- *Corresponding Author:
- Reshmi Chembrammal
Cell and Molecular Biology Division, Department of Botany, University of Calicut, Thenhipalam, Kerala 673635, India
E-mail: reshmibalan824@gmail.com
| Date of Received | 08 September 2020 |
| Date of Revision | 09 June 2022 |
| Date of Acceptance | 05 April 2023 |
| Indian J Pharm Sci 2023;85(2):511-517 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Modern food habits in humans have increased the frequency of diseases especially colon cancer. The present findings suggest the potential efficacy of two endemic Strobilanthes species of Southern Western Ghats. The shoot extract of the plants showed high radical scavenging activity in hydroxyl scavenging assay at an half maximal inhibitory concentration of 375.24±2.03 and 375.24±2.03 μg/ml against gallic acid for Strobilanthes anamallaica and Strobilanthes virendrakumarana respectively. Their activity in the superoxidefree radical assay was moderate when compared with the standard. Among the two species, Strobilanthes virendrakumarana highly impacted colon cancer cells (DLD1) with an Lethal concentration 50 of 111.99±4.91 μg/ml. These concentrations do not affect the normal cell line L929 under consideration. Hence, the endemic plant of Southern-Western Ghats, viz., Strobilanthes virendrakumarana is a promising candidate against colon cancer cells.
Keywords
Strobilanthes anamallaica, Strobilanthes virendrakumarana, antioxidants, colon cancer, endemic, anticancer
In the living organism, oxygen is involved in an integrated series of oxidation-reduction and enzymatic processes. These are essential components of biological systems[1]. The problem arises in uncoupled electron flow which eventually produces free radicals. Oxygen-centered free radicals are called Reactive Oxygen Species (ROS). They are essential for maintaining a balance between oxidative stress and antioxidant protection[2]. They are considered as a necessary evil due to their benefit as well as detrimental effects.
Normal cell functioning requires ROS at its physiological concentrations. In many human cancers, there is an increase in ROS stress which eventually causes damage to normal cells. The detoxification of excess ROS is done by various cellular enzymatic and non-enzymatic mechanisms. The harmful effects caused by them may be balanced by antioxidants. Hence antioxidants protect the biomolecules including lipids, carbohydrates, nucleic acids and proteins. From the word itself ‘antioxidants’ convey the functional duty as a bodyguard to the oxidative stress caused by the oxidants. The cumulative effect caused by ROS or free radicals can be scavenged using the potential activity of antioxidants. Plants are good source for the same and the less negative effect makes them more reliable by the modern man.
Strobilanthes anamallaica (S. anamallaica ) is a shrub with 1-2 m height having slender branches. The plant is endemic to Southern Western Ghats, common along the margins of evergreen forests at 1500-2000 m above sea level. The flowering periodicity is about 8 y. Strobilanthes virendrakumarana (S. virendrakumarana) is a large shrub member in the genus Strobilanthes. It is very common in semi-evergreen and moist deciduous forests at low elevations but endemic to Kerala. The vernacular name in Malayalam is ‘chorukurinji’ as the shape and color of the flower bud is similar to that of cooked rice. Morphologically it can be identified easily by the yellow-red glands on the lower surface of leaves[3]. The flowering periodicity of the plant is about 10 y. These two South Western Ghat endemic species of Strobilanthes were screened for their protective efficacy against free radicals by various methods in parallel and anti-inflammatory properties against human colon cancer cells.
The screening of plant and plant-derived compounds requires proper methods and need to focus on the kinetics of their reaction. Each antioxidant assay has its chemical principle to trap the free radicals and convert them into visible changes in the assay system. The present study thus focuses on the protective efficacy of the plant’s shoot extracts as well as the antiproliferative efficacy through cell line studies.
