- *Corresponding Author:
- Dong Peng
Department Medical Imaging Center, Xian People’s Hospital (Xi’an Fourth Hospital), Xian 710004, China
E-mail: shenbzaxhiz53376@163.com
| Date of Received | 15 February 2023 |
| Date of Revision | 20 November 2023 |
| Date of Acceptance | 06 May 2024 |
| Indian J Pharm Sci 2024;86(3):991-999 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the radiobiological effects and related mechanisms of brain tissue after craniocerebral irradiation in young rats. Twenty-seven young male rats were randomly divided into high dose group (group C) (n=9), low dose group (group B) (n=9) and control group (group A) (n=9). The brains of rats in the group C were irradiated with 20Gy, while those in the group B were irradiated with 3Gy. The changes of body weight and organ index of rats in the three groups after craniocerebral irradiation were recorded. 1 w later, the rats were killed, the brain tissue was taken out, and the brain tissue sections were stained with hematoxylin and eosin. The migration ability was detected by cell scratch test, the levels of oxidative stress, inflammatory factors and apoptosis factors in brain tissue were observed, and the radiobiological effects and possible mechanism of brain injury were analyzed. At 3 d and 7 d after craniocerebral irradiation, the body weight of rats in the group B and group C was lower than that in the group A, and that in the group C was lower than that in the group B. Compared with the group A, the thymus and spleen organ index of the group B and the group C decreased and the decrease degree of the group C was greater than that of the group B. The normal nerve cells in the hippocampus of rats have large and round nucleoli, clear nucleoli and intact cell membrane. The pathological sections of the group B showed nerve cell injury, swelling, deformation, pale cytoplasm and tissue edema. The results of scratch test showed that the cell migration distance between the group B and the group C was larger than that in the group A at 24 h and 48 h, and the migration distance in the group C was larger than that in the group B. Compared with the group A, the serum superoxide dismutase activity decreased and the malondialdehyde content increased in the group B, while the superoxide dismutase activity and malondialdehyde content in the group C increased compared with the group B. Compared with the group A, the concentrations of tumour necrosis factor-alpha and interleukin-6 in the serum of the group B were increased, and these in the group C were higher than those of the group B. Compared with the group A, the apoptotic factors Bcl-2-associated X-protein and caspase-3 in the group B increased, while the content of B-cell lymphoma 2 decreased. Compared with the group B, the apoptotic factors Bcl-2-associated X-protein and caspase-3 increased and the content of B-cell lymphoma 2 decreased in the group C. Brain irradiation caused radiation-induced pathological damage to the brain tissue of young rats, resulting in weight loss and damage to immune organs in a dose-dependent manner. Its mechanism can be mediated by promoting inflammation, oxidative stress and apoptosis.
Keywords
Juvenile rat, brain irradiation, brain tissue, radiobiology, mechanism
With the wide application of radiotherapy in clinic, more and more attention has been paid to the radiobiological effects of brain[1]. However, there is a relative lack of research on the effects and related mechanisms of radiobiological effects on the brain tissue of young rats after craniocerebral irradiation[2]. As an important structure in the central nervous system, brain tissue plays an important role in motor coordination and balance control[3]. However, although the sensitivity of brain tissue to radiation has been widely recognized, the study
of radiobiological effects in brain tissue of young rats after craniocerebral irradiation is relatively limited. Therefore, it is necessary to understand these effects and their related mechanisms[4]. Young rats are widely used in radiobiological research because of their high sensitivity to radiation at the stage of neurodevelopment, and radiobiological effects can be evaluated by monitoring behavior and nerve growth factor expression in adulthood[5]. Therefore, this study aimed to explore the radiobiological effects and related mechanisms of brain tissue after craniocerebral irradiation in young rats. In this study, we will explore the effects of radiation on the cellular and molecular levels of brain tissue of young rats by means of histology and molecular biology, and further clarify these mechanisms. Through this study, we hope to provide a new experimental basis for the evaluation of brain tissue effects and related mechanisms in radiotherapy, so as to further improve the safety and effectiveness of radiotherapy.
Materials and Methods
Experimental animal:
27 healthy male Sprague-Dawley (SD) young rats, Specific-Pathogen-Free (SPF) grade, 2 mo old, weighing 298-413 g, provided by the Department of Experimental Animal Science, Medical Department of Peking University, certificate number: SCXK (Beijing) 2011-0012. The animals were fed adaptively for 2 w after purchase. The rats were reared in separate cages, temperature 20°- 26°, humidity 40°-70°, ammonia concentration ≤14 mg/m3, noise ≤60 dm (A), natural light for 12 h a day, and free to eat and drink.
