- *Corresponding Author:
- V. Soni
Department of Herbal Drug Research, B. R. Nahata College of Pharmacy and Contract Research Center, Mandsaur-458 001, India
E-mail: vishalpanacea@rediffmail.com
| Date of Submission | 04 May 2016 |
| Date of Revision | 07 April 2017 |
| Date of Acceptance | 16 November 2017 |
| Indian J Pharm Sci 2018;80(1):79-84 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Antifertility herbs are used to prevent conception or fertilization. This study is aimed to evaluate some antifertility herbs Bambusa arundinacea (leaf), Bauhinia racemosa Lam. and Ficus racemosa (bark) qualitatively and quantitatively in term of their phytoconstituents. The total phenolic content was determined by Folin-Ciocalteu reagent using gallic acid as a standard, total flavonoid content by aluminum chloride assay using quercetin as a standard, total tannin content by Folin-Denis' reagent and alkaloid content was determined as equivalents to atropine. Saponin content was determined in crude powder of drugs. The extracts were screened for presence of various phytoconstituents using preliminary chemical tests and TLC was performed for qualitative analysis. Among the four extracts, ethanol extract of B. arundinacea leaves showed maximum amount of phenolic content (2.24±0.34mg/g) and flavonoid content (4.65±0.74 mg/g) as compared to F. racemosa and B. racemosa bark extracts whereas total tannin content was found maximum in F. racemosa bark (8.96±1.54 mg/g). Preliminary phytochemical screening revealed the presence of alkaloids, glycosides, flavonoids, carbohydrates, tannins, phenols and saponins. Thin layer chromatographic studies of various extracts constituted different colored phytochemical compounds with different Rf values. This study gave an insight to the Aerva, the phytoconstituents present in the plant and useful for quantification of the compound in herbal formulation.
Keywords
Antifertility herbs, phenolics, flavonoids and phytochemical screening
Plants synthesize compounds with biological activity, namely antioxidant, as secondary products, which are mainly phenolic compounds serving in plant defense mechanisms to counteract reactive oxygen species (ROS) in order to avoid oxidative damage[1]. Many epidemiological studies have shown that the consumption of phenolics-rich foods is associated with the prevention of chronic diseases[2]. In addition to their antioxidant properties, these compounds have been reported to be potential candidates in lowering cardiovascular diseases[3], anticarcinogenic[4,5], antiallergenic, antiarthrogenic, antiinflammatory, antimicrobial and antithrombotic effects[6]. Plant phenolics, in particular phenolic acids, tannins and flavonoids are known to be antioxidants and occur in vegetables, fruits, nuts, seeds, roots and barks[7]. Fertility regulation with plant or plant preparation has been revealed in indigenous system of medicines of many countries. It is difficult to establish quality control parameters of plant-based drug due to complex nature and variability of chemical constituents[8]. So, modern analytical techniques should be implicated to overcome this problem. The aim of the present study was to evaluate few antifertility herbs qualitatively and quantitatively for estimation of phytoconstituents.
Ficus racemosa commonly known as Gular, Udambara belongs to the family Moraceae grows in evergreen forests, moist localities and bank of streams, deciduous forests, to the elevation of 1800 m above sea level. Well drained medium to heavy soil for successful cultivation. Propagation is done by stem and root suckers. It grows up to 18 m high[9].
Bambusa arundinacea (family: Graminae) is a highly reputed Ayurvedic medicinal tree commonly known as the Bamboo, distributed throughout the moist parts of India, up to an altitude of 1250 m particularly near river banks in Central and South India ascending up to 1100 m on the Nilgiris, also cultivated in many places in North-West India and Bengal. It also occurs in Sri Lanka, Malaya, Peru and Myanmar (Burma)[10]. Bamboo prefers loamy and sandy loamy soils. Best period for cultivation is end of the dry season, a few months prior to the start of the wet harvested on maturity during January-February. B. racemosa Lam. (the Sonpatta tree) is mostly present in low land and drier forest types of northwestern South America, extending to Brazil and Argentina[11]. B. racemosa is very common in foothills up to 1000 m in India and Sri Lanka. B. racemosa Lam is a small, crooked, bushy, deciduous tree with drooping branches, which can grow in poor and very harsh climatic conditions. The deciduous tree is propagated easily from seed[12].