Materials and Methods
Collection and extraction of material:
S. anamallaica J. R. I. Wood was collected from Nelliyampathy hills of Palakkad district. S. virendrakumarana Venu and P. Daniel was from Bhothathankettu of Ernakulam district. After proper taxonomic identification, a voucher specimen was prepared and deposited in the herbarium of Calicut University, Calicut (CALI-123781, CALI-123780). The collected shoot system of the plants was cleaned, shade dried and made into small pieces for easy grinding. The powdered samples were subjected to extraction in methanol using the Soxhlet apparatus. The shoot extract was filtered and concentrated. Different concentrations of the extract such as 125, 250 500, 1000, 2000 µg/ml were prepared for all the assays. The percentage of inhibition was calculated using the formula.
Inhibition (%)=Ic-Is/Ic×100
Where, Ic and Is is the inhibition percentage of control and sample respectively. Each shoot extract was tested in triplicates.
Hydroxyl radical scavenging assay:
Different concentrations of the shoot extract and standard were prepared. Gallic acid (10 mg/ml) is taken as the standard in this assay. The reaction mixture consists of 2.8 mM of 2 deoxy 2 ribose, 100 µM of FeCl3, Ethylenediamine Tetraacetic acid (EDTA) (100 µM), 1 mM H2O2 and 100 µM of ascorbic acid in KH2PO4-KOH buffer (20 mM of pH 7.4)[4]. To the prepared concentrations of the extract and standard 50 µl of the reaction mixture was added. The final volume is made up to 1 ml. An equivalent amount of distilled water without a test sample was taken as control. It was followed by incubation at 37° for 1 h. After that 1 ml of trichloroacetic acid (2.8 %) and 1 ml of aqueous thiobarbituric acid (1 %) were added. It was then incubated for 15 min at 90°. It was allowed to cool. Absorbance was measured at 532 nm against an appropriate blank solution.
Super oxide free radical scavenging activity:
The assay was carried out using ascorbic acid (10 mg/ml) as the standard. Different concentrations of the shoot extract and standard were taken. The reaction mixture consists of 0.05 ml of riboflavin (0.12 mM), 0.2 ml of EDTA solution (0.1 M) and 0.1 ml of Nitro-Blue Tetrazolium (NBT) solution (1.5 mM). The reaction mixture was diluted up to 2.64 ml using phosphate buffer (0.067 M). Control without the test compound but an equal amount of distilled water was taken. It was incubated in fluorescent light for 5 min and absorbance was measured at 560 nm. The absorbance was also measured at the same wavelength after 30 min of illumination. The change in Optical Density (OD) was calculated[5].
Antiproliferative efficacy:
Cell seeding: The Human Colorectal Adenocarcinoma cells (DLD1 cells) and The normal cell line cell line (L929 cell line) were procured from National Centre for Cell Sciences (NCCS), Pune, India and maintained in Dulbecco's Modified Eagle Medium (DMEM) (Sigma-Aldrich, USA) medium. It was supplemented with 10 % Fetal Bovine Serum (FBS), L-glutamine, sodium bicarbonate (Merck, Germany) and an antibiotic solution containing: Penicillin (100 µg/ml), Streptomycin (100 µg/ml), and Amphoteracin B (2.5 µg/ml). 2 d old confluent monolayer of cells was trypsinized and the cells were suspended in a 10 % growth medium. The 100 µl cell suspension was seeded in a culture plate and incubated at 37° in a humidified 5 % CO2 incubator.
Extract preparation and anticancer evaluation:
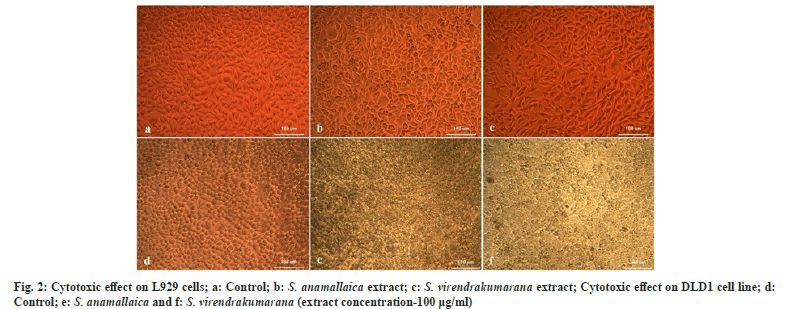
1 mg of the shoot extract was weighed and dissolved in 1 ml DMEM using a cyclomixer. The sample solution was filtered through a Millipore syringe filter to ensure sterility. Different concentrations of the shoot extract (100, 50, 25, 12.5, 6.25 µg in 500 µl of 5 % DMEM) were prepared. From this 100 µl of each concentration of the prepared extract was added in triplicates to the respective wells. In the same condition, untreated controls were also maintained. Microscopic observation was done after 24 h of treatment with the help of an inverted phase-contrast tissue culture microscope (Olympus CKX41 with Optika Pro5 CCD camera).