Experimental instruments and reagents:
Absolute ethanol (purchased from Shanghai Chemical Plant), methanol (purchased from Xilong Chemical Co., Ltd.), rat Malondialdehyde (MDA), Enzyme Linked Immunosorbent Assay (ELISA) kit, rat Superoxide Dismutase (SOD), ELISA kit, rat Tumour Necrosis Factor-Alpha (TNF-α), ELISA kit, rat Interleukin-6 (IL-6), ELISA kit, rat caspase-3, ELISA kit (purchased from Shanghai Enzymatic Union Biotechnology Co., Ltd.), -20° cryogenic refrigerator (purchased from Haier Company), Co60-Gamma (γ) ray radioactive source (from Chinese Academy of military Medical Sciences). 37° incubator (purchased from Sanyo Electric).
Experimental method:
27 young rats were randomly divided into high dose group (group C) (n=9), low dose group (group B) (n=9) and control group (group A) (n=9). All the rats in the three groups were fed adaptively for 2 w before irradiation, and were fed with standard maintenance diet and pure water. The 1 ml solution was given with 3.6 % chloral hydrate per 100 g body weight, and the anesthetized rats were anesthetized by intraperitoneal injection of ether in a fixed position, fixed on the operating table, exposed the brain tissue area in an appropriate way, and locally irradiated with radiotherapy equipment. The radiation dose was 20Gy in group C and 3Gy in group B. The irradiation time was 3 w, once a day. After irradiation, the rats were released and transported back to the cage to continue feeding until the animals were killed.
Disposal of rats and extraction and preservation of brain tissue:
1 w after craniocerebral irradiation, the experimental rats were killed. Before the experiment, the rats were anesthetized with 10 % chloral hydrate (350 mg/kg, intraperitoneal injection). After anesthesia, the rats were dissected and the brain tissue was removed quickly. The obtained brain tissue was placed in an appropriate container and immediately stored in a refrigerator at -80° to maintain the stability and biological activity of the tissue. Brain tissue that requires some specific experiments can also be fixed in 4 % paraformaldehyde. For the brain tissue that needs histological examination, the brain tissue is embedded in paraffin and sectioned for Hematoxylin and Eosin (H&E) staining.
Pathological section staining of rat brain tissue (H&E staining):
The brain tissue of the executed rats was taken out and fixed in 10 % neutral buffer formaldehyde and soaked for 24 h. The fixed brain tissue was dehydrated. Soak in 70 % ethanol for 30 min, then soak in 80 %, 95 % and 100 % ethanol for 30 min each. The brain tissue after dehydration was placed in a transparent agent (xylene) and the soaking time was 30 min. The transparent brain tissue was immersed in molten wax for 1 h, and the brain tissue impregnated with molten wax was put into a paraffin mold. The paraffin-solidified brain tissue was cut into thin slices with a thickness of 5 to 10 microns. The cut brain tissue slices were continuously placed on the pretreated glass slides. The brain slices were dewaxed in an oil remover. The dewaxing temperature was 60°-65° and the dewaxing time was about 30 min. The dewaxed brain tissue slices were stained. Soak 5~10 min in hematoxylin (hematoxylin violet) solution and rinse it in tap water. Soak the 1~2 min in eosin solution and rinse the sheet in tap water. The dyed slices were dehydrated in different concentrations of ethanol, the soaking time was 95 % ethanol 1 min, absolute ethanol 1 min, and then 1~2 min was soaked in xylene. The dehydrated slices were sealed with a sealant. Finally, the stained pathological sections of rat brain tissue were observed and photographed under the microscope.
Cell scratch test to detect migration ability:
Brain tissue cells were cultured in a petri dish to a suitable density and state. Using a thin distance (200 μl straw can absorb liquid at one end with a thin and straight caliber), gently draw a slender straight line on the cell layer in the petri dish. The width of scratches is generally 100-200 μm. After the scratch preparation is completed, carefully remove the culture medium from the petri dish and gently wash the cell layer with Phosphate Buffer Solution (PBS) buffer to remove the cell fragments and residues in the culture medium. A new medium was added to enable the cells to continue to grow and migrate. The central area of the petri dish should be kept clear in order to observe the repair of scratches. The repair of scratches was observed and recorded by microscope at 24 h and 48 h after scratch preparation. Phase contrast microscope and fluorescence microscope were used to observe the migration of cells.