Already several scientific papers have been published related to fertility control from medicinal plants, but still number of plants are yet to be screened for their efficacy[13]. In the area of female fertility regulation, the development of orally active antifertility agents has been the main area of focused research for the last six decades. According to the literature, flavonoids and saponins are known to exhibit antifertility activity[14]. Thus it is imperative that mankind turns toward alternative system of medicines. B. arundinacea, F. racemosa and B. racemosa elected on the basis of traditional claim for quantification of active component, which may be responsible for antifertility potential[15]. Tribal women around Salem in Tamilnadu chew leaves of B. arundinacea in the morning and evening for 1-3 d to induce abortion of an early conception. Pseudostem is crushed and mixed with jaggery and made into pills. Each pill is taken early in the morning for nine days after menstruation. Antifertility effect of an ethanol extract of B. arundinacea tender shoots (BASE) caused a reduction in fertility of male rats[16]. The bark of F. racemosa is traditionally used to induce threatened abortion and also recommended in urological disorders, diabetes, leprosy, asthma and piles. The bark extract of B. racemosa Lam. commonly known as Jhinjha is used by female of Rajasthan to develop sterility as contraceptive[17].
The present evaluation of various quantitative standards could help in standardizing the drug for various pharmacological activities, to ascertain its identity, to establish the quality and purity of this plant material in closely related species and to check adulteration.
Materials and Methods
Collection and authentication of plant material
Leaves of B. arundinacea, bark of F. racemosa and B. racemosa were collected from the garden, Jhalawar district, Rajasthan in the months of November, December and May, 2010, respectively. The plants were identified at the K.N.K College of Horticulture. Voucher specimens (BRNCP/B/08/2010, BRNCP/F/04/2010 and BRNCP/F/09/2010) were stored in the herbarium of Pharmacognosy Department, B. R. Nahata College of Pharmacy and Contract Research Center, Mandsaur, Madhya Pradesh, India.
Determination of loss on drying
Collected sample were spread in thin layer and allow drying in shade and bark were cut in to small piece and subjected to shade drying day in morning hours. About 10 g of the sample was weighed and placed in a tarred evaporating dish. It was dried at 105° for 5 h and at 1 h interval until difference two successive weighing corresponded to not more than 0.25 %[18].
Preparation of extract
Dried leaves of B. arundinacea were coarsely powdered. The powdered drug was extracted using ethanol for 72 h each in a Soxhlet apparatus. After that the extract was filtered and solvent was evaporated under reduced pressure until producing solid mass. The marc obtained was further extracted with 50 % ethanol for 72 h. The ethanol extracts were filtered and solvent was evaporated under reduced pressure to obtain solid masses. Percent yields of both extracts were calculated. The bark of F. racemosa and B. racemosa were dried, powdered using grinder and extracted in 50 % ethanol and methanol at 37° for 72 h, respectively. It was filtered and lyophilized. The yield of the lyophilized powder was calculated. The brownish yellow powder was kept in dark clean jar in room temperature for further experiments[19].
Qualitative chemical examination
Chemical tests for the screening and identification of bioactive chemical constituents like alkaloids, carbohydrates, glycosides, saponins, phenolic compounds, phytosterols, proteins, amino acids, flavonoids, and tannins, in the medicinal plants under study were carried out. The method of Harborne was adopted for the preliminary phytochemicals screening[20].
Determination of total phenolic content
The total phenolic content of extract was measured using Folin-Ciocalteu reagent. The extract was solubilized in distilled water. After that 100 μl of sample was mixed with Folin-Ciocalteu reagent (500 μl), sodium carbonates (400 μl) and distilled water (5 ml). This solution was kept at room temperature for 30 min, and the absorbance of solution was measured at 760 nm. Total phenolic content was concluded using gallic acid as standard[21].
Determination of total flavonoid content
The aqueous extract about 500 μl was mixed with ethanol (1.5 ml), aluminum nitrate (100 ml, 10 %), potassium acetate (100 ml, 1 M) and water (2.8 ml). The solution was kept at ambient temperature for 40 min, and measured the absorbance of solution using spectrophotometer. Total flavonoid content was recorded according to a standard established curve with quercetin[22].