3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) (15 mg) was completely dissolved in a 3 ml phosphate buffer solution and sterilized by filter sterilization. After 24 h of the incubation period, the sample content in wells was removed and 30 µl of prepared MTT solution was added to all test and control wells. The plate was gently shaken well and incubated at 37° in a humidified 5 % CO2 incubator for 4 h. Then the supernatant was removed and 100 µl of MTT solubilization solution (Dimethyl Sulphoxide (DMSO) Sigma-Aldrich, USA) was added and mixed gently by pipetting up and down in order to solubilize the formazan crystals. The absorbance values were measured by using a microplate reader at a wavelength of 540 nm[6].The percentage of viability was calculated using the formula.
Viability (%)=Mean OD of samples/Mean OD of control×100
Apoptosis detection using double staining:
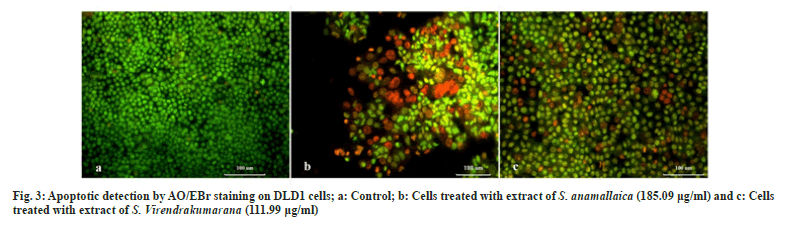
Acridine Orange (AO) and Ethidium Bromide (EtBr) are DNA binding fluorescent dyes (Sima-Aldrich, USA). They were used in the morphological detection of apoptotic and necrotic cells[7]. After proper culturing and seeding of DLD1 cell lines, Lethal Concentration 50 (LC50) of the two samples were added separately and kept for 24 h. The LC50 concentrations were 185.09 and 111.999 µg/ml of S. anamallaica and S. virendrakumarana respectively. It is followed by a wash in cold Phosphate Buffer Solution (PBS) and then stained with a mixture of AO (100 μg/ml) and EtBr (100 μg/ml) at room temperature for 10 min. The stained cells were washed twice with 1X PBS and observed in a blue filter of a fluorescent microscope (Olympus CKX41 with Optika Pro5 CCD camera).
Statistical analysis:
The experimental values were statistically analysed using SPSS 20 (SPSS Inc., Chicago, IL, USA) to determine the mean separation and significance of treatments. The resulted datas were subjected to one way Analysis of Variance (ANOVA) and Duncan’s multiple range tests for validation. The values were expressed in mean±Standard Error (SE). The differences between corresponding controls and treatments were considered statistically significant at p<0.05.
Results and Discussion
To counteract the adverse effect of oxidative stress body has several counter mechanisms. In animals, antioxidants are one among them which are produced in situ, or externally supplied through foods[8]. Plants can acclimate to oxidative stress by increasing the expression of genes involved in antioxidant systems. They exhibit both enzymatic and non-enzymatic metabolites to protect the cell from oxidative injury[9]. Several studies have suggested that differences in oxidative damage tolerance may be partially due to the higher constitutive antioxidant enzyme activities intolerant versus intolerant species[10,11]. Protective efficacy is a cumulative mechanism hence single assays cannot judge the potentiality of an extract.