Detection of biochemical indexes in rat brain tissue:
The levels of Oxidative Stress (OS), inflammatory factors and apoptosis factors in rat brain tissue were detected to evaluate the degree of brain tissue damage caused by radiation. In this experiment, the level of OS was investigated by detecting the levels of SOD and MDA, and the level of inflammatory factors was detected by TNF-α and IL-6. SOD, MDA, TNF-α and IL-6 were all treated by ELISA kit. A small amount of ground brain tissue powder was weighed and put into a centrifuge tube, then Radioimmunoprecipitation Assay (RIPA) lysate was added. After 30 min on the ice, 5000 r/min centrifuged for 10 min, and the supernatant was obtained. Use commercial SOD test kit and follow the instructions.
Detection of organ index in rats:
1 w after craniocerebral irradiation, the rats were killed and each organ was taken out for detection. The fat organs such as spleen, liver and kidney were weighed and their weights were recorded. The corresponding organ index was obtained by dividing the weight of fat organs by the body weight of rats. Organ index is generally used to evaluate the burden or changes of fat organs. Usually, the change of organ index can reflect the physiological or pathological state of adipose organs. The weight ratio of fat organs (such as spleen, liver and kidney) to the body weight of rats. Organ index can be used to evaluate the burden or changes of fat organs. The increased organ index may indicate the swelling or proliferation of organs, while the decreased organ index may be related to organ dysfunction or injury. The changes of organ index can reflect the physiological or pathological state of adipose organs.
Detection of related protein expression by Western blot:
The brain tissue was added with RIPA lysate to split the cells, and the protein was quantified. The protein samples were taken by Sodium Dodecyl Sulphate (SDS)-polyacrylamide gel electrophoresis and transferred to Polyvinylidene Fluoride (PVDF) membrane. 5 % skimmed milk powder was sealed, and the first antibody was added to incubate in the refrigerator overnight. The corresponding second antibody was added to incubate at room temperature, and the Enhanced Chemiluminescence (ECL) method was used for coloration.
Statistical method:
The data were processed by 20.0 statistical software, the measurement data were described by (statistical x±s), the means were compared by independent sample t-test, and the counting data were described by percentage, using Chi-square (χ2) test, the difference was statistically significant (p<0.05). Compared with control group, ap<0.05 and compared with low-dose group, bp<0.05.