Determination of crude saponin content
Add 20 g drug powder in a conical flask containing 100 ml of 20 % aqueous ethanol. The solution was heated for 4 h with constant stirring at 55°. Solution was filtered and marc was extracted with 200 ml 20 % ethanol. After that both extracts were mixed and solvent was evaporated till 40 ml volume of extract. The concentrate was extracted with 20 ml of diethyl ether in separating funnel. The aqueous layer was recovered while the ether layer was discarded. The aqueous extracts were purified by adding 60 ml n-butanol. Further it was washed with twice 10 ml of 5 % aqueous NaCl. The solution was dried and the saponin content was calculated as percentage[23].
Determination of tannin content
Stock solution of 1 mg/ml of tannin acid was prepared by dissolving 100 mg of accurately weighed tannic acid in water. About 1-10 ml aliquots were taken in clear test tube and 0.5 ml of Folin-Denis reagent, 1 ml of sodium carbonate solution were added to each test tube. Each tube was made upto 10 ml with distilled water. All the reagents in each tube were mixed well and kept undisturbed for about 30 min and read at 760 nm against blank reagent[24].
Determination of alkaloid
The plant extract (1 mg) was dissolved in dimethyl sulphoxide (DMSO), 1 ml of 2 N HCl was added and filtered. This solution was transferred to a separating funnel, 5 ml of bromocresol green solution and 5 ml of phosphate buffer were added. The mixture was shaken with 1, 2, 3 and 4 ml chloroform by vigorous shaking and collected in a 10-ml volumetric flask and diluted to the volume with chloroform. A set of reference standard solutions of atropine (20, 40, 60, 80 and 100 μg/ml) were prepared in the same manner as described earlier. The absorbance for test and standard solutions were determined against the reagent blank at 470 nm with an UV/Vis spectrophotometer. The total alkaloid content was expressed as mg of AE/g of extract[25].
Thin layer chromatography (TLC)
Each solvent extract was subjected to TLC as per conventional one-dimensional ascending method using silica gel 60F254, 7×6 cm (Merck) were cut with ordinary household scissors. Plate markings were made with soft pencil. Glass capillaries were used to spot the sample for TLC. Applied sample volume was 1-μl by using capillary at a distance of 1 cm at 5 tracks. In the twin trough chamber with different solvent systems; ethyl acetate:acetic acid (9:1), hexane:ethyl acetate:acetic acid (5:4:1), solvent system I and II, respectively. After pre-saturation with mobile phase, a 20-min development run was used. After the run, plates were dried and sprayed with freshly prepared iodine reagent to detect the bands. The movement of the active compound was expressed by its retention factor (Rf), values were calculated for different samples[26].
Results and Discussion
For the pharmacological study of novel drugs, the essential information regarding the chemical constituents is generally provided by the qualitative phytochemical screening of plant extracts. The qualitative tests of extracts showed significant indication about the presence of metabolites. Standardization is an essential measurement for ensuring the quality control of the herbal drugs[27]. Moisture contents and LOD were used to find out the amount of moisture including volatile contents of the tested drug. The hydrolysis of the active ingredients of drug may occur due to higher moisture content in the drug, which leads to poor quality and efficacy. The final processes of dryness and removal rate of moisture contents are of great importance. The secondary metabolites are compounds, which are responsible for therapeutic efficacy of the drugs. Tannins bind to proteins and inhibit the protein synthesis[24]. Therefore, the current preliminary phytochemicals screening might be proved valuable in the detection and further quantitative analysis of these therapeutically important compounds. The phenolic and flavonoid compounds are important antioxidants, which also include antimicrobial, antiallergic, antiinflammatory and anticancer agents. These secondary metabolites play a vital role in reproduction and growth. These compounds also provide protection against harmful pathogenic microbes and predators[2,3]. According to the literatures, flavonoids, phenolic and saponins are known to exhibit antifertility activity[14]. Therefore, quantitative analysis of such vital compounds is extremely significant to determine the quality of drugs. The total quantitative analysis of phenolic and flavonoid contents of some antifertility herbs was carried out by UV spectroscopic method.