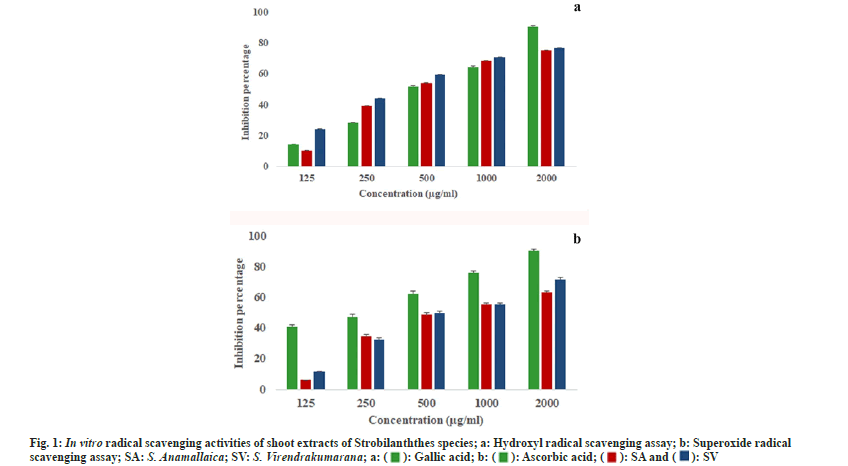
The hydroxyl radicals formed from the Fenton reaction degrade 2-deoxy-d-ribose in the reaction mixture. This degradation is determined photometrically after the addition of 2-thiobarbituric acid which eventually reacts and produces a pink color. The quenching of radicals in this assay was also dose dependent. In the least concentrations, S. virendrakumarana showed the highest percentage of inhibition (24.23 %) when compared with the other species. But at the highest concentration, both species showed almost an equal percentage of inhibition (fig. 1a).
The maximum percentage of inhibition was 76.72±0.41 µg/ml for 2000 µg/ml of S. virendrakumarana shoot extract. All the half maximal Inhibitory Concentration (IC50) values were within the third concentration selected (Table 1). When compared with the standards, the IC50 values were less for the plant shoot extracts. In 100 and 200 µg/ml concentrations, the inhibition percentage seems to be almost equal in both species. The IC50 value for S. virendrakuamarana was less than the standard (gallic acid) used. But it was higher in the case of S. anamallaica. It implies the extreme protective efficiency of the shoot extract to replace the highly proved synthetic antioxidants. The in vitro analysis of ethyl acetate and n-Butanol flower extracts of S. kunthianus proves that it is a promising free radical scavenger in antioxidantassays[12].
| Plant | IC50±SE (µg/ml) | |
|---|---|---|
| Hydroxyl radical scavenging activity | Super oxide free radical scavenging activity | |
| S. anamallaica | 432.82±2.03 | 754.58±2.05 |
| S. virendrakumarana | 375.24±2.03 | 749.47±2.04 |
| Standard | 477.49±2.36 | 283.48±2.62 |
Note: IC50: Concentration of the sample for 50 % inhibition, values are expressed in mean±standard error
Table 1: In Vitro antioxidant activities of standards and plant extracts.
Superoxide is biologically important as it can form singlet oxygen and hydroxyl radicals. Overproduction of superoxide anion radical contributes to redox imbalance and is associated with harmful physiological consequences. The standard used in superoxide free radicals scavenging activity was ascorbic acid. In this assay, the ribofalvin-Nicotinamide Adenine Dinucleotide (NADH) system will generate superoxide radicals by the oxidation of NADH. When the shoot extract is added to the reaction mixture, H+ ions are generated. These will reduce the NBT in the assay system producing a blue-colored formazan product. This blue formazan is quantified in respect of the potential antioxidant activity of the plant extract. The quenching of radicals in this assay was also dose dependent.
In the least concentration, S. virendrakumarana showed the highest percentage of inhibition. But when the concentrations of the shoot extracts increased, both the species exhibited tremendous increment in free radical capturing (fig. 1b). The IC50 values were high while comparing with the hydroxyl scavenging assay performed. Regarding the IC50 value, the standard has a higher estimate when compared with the plant extracts. Likewise, the maximum quenching in ethanolic extract of S. barbatus was reported as 70.45 % at a concentration of 2000 μg/ml against ascorbic acid but in hydroxyl radical scavenging it was only 57.12 % for the same[13]. Since these two species were analyzed for the first time, the high protective efficacy was in consistent with the previously reported species of the genus[14,15]. Strobilanthes has been suggested as a herbal alternative after proper analysis of antioxidants in it[16]. The herbal tea from dried leaves increased the defense system and reduced the blood glucose level[17,18].