Results and Discussion
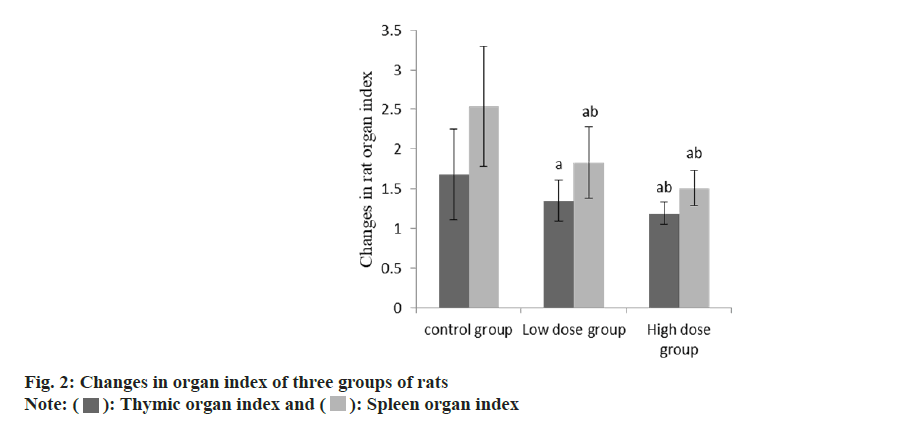
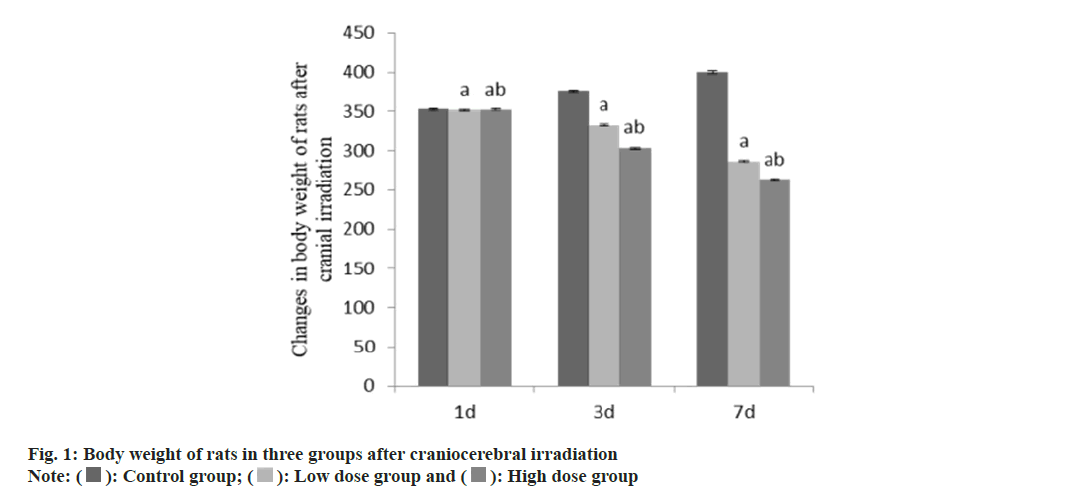
At 3 d and 7 d after craniocerebral irradiation, the body weight of rats in group B and group C was lower than that in group A, and that in group C was lower than that in group B (Table 1 and fig. 1). Compared with the group A, the thymus and spleen organ index of the group B and the group C decreased, and the decrease degree of the group C was higher than that of the group B (p<0.05) (Table 2 and fig. 2).
| Group | n | Weight (g) | ||
|---|---|---|---|---|
| 1 d | 3 d | 7 d | ||
| A | 9 | 353.17±1.27 | 376.58±1.47 | 400.55±1.53 |
| B | 9 | 351.69±1.31a | 332.64±1.25a | 286.58±1.26a |
| C | 9 | 352.26±1.28ab | 303.12±1.16ab | 263.24±1.05ab |
| F | 3.30 | 7278.23 | 28969.55 | |
| p | 0.067 | <0.001 | <0.001 | |
Note: Compared with control group B, ap<0.05 and compared with low-dose group C, bp<0.05
Table 1: Body weight of rats in three groups after craniocerebral irradiation
| Group | n | Thymic organ index | Spleen organ index |
|---|---|---|---|
| A | 9 | 1.68±0.57 | 2.54±0.76 |
| B | 9 | 1.35±0.26a | 1.83±0.45a |
| C | 9 | 1.19±0.14ab | 1.51±0.22ab |
| F | 4.09 | 9.06 | |
| p | 0.030 | 0.001 |
Note: Compared with control group B, ap<0.05 and compared with low-dose group C, bp<0.05
Table 2: Changes in organ index of three groups of rats
The nuclei of normal neurons in rat hippocampus are large and round, transparent, with 1-2 obvious nucleoli, clear nucleoli and intact cell membrane. By comparison, it was found that the pathological sections of the group B had certain damage, that is, the nerve cells swelled, lost the typical circle and became multi-stage shape; the position of the nucleus shifted and showed eosinophilic staining, and the structure became unclear; the staining of the cytoplasm was light and pale and homogeneous; there was brain edema, loose tissue and interstitial edema; some of the more severe cells could be seen that the nucleus was heavily stained and showed dark red. Some even appear nerve cell pyknosis, inclusion body shrinking and deformation and so on. The interstitial edema in the group C was more obvious, the nerve cells showed nuclear fragmentation and nuclear lysis and necrosis; the number of necrotic and apoptotic cells in the hippocampal dentate gyrus increased, and some cells disintegrated, resulting in unclear contours; neurodegenerative changes were found in nerve cells of hippocampal dentate gyrus.