The present investigation has been carried out to determine phytoconstituents present in various solvent extracts of B. arundinacea leaves, bark of F. racemosa and B. racemosa. The loss on drying was found maximum in B. arundinacea leaves (9.56±1.34) followed by bark of F. racemosa (7.34±0.64) and B. racemosa (6.78±1.24). Various extracts of sample were prepared by continuous hot percolation methods. B. racemosa B-M shows highest percent yield (9.5 % w/w) followed by F. racemosa B-HA (7.6 % w/w), B. arundinacea L-E (6.5 % w/w) and B. arundinacea L-HA (5.8 % w/w). The appearance and percent extract ability of all the extracts are shown in Table 1. The results of preliminary phytochemical screening revealed the presence of carbohydrates, tannins, glycosides, flavonoid, phenolics, saponins and volatile oils. The result of preliminary phytochemical screening of various extracts revealed the presence of various phytoconstituents, as shown in Table 2.
| Plant extracts | Parameters | ||
|---|---|---|---|
| Colour | Consistency | Yield (% w/w) | |
| Ba. L-E | Brown | Semisolid, sticky | 6.5 |
| Ba. L-HA | Dark brown | Semisolid | 5.8 |
| Fr. B-HA | Brown | Powder form | 7.6 |
| Br. B-M | Yellow brown | Semisolid | 9.5 |
Table 1: Percent Yield of Extracts
| Plant extracts | Phytoconstituents |
|---|---|
| Ba. L-E | Steroids, fats |
| Ba. L-HA | Alkaloid, glycoside, flavonoids, carbohydrates, tannins, phenolic, saponin |
| Fr.B-HA | Carbohydrates, alkaloids,proteins, anthraquinones, phenolic, saponin, flavonoids, tannins |
| Br. B-M | Carbohydrates, steroids, flavonoids, triterpenoids, tannins |
Table 2: Preliminary Phytochemical Studies of Various Extracts of Plants
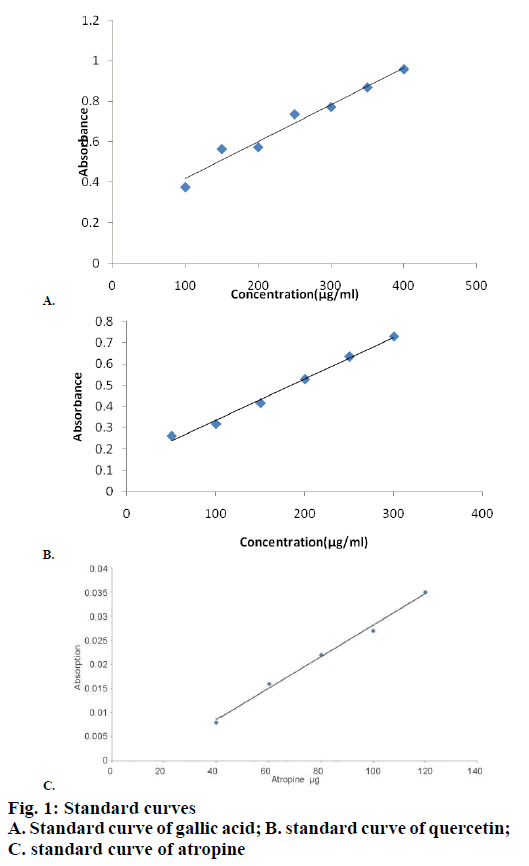
Total phenolic, flavonoid, tannins and alkaloid contents of all the extracts were determined by UV spectrophotometric method. The total phenolic and flavonoid contents were found maximum in B. arundinacea L-E 2.24±1.34 and 4.65±0.74 mg/100 g, respectively results shown in Table 3. The given values are mean±SD of three different determinations. B. racemosa bark and B. arundinacea leaves, shown in Table 3. Using the standard plot of quercetin (y=0.001+0.142, R2=0.992), the flavonoid contents of various extract of B. arundinacea leaves, bark of F. racemosa and B. racemosa were found ranging from 1.34 to 4.6 mg quercetin equivalent/g of dry sample. Phenolic compounds modulate the enzyme system due to their interaction with various biomolecules. They are essential for the growth and reproduction of plants, and are produced as a response for defending injured plant against pathogens. Using the standard plot of gallic acid (y=0.001+0.237, R2=0.968), the phenolic contents of various extract of B. arundinacea leaves, bark of F. racemosa and B. racemosa were found ranging from 0.66 to 2.24 mg quercetin equivalent/g of dry sample. The calibration plot for the determination of phenols and flavonoids, are shown in Figure 1A, B and C. Total saponin contents of F. racemosa bark was found to be more (2.42±0.24 % w/w), results presented in Table 4. The total tannin content of F. racemosa B-HA was found to be more (8.96±1.54 % w/w). The alkaloid content was examined in plant extracts and expressed in terms of atropine equivalent as mg of AE/g of extract (y=0.006x–0.003, R2=0.997). The highest concentration of alkaloid was measured (46.34±0.86) in B. arundinacea L-HA extracts (Table 5).