The shoot extract concentrations were treated on one normal and one colon cancer cell line to assess the viable cells within the population. The LC50 values will determine the lethal activities of potential of phytocompound in an extract against cancer cell lines DLD1 (Table 2). The L929 cell lines were used for screening the effect of the extract on normal cells. It was found that the plant extract doesn’t affect the normal cells in any way. The cytotoxic cell lines reveal low LC50 values in DLD1 when compared with normal ones that indicate the potential phytocompounds in the shoot methanolic extract that can act against the cancer cells. The initial concentration does not affect the cells considerably in both L929 and DLD1. But the S. virendrakumarana extract at its 100 µg/ml concentration has tremendously squashed the cancer cells enormously (fig. 2).
| Plant | LC50±SE (µg/ml) | |
|---|---|---|
| DLD | L929 | |
| S. anamallaica | 185.09±2.89 | 264.03±4.91 |
| S. virendrakumarana | 111.99±4.91 | 189.79±2.32 |
Note: LC50: Concentration of the sample for 50 % lethality, values are expressed in mean±standard error
Table 2: Cytotoxic Potential of Strobilanthes species on DLD1 and L929 Cell Lines.
In L929 cells, the LC50 value was found to be 264.03±4.91 and 189.79±2.32 µg/ml for S. anamallaica and S. virendrakumarana respectively. In colon cancer cell lines, 50 % death was induced by a very low concentration of S. virendrakumarana shoot extract with a value of 111.99±4.91 µg/ml. In the previous studies, five different leaf extracts of S. crispus (hexane, chloroform, ethyl acetate, methanol, and aqueous) in CNE-1 cells, the ethyl acetate extract showed the strongest anti-proliferative effect on the cells with an LC50 value of 119.00±48.10 µg/ml[19]. The antiglycolytic activities of S. crispus fraction and its bioactive components on triple-negative breast cancer cells (MDA-MB-231) are attributed to the bioactivity of the plant[20]. The plant was cytotoxic against human liver cancer (Hep G2) and breast cancer (MCF-7) with an LC50 value of 0.3 and 24.8 µg/ml. Hence with the supporting evidences obtained from the literature clearly indicate the bioactivity of the Strobilanthes species under investigation.
The morphological visualization may act as the foundation for proving the efficacy. AO and EtBr are DNA binding fluorescent dyes (AO/EtBr). They were used in the morphological detection of apoptotic and necrotic cells. The evident observation of apoptosis by the LC50 concentration of the shoot methanolic extracts of Strobilanthes species has resulted from AO/EtBr staining[21]. The non-viable cells take up EtBr dye which is intercalated into DNA, when cells have altered the cell membranes and emit red fluorescence. But the viable cells take up the AO stain and emit green fluorescence when intercalated into DNA[22]. This gives a morphological differentiation of viable and non-viable cells that are resulted from the treatment of plant extract in colon cancer cells. The dead non-viable cells were seen in red and viable cells in green color (fig. 3).
The findings of Chong et al.[23] suggest that the leaf extract of Strobilanthes can induce apoptosis and DNA fragmentation on hormone-dependent cancer cell lines. Moreover, it is powerful in reducing hepatic necrosis in rats by inhibiting the enzymes involved in boosting carcinogens[24]. But its leaf extract was reported to be non-toxic to normal Chang liver cell line[25]. Likewise in colon cancer cells, S. virendrakumarana is having a very low concentration for 50 % damage and this concentration is not lethal for normal cell lines too. Hence the extract of S. virendrakumarana acts as a promising candidate for cancer treatments. The previous studies in the genus against cancer cells proved their efficacy in human liver cancer (Hep G2), breast cancer (MCF-7), triple-negative breast cancer cells (MDA-MB-231), etc. But it was not toxic against colon cancer cell Caco-2[26,27]. So, the present findings are in contradiction to the previous findings and prove to be useful in colon cancer studies.