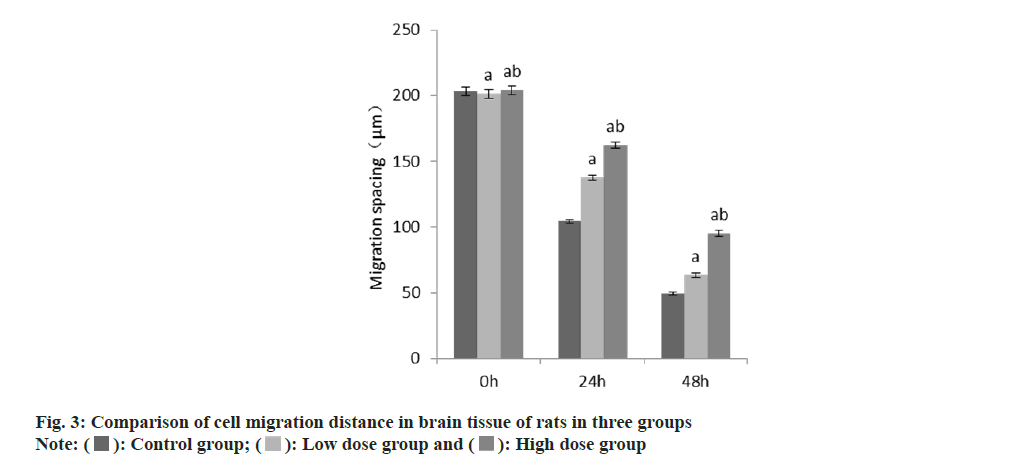
The results of scratch test showed that at 24 h and 48 h, the cell migration distance between the group B and the group C was larger than that in the group A, and this in the group C was larger than that in the group B (Table 3 and fig. 3).
| Group | n | Migration spacing (μm) | ||
|---|---|---|---|---|
| 0 h | 24 h | 48 h | ||
| A | 9 | 203.27±3.35 | 104.32±1.46 | 49.67±1.03 |
| B | 9 | 201.37±3.49a | 137.67±2.08a | 63.25±1.54a |
| C | 9 | 204.16±3.38ab | 162.48±2.55ab | 95.31±2.17ab |
| F | 1.57 | 1774.36 | 1821.40 | |
| p | 0.228 | <0.001 | <0.001 | |
Note: Compared with control group B, ap<0.05 and compared with low-dose group C, bp<0.05
Table 3: Comparison of cell migration distance in brain Tissue of rats In three groups
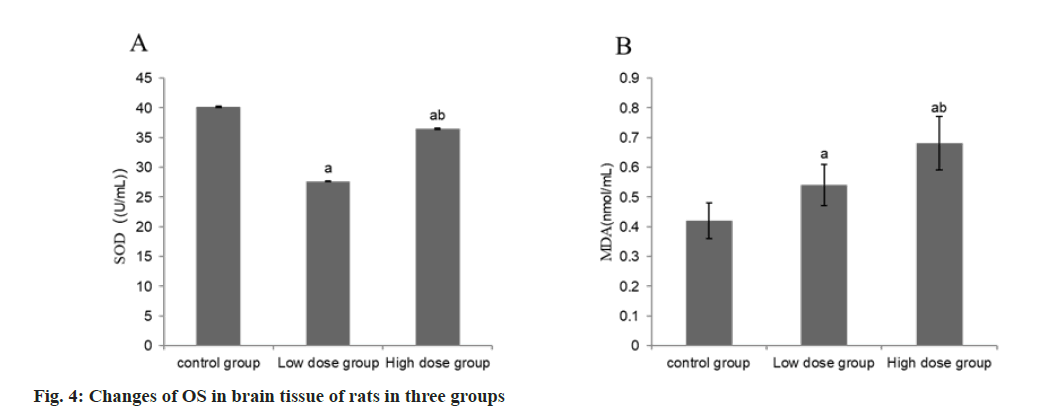
Compared with the group A, the serum SOD activity decreased and the MDA content increased in the group B, while the SOD activity and MDA content in the group C increased compared with the group B (p<0.05) (Table 4 and fig. 4).