| Plant extracts | Total phenolic content (mg/100g) | Total flavonoid content (mg/100g) | Total tannins content (% w/w) |
|---|---|---|---|
| Ba. L-E | 2.24±0.34 | 4.65±0.74 | 2. 34±0.34 |
| Ba. L-HA | 1.45±0.55 | 4.22±0.64 | 4.34±0.76 |
| Fr. B-HA | 0.72±0.36 | 0.84±1.34 | 8.96±1.54 |
| Br. B-M | 0.66±2.12 | 1.34±1.25 | 7.55±0.84 |
Table 3: Total Phenolic, Flavonoid and Tannin Contents of Extracts
| Plant Material | Total saponin content (% w/w) |
|---|---|
| Ba. L | 1.14±0.54 |
| Fr. B | 2.42±0.24 |
| Br. B | 2.31±0.48 |
Table 4: Total Saponin Content of Extracts
| Plant extracts mg of AE/g |
Alkaloid content (%w/w) |
|---|---|
| Ba. L-E | 19.15±0.44 |
| Ba. L-HA | 46.34±0.86 |
| Fr.B-HA | 28.96±1.24 |
| Br. B-M | 31.53±0.64 |
Table 5: Alkaloid Contents in the Plant Extracts
A large number of solvent systems were tried to achieve a good resolution. Finally, the solvents hexane:ethyl acetate:acetic acid was used. Thin layer chromatographic studies the all extracts through solvent system I (hexane:acetic acid; 9:1), 3 spots were visible and Rf values were 0.26, 0.46 and 0.52, respectively. In solvent system II (hexane:ethyl acetate:acetic acid; 5:4:1), 1 spot was detected and the Rf value was 0.90.
TLC profiling of all three extracts gives an impressive result that directing towards the presence of number of phytochemicals. Various phytochemicals gives different Rf values in different solvent systems. The selection of appropriate solvent system for a particular plant extracts can only be achieved by analysing the Rf values of compounds in different solvent system. Different Rf values of the compound also reflect an idea about their polarity. This information will help in selection of appropriate solvent system for further separation of compound from these plant extracts.
The present investigation revealed that the B. arundinacea leaves, bark of B. racemosa and F. racemosa extracts contain significant amount of phenols, flavonoids, tannins and saponins. The outcome of these findings might be useful as a diagnostic tool for the evaluation of these antifertility herbs. Further deeper studies have to be carried out to isolate new natural molecules.
Acknowledgements
The authors thank the Director, B. R. Nahata College of Pharmacy, Mandsaur College of Pharmacy and Management for providing all necessary facilities to carry out this research work. The authors also thank Dr. S. N. Mishra, K. N. K. College of Horticulture for identifying and authenticating the plant.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Stankovic MS. Total phenolic content, flavonoid concentration and antioxidant activity of Marrubiumper egrinum L. extracts. Kragujevac J Sci 2011;33:63-72.
- Rice-Evans CA, Miller NJ, Paganga G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 1996; 20: 933-56.
- Gautam B, Vadivel V, Stuetz W, Biesalski HK. Bioactive compounds extracted from Indian wild legume seeds: antioxidant and type II diabetes related enzyme inhibition properties. Int J Food Sci Nutr 2011;15:1-4.
- Meghashri S, Kumar V, Gopal S. Antioxidant properties of a novel flavonoid from leaves of Leucas aspera. Food Chem 2010;122:105-10.