Colon cancer which affects the large intestine is the most alarming dreadful disease among malignancies. Among the multiple factors leading to colon cancer, high-fat diets of modern food habits are a core reason[28]. The results of the current study can be used for further advancement in cancer treatment studies. In this study, while comparing the LC50 values of both endemic plants on normal and colon cancer cells, S. virendrakumarana seems to be a better candidate for further studies.
Acknowledgements:
The first author acknowledges the financial grant supported by the Council of Scientific and Industrial Research (CSIR) in the form of Senior Research Fellowship (09/043(0186)/2017-EMR-1). The authors are thankful for the taxonomic identification of the plant specimen by senior scientist Dr. K M Prabhukumar, Plant Diversity, Systematics and Herbarium, CSIR-NBRI, Lucknow and Dr. A. K. Pradeep, Assistant Professor, Department of Botany, University of Calicut. The first author’s gratitude towards Dr. Maya C Nair, Assistant Professor, Department of Botany, Govt. Victoria College and Mr. Amal MS, Mr. Rahul KP during the specimen collection are greatly mentioned.
Conflict of interest
All the authors declares that there is no conflict of interest.
References
- Gulcin İ. Antioxidants and antioxidant methods: An updated overview. Arch Toxicol 2020;94(3):651-715.
[Crossref] [Google Scholar] [PubMed]
- Öztaskın N, Taslimi P, Maraş A, Gülcin İ, Göksu S. Novel antioxidant bromophenols with acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase inhibitory actions. Bioorg Chem 2017;74:104-14.
[Crossref] [Google Scholar] [PubMed]
- Augustine J. Strobilanthes Bl. in the Western Ghats, India: The Magnificent role of nature in speciation. Malabar Natural History Society; 2018.
- Caillet S, Yu H, Lessard S, Lamoureux G, Ajdukovic D, Lacroix M. Fenton reaction applied for screening natural antioxidants. Food Chem 2007;100(2):542-52.
- Valentão P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, Bastos ML. Antioxidative properties of cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical, and hypochlorous acid. J Agric Food Chem 2002;50(17):4989-93.
[Crossref] [Google Scholar] [PubMed]
- Talarico LB, Zibetti RG, Faria PC, Scolaro LA, Duarte ME, Noseda MD, et al. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds Gymnogongrus griffithsiae and Cryptonemia crenulata. Int J Biol Macromol 2004;34(1-2):63-71.
[Crossref] [Google Scholar] [PubMed]
- Zhang JH, Yu J, Li WX, Cheng CP. Inhibited apoptosis in rat corpus luteal cells by flow cytometry and fluorochromes. Chin J Physiol 1998;41(2):121-6.
[Google Scholar] [PubMed]
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4(2):89-96.
[Google Scholar] [PubMed]
- Vranová E, Inzé D, Van Breusegem F. Signal transduction during oxidative stress. J Exp Bot 2002;53(372):1227-36.
[Crossref] [Google Scholar] [PubMed]
- Xu S, Li J, Zhang X, Wei H, Cui L. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot 2006;56(3):274-85.
- Zhao X, Nishimura Y, Fukumoto Y, Li J. Effect of high temperature on active oxygen species, senescence and photosynthetic properties in cucumber leaves. Environ Exp Bot 2011;70(2-3):212-6.
- Singh B, Das S, Maithi A. Antioxidant Property for lipophilic extract of Strobilanthes kunthiana flowers. Indian J Res Pharm Biotechnol 2014;2(1):1005.
- Subbulekshmi KO, Godwin SE, Vahab AA. Phytochemical and in vitro antioxidant activity of ethanolic extract of Strobilanthes barbatus nees leaves. Asian J Pharm Res Dev 2015;3(2):13-20.
- Sundaram V, Sadhasivam S, Chandrasekaran S, Nanjian R, Pandian A. Strobilanthes heyneanus root extract as a potential source for antioxidant and antimicrobial activity. Future J Pharm Sci 2021;7:1-2.
