.
- Hua S, Zhang Y, Liu J, Dong L, Huang J, Lin D, et al. Ethnomedicine, phytochemistry and pharmacology of Smilax glabra: An important traditional Chinese medicine. Am J Chin Med 2018;46(2):261-97.
- Liu C, Kang Y, Zhou X, Yang Z, Gu J, Han C. Rhizoma smilacis glabrae protects rats with gentamicin-induced kidney injury from oxidative stress-induced apoptosis by inhibiting caspase-3 activation. J Ethnopharmacol 2017;198:122-30.
- Gan D, Ma L, Jiang C, Wang M, Zeng X. Medium optimization and potential hepatoprotective effect of mycelial polysaccharides from Pholiota dinghuensis Bi against carbon tetrachloride-induced acute liver injury in mice. Food Chem Toxicol 2012;50(8):2681-8.
- Lu Y, Hu D, Ma S, Zhao X, Wang S, Wei G, et al. Protective effect of wedelolactone against CCl4 induced acute liver injury in mice. Int Immunopharmacol 2016;34:44-52.
- Liu Q, Zhu M, Geng X, Wang H, Ng TB. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the edible mushroom Oudemansiella radicata. Molecules 2017;22(2):234.
- Dong YR, Cheng SJ, Qi GH, Yang ZP, Yin SY, Chen GT. Antimicrobial and antioxidant activities of Flammulina velutipes polysacchrides and polysacchride-iron (III) complex. Carbohydr Polym 2017;161:26-32.
- Liu Y, Zheng D, Su L, Wang Q, Li Y. Protective effect of polysaccharide from Agaricus bisporus in Tibet area of China against tetrachloride-induced acute liver injury in mice. Int J Biol Macromol 2018;118:1488-93.
- Wree A, Marra F. The inflammasome in liver disease. J Hepatol 2016;65(5):1055-6.
- Boaru SG, Borkham-Kamphorst E, Tihaa L, Haas U, Weiskirchen R. Expression analysis of inflammasomes in experimental models of inflammatory and fibrotic liver disease. J Inflamm 2012;9(1):1-6.
- Watanabe A, Sohail MA, Gomes DA, Hashmi A, Nagata J, Sutterwala FS, et al. Inflammasome-mediated regulation of hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 2009;296(6):1248-57.
- de Vasconcelos NM, Van Opdenbosch N, Lamkanfi M. Inflammasomes as polyvalent cell death platforms. Cell Mol Life Sci 2016;73(11):2335-47.
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 2002;10(2):417-26.
- Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol 2010;30(5):628-31.
- Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci 2014;1319(1):82-95.
- Haneklaus M, O’Neill LA, Coll RC. Modulatory mechanisms controlling the NLRP3 inflammasome in inflammation: recent developments. Curr Opin Immunol 2013;25(1):40-5.
- Xian W, Jinni W, Yong P, Zuliang H. Analgesic and anti-inflammatory activities of the Smilax glabra Roxb. leaves extract. J Youjiang Med Univ Natl 2015;2:46-61.
- Meng QF, Li YB. Immunosuppressive effect of Tufuling astilbin and its monomer, Yunnan. J Tradit Chin Med Mater Med 2014;10:94-5.