| Group | n | SOD (U/ml) | MDA (nmol/ml) |
|---|---|---|---|
| A | 9 | 40.17±0.15 | 0.42±0.06 |
| B | 9 | 27.63±0.08a | 0.54±0.07a |
| C | 9 | 36.46±0.11ab | 0.68±0.09ab |
| F | 32.58 | 27.54 | |
| p | <0.001 | <0.001 |
Note: Compared with control group B, ap<0.05 and compared with low-dose group C, bp<0.05
Table 4: Changes of OS in brain tissue of rats in three groups
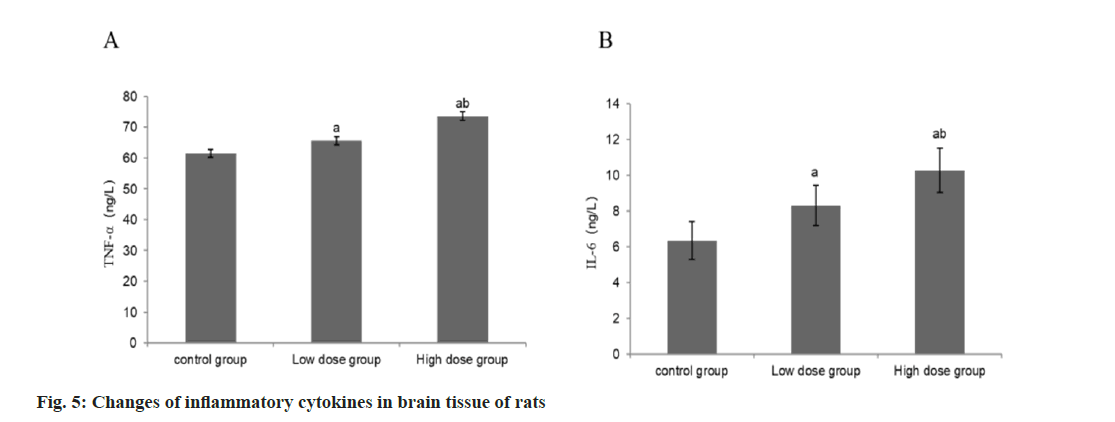
Compared with the group A, the concentrations of TNF-α and IL-6 in the group B were higher than those in the group B, and the concentrations of TNF-α and IL-6 in the group C were higher than those in the group B (Table 5 and fig. 5).
| Group | n | TNF-α (ng/l) | IL-6 (ng/l) |
|---|---|---|---|
| A | 9 | 61.43±1.22 | 6.34±1.05 |
| B | 9 | 65.63±1.28a | 8.37±1.14a |
| C | 9 | 73.57±1.46ab | 10.27±1.23ab |
| F | 195.17 | 26.64 | |
| p | <0.001 | <0.001 |
Note: Compared with control group B, ap<0.05 and compared with low-dose group C, bp<0.05
Table 5: Changes of inflammatory cytokines in Brain tissue of rats
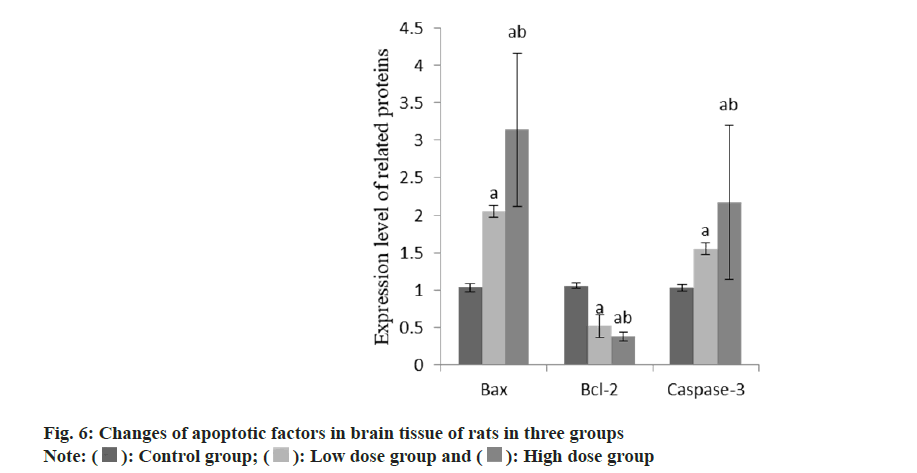
Compared with the group A, the apoptotic factors Bcl-2-Associated X-Protein (Bax) and caspase-3 in the group B increased, while the content of B-Cell Lymphoma 2 (BCL-2) decreased. Compared with the group B, the apoptotic factors Bax and caspase-3 in the group C increased and the content of Bcl-2 decreased (Table 6 and fig. 6).