- Ahmad N, Fazal H, Abbasi BH, Rashid M, Mahmood T, Fatima N. Efficient regeneration and antioxidant potential in regenerated tissues of Piper nigrumL. Plant Cell Tissue Organ Cult 2010;102:129-34.
- McDonald S, Prenzler PD, Antolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem 2001;73:73-84.
- Buer CS, Imin N, Djordjevic MA. Flavonoids: new roles for old molecules. J Integr Plant Biol 2010;1:98-111.
- Kumar D, Kumar A, Prakash O. Potential antifertility agents from plants: A comprehensive review. J Ethnopharmacol 2012;140:1-32.
- Faiyaz A, Mysore RA, Asna U, Kodangala KB. Ficus racemosa bark: Nutrient composition, physicochemical properties and its utilization as nutra tea. Int J Nutr Metab 2010; 2:33-9.
- Maurya R, Srivastava S, Kulshreshta DK, Gupta CM. Traditional Remedies for Fertility Regulation. Curr Med Chem 2004;11:1431-50.
- Jain R, Yadav N, Bhagchandani T, Jain SC. A new pentacyclic phenol and other constituents from the root bark of B. racemosa Lamk. Nat Prod Res 2013;20:1870-76.
- El-Hossary GA, Selim MA, Sayed AE, Khaleel AE. Study of the flavonoid content of Bassia muricata and Bauhinia racemosa. Bull Fac Pharm Cairo Univ 2000;38:93-7.
- Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effect on mouse splenocyte proliferation. Food Chem 2007;101:140-7.
- Jain A, Kateva SS, Galav PK, Sharma P. Medicinal plant diversity of Sitamata wildlife santury, Rajasthan, India. J Ethanopharmacol 2005;102:143-57.
- Kiruba S, Jeeve S, Manohardas S, Kannan D. Bamboo seed as a mean to sustenance of the indigenous community. Indian J Tradit know 2007;6:199-203.
- Vanithakumari G, Manonayagi S, Padma S, Malini T. Antifertility effect of Bambusa arundinaceashoot extracts in male rats. J Ethnopharmacol 1989;25:173.
- Kumar RV, Venkatraji Reddy G, Krishna Reddy M. Medicinal plants having fertility related and pharmacological activities. Int J Pharm Med Bio Sci 2012:124-28.
- Scott PM. Natural poisons. In: Helrich K, editor. Official methods of analysis of the Association of Official Analytical Chemists. 15th ed. Arlington: AOAC; 1990. p.1184-213.
- Yadav SK, Deepika, Jalalpure SS, Saini P. Phytochemical screening of various extracts of stem bark of B. racemosaplant. Int J Pharma Res 2010; 2: 1-6.
- Khandelwal KR. Practical pharmacognosy, techniques and experiments. 17th ed. Pune: Nirali Prakashan Publishers; 2007, p. 149-56.
- Madaan R, Bansal G, Kumar S, Sharma A. Estimation of total phenols and flavonoids in extracts of Actaeaspicata roots and antioxidant activity studies. Indian J Pharm Sci 2011;73:666-9.
- Mohsen MS, AmmarSMA. Total phenolic contents and antioxidant activity of corn tassel extracts. Food Chem 2008;112:595-98.
- Eleazu CO, Eleazu KC, Awa E, Chukwuma SC. Comparative study of the phytochemical composition of the leaves of five Nigerian medicinal plants. J Biotechnol Pharm Res 2012;3:42-6.
- Polshettiwar SA, Ganjiwale RO, Wadher SJ, Yeole PG. Spectophotometric estimation of total tannins in some ayurvedic eye drops. Indian J Pharm Sci 2007;69:574-6.
- Tambe VD, Bhambar RS. Estimation of total Phenol, tannin, alkaloid and flavonoid in Hibiscus tiliaceusLinn. Wood Extracts. J Pharmacogn Phytochem 2014;2:41-4.
- Agrawal SS, Paridhavi M. Herbal drug Technology, 1st edn., Hyderabad, India: Universities Press: 2007. p. 491.
- Ahmad A, Husain A, Mujeeb M, Khan SA, Alhadrami HA , Bhandari A. Quantification of total phenol, flavonoid content and pharmacognostical evaluation including HPTLC fingerprinting for the standardization of Piper nigrum Linn fruits. Asian Pac J Trop Biomed 2015;5:101-07.