- Balasubramaniam G, Sekar M, Varadarajan M, Badami S. Antioxidant and hepatoprotective activities of Strobilanthes kunthianus against carbon tetrachloride-induced hepatotoxicity in rats. Pharmacogn J 2020-12(5):1143-1151.
- Prabakaran R, Kirutheka E. GCMS, phytochemicals and antioxidant activities of in vitro callus extracts of Strobilanthes kunthiana (Nees) T. Anderson ex Benth: An endemic plant of Acanthaceae. Brazil J Biol Sci 2018;5(10):359-72.
- Ismail M, Manickam E, Danial AM, Rahmat A, Yahaya A. Chemical composition and antioxidant activity of Strobilanthes crispus leaf extract. J Nutr Biochem 2000;11(11-12):536-42.
[Crossref] [Google Scholar] [PubMed]
- Fadzelly AM, Asmah R, Fauziah O. Effects of Strobilanthes crispus tea aqueous extracts on glucose and lipid profile in normal and streptozotocin-induced hyperglycemic rats. Plant Foods Hum Nutr 2006;61:6-11.
[Crossref] [Google Scholar] [PubMed]
- Koh RY, Sim YC, Toh HJ, Liam LK, Ong RS, Yew MY, et al. Cytotoxic and apoptogenic effects of Strobilanthes crispa Blume extracts on nasopharyngeal cancer cells. Mol Med Rep 2015;12(4):6293-9.
[Crossref] [Google Scholar] [PubMed]
- Muhammad SN, Yaacob NS, Safuwan NA, Fauzi AN. Antiglycolytic activities of Strobilanthes crispus active fraction and its bioactive components on triple-negative breast cancer cells in vitro. Anticancer Agents Med Chem 2022;22(7):1363-9.
[Crossref] [Google Scholar] [PubMed]
- Jambunathan S, Bangarusamy D, Padma PR, Sundaravadivelu S. Cytotoxic activity of the methanolic extract of leaves and rhizomes of Curcuma amada Roxb against breast cancer cell lines. Asian Pac J Trop Med 2014;7:S405-9.
[Crossref] [Google Scholar] [PubMed]
- Ciniglia C, Pinto G, Sansone C, Pollio A. Acridine orange/Ethidium bromide double staining test: A simple in vitro assay to detect apoptosis induced by phenolic compounds in plant cells. Allelopathy J. 2010;26(2):301-8.
- Chong HZ, Rahmat A, Yeap SK, Md Akim A, Alitheen NB, Othman F, Gwendoline-Ee CL. In vitro cytotoxicity of Strobilanthes crispus ethanol extract on hormone dependent human breast adenocarcinoma MCF-7 cell. BMC Complement Altern Med 2012;12:35.
[Crossref] [Google Scholar] [PubMed]
- Hanachi P, Fauziah O, Asmah R. Lesion scoring and p450 isoenzyme activityin liver of hepatocarcinogenesis rats treated with Strobilanthes crispus. Iran J Cancer Preve 2008;1(1):11-15.
- Rahmat A, Edrini S, Akim AM, Ismail P, Yap TY, Fadzelly AM. Anticarcinogenic properties of Strobilanthes crispus extracts and its compounds in vitro. Int J Cancer Res 2006;2(1):47-9.
- Muhammad SN, Yaacob NS, Safuwan NA, Fauzi AN. Antiglycolytic activities of Strobilanthes crispus active fraction and its bioactive components on triple-negative breast cancer cells in vitro. Anticancer Agents Med Chem 2022;22(7):1363-9.
[Crossref] [Google Scholar] [PubMed]
- Endrini S, Rahmat A, Ismail P, Taufiq-Yap YH. Comparing of the cytotoxicity properties and mechanism of Lawsonia inermis and Strobilanthes crispus extract against several cancer cell lines. J Med Sci 2007;7(7):1098-102.
- Aiello P, Sharghi M, Mansourkhani SM, Ardekan AP, Jouybari L, Daraei N, et al. Medicinal plants in the prevention and treatment of colon cancer. Oxid Med Cell Longev 2019;2019:2075614.
[Crossref] [Google Scholar] [PubMed]
 .
.