| Group | n | Bax | Bcl-2 | Caspase-3 |
|---|---|---|---|---|
| A | 9 | 1.04±0.06 | 1.05±0.03 | 1.03±0.04 |
| B | 9 | 2.05±0.08a | 0.52±0.15a | 1.55±0.08a |
| C | 9 | 3.14±1.02ab | 0.38±0.06ab | 2.17±1.03ab |
| F | 28.35 | 70.23 | 8.23 | |
| p | <0.001 | <0.001 | 0.002 |
Note: Compared with control group B, ap<0.05 and compared with low-dose group C, bp<0.05
Table 6: Changes of apoptotic proteins in brain tissue of rats in three groups
The results showed that the brain tissue of rats in the irradiation group showed obvious pathological damage, in which the pathological changes such as nerve cell swelling, nuclear displacement, cytoplasmic pallor, brain edema and interstitial edema increased. In addition, the migration ability of cells in the irradiation group decreased, indicating that irradiation can inhibit the migration of brain cells. The effects of radiotherapy on the brain of young rats are mainly caused by radiobiological effects of brain tissue, including neuronal apoptosis, glial cell injury, vascular disorder and inflammation[6]. Radiotherapy can lead to neuronal apoptosis in the brain tissue of young rats. The study found that there were a large number of neuronal apoptosis in the brain tissue of young rats after craniocerebral irradiation[7]. This may be due to the Deoxyribonucleic Acid (DNA) damage caused by radiotherapy, which interferes with the self-regulation mechanism of cells, which leads to the enhancement of apoptosis. In addition, radiation therapy can also cause damage to glial cells in brain tissue. Glial cells play an important role in maintaining the normal function of nervous system and repairing damaged tissue[8]. Radiotherapy can lead to the injury and apoptosis of glial cells, affecting their function and repair ability, thus further damaging the normal function of brain tissue. Radiotherapy can also cause inflammation and vascular disorders in brain tissue[9]. Radiotherapy increases the release of inflammatory factors in brain tissue and promotes the occurrence of inflammatory reaction. At the same time, radiotherapy causes damage to cerebral vessels, resulting in vascular disorders and reduced blood perfusion. These inflammatory reactions and vascular disorders can further aggravate brain damage and dysfunction[10].
At the cellular and molecular level, the level of OS in the brain tissue of the irradiated group increased, which was characterized by the decrease of SOD activity and the increase of MDA content. This shows that irradiation produces OS on brain tissue, which may lead to cell membrane damage and apoptosis. The radiobiological effects caused by radiotherapy are closely related to OS. Radiotherapy can increase the level of OS in brain tissue, produce a large number of reactive oxygen free radicals, and then cause lipid peroxidation and cell oxidative damage[11]. Environmental factors such as diet, exercise and exposure to harmful substances can affect the level of OS in brain tissue. The effect of OS on brain function is mainly achieved by affecting DNA damage and repair. Radiotherapy can cause double strand breaks of DNA in brain tissue and destroy the structure and function of DNA[12]. DNA repair system in brain tissue plays an important role in repairing DNA damage caused by radiotherapy. However, higher doses of radiotherapy may exceed the repair capacity of the brain DNA repair system, resulting in DNA damage that cannot be repaired, leading to apoptosis and dysfunction[13]. Cell cycle regulation also plays an important role in brain injury caused by radiotherapy. Radiotherapy can cause abnormal cell cycle in brain tissue and interfere with cell proliferation and differentiation.
In addition, the levels of inflammatory cytokines TNF-α and IL-6 in brain tissue of rats in irradiation group also increased, which further indicated that radiation induced inflammatory response. Inflammatory response and immune regulation induced by radiotherapy also play an important role in the repair of brain tissue. Radiotherapy increases the release of inflammatory factors in brain tissue and activates the response of immune cells. Immune regulation plays critical role in brain tissue repair, which can regulate inflammatory response and promote tissue repair[14,15]. Compared with the group A, the apoptosis factors Bax and caspase-3 in the group B increased, while the content of Bcl-2 decreased. Compared with the group B, the apoptosis factors Bax and caspase-3 increased and the Bcl-2 content decreased in the group C. The results showed that the brain tissue of young rats mediated apoptosis after craniocerebral irradiation. However, this study also has some limitations. First of all, only male rats were used in this study, and no female rats were involved, so the effect of gender differences on radiobiological effects is not clear. Secondly, this study did not further study the effects of different radiation doses and frequencies on the radiobiological effects of brain tissue. These factors can be taken into account in future studies to more comprehensively assess the effects of radiotherapy on brain tissue.
To sum up, craniocerebral irradiation caused radiation-induced pathological damage to the brain tissue of young rats, resulting in weight loss and immune organ damage in a dose-dependent manner. Its mechanism can be mediated by promoting inflammation, OS and apoptosis.
Conflict of interests:
The authors declared no conflict of interests.
References
- Lin B, Huang D, Gao F, Yang Y, Wu D, Zhang Y, et al. Mechanisms of FLASH effect. Front Oncol 2022;12:995612.
[Crossref] [Google Scholar] [PubMed]
- Xu W, Li F, Liu Z, Xu Z, Sun B, Cao J, Liu Y. MicroRNA-27b inhibition promotes Nrf2/ARE pathway activation and alleviates intracerebral hemorrhage-induced brain injury. Oncotarget 2017;8(41):70669.
[Crossref] [Google Scholar] [PubMed]
- Dong K, Du Y, Rinkevich F, Wang L, Xu P. The Drosophila Sodium Channel 1 (DSC1): The founding member of a new family of voltage-gated cation channels. Pestic Biochem Physiol 2015;120:36-9.
[Crossref] [Google Scholar] [PubMed]
- Wang A, Fang S, Zhong L, Lu M, Zhou H, Huang W, et al. Shikonin, a promising therapeutic drug for osteoarthritis that acts via autophagy activation. Int Immunopharmacol 2022;106:108563.
[Crossref] [Google Scholar] [PubMed]
- Laemmerer A, Kirchhofer D, Madlener S, Loetsch-Gojo D, Jaunecker CN, Gabler L, et al. HGG-50. Specific sensitivity of pediatric high-grade glioma with ATRX inactivation to PARP inhibitor combinations. Neuro Oncol 2022;24(1):i73.
- Bi R, Bao C, Jiang L, Liu H, Yang Y, Mei J, et al. MicroRNA-27b plays a role in pulmonary arterial hypertension by modulating peroxisome proliferator-activated receptor γ dependent Hsp90-eNOS signaling and nitric oxide production. Biochem Biophys Res Commun 2015;460(2):469-75.
[Crossref] [Google Scholar] [PubMed]
- Kool MJ, Onori MP, Borgesius NZ, van de Bree JE, Elgersma-Hooisma M, Nio E, et al. CAMK2-dependent signaling in neurons is essential for survival. J Neurosci 2019;39(28):5424-39.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Ran S, So KF, Zhang L. Molecular mechanisms of glial cells in brain disorders following physical exercise. Stress Brain 2023;3(4):179-90.
- Liang XS, Qian TL, Xiong YF, Liang XT, Ding YW, Zhu XY, et al. IRAK-M ablation promotes status epilepticus-induced neuroinflammation via activating M1 microglia and impairing excitatory synaptic function. Mol Neurobiol 202360(9):5199-213.
[Crossref] [Google Scholar] [PubMed]
- Casali M, Lauri C, Altini C, Bertagna F, Cassarino G, Cistaro A, et al. State of the art of 18F-FDG PET/CT application in inflammation and infection: A guide for image acquisition and interpretation. Clin Transl Imaging 2021;9(4):299-339.
[Crossref] [Google Scholar] [PubMed]
- Effects of reduced glutathione on radiation lung injury, oxidative stress factor,TGF-β1 and ICAM-1 in lung cancer patients treated with radiotherapy.
- Sun J, Teng M, Wu F, Zhao X, Li Y, Zhao L, et al. Adverse health effects and stresses on offspring due to paternal exposure to harmful substances. Crit Rev Env Sci Technol 2023;53(10):1059-84.
- Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol 2009;4:71-95.
[Crossref] [Google Scholar] [PubMed]
- Wang L, Wang A, Fu Q, Shi Z, Chen X, Wang Y, et al. Ferroptosis plays an important role in promoting ionizing radiation-induced intestinal injuries. Biochem Biophys Res Commun 2022;595:7-13.
[Crossref] [Google Scholar] [PubMed]
- Liu T, You J, Gao Q, Zhang Y, Xu W, Kong D, et al. The Efficacy and mechanism of Qinghua Jianpi recipe in inhibiting concertation of colorectal adenoma based on inflammatory cancer transformation. J Immunol Res 2023;2023.
 ): Control group; (
): Control group; (  ): Low dose group and (
): Low dose group and ( ): High dose group
): High dose group