- *Corresponding Author:
- S. Kollipara
Formulation Development and Pharmacokinetics Laboratory, Pharmacy Group, Birla Institute of Technology and Science, Pilani-333 031, India
E-mail: sivacharan.kollipara@ranbaxy.com
| Date of Submission | 16 December 2009 |
| Date of Revision | 15 July 2010 |
| Date of Acceptance | 30 July 2010 |
| Indian J Pharm Sci 2010, 72 (4): 465-470 |
Abstract
A simple and fast reversed phase liquid chromatographic method was developed for estimation of paclitaxel in commercially available parenteral formulation and nanoparticles. Separations were carried out using mobile phase consisting of acetonitrile and 20 mM potassium dihydrogen phosphate (45:55, v/v) on Lichrocart® C 18 analytical column at a flow rate of 1 ml/min and detection wavelength of 230 nm. The developed method exhibited linearity over an analytical range of 50-2000 ng/ml with regression equation, mean peak area= 137.58 concentration (ng/ml)+1765.94, (R 2 =0.9999). The method demonstrated selectivity with no interfering peaks eluting near the vicinity of drug peak. The method was found to be sensitive with detection and quantification limits of 7.57 ng/ml and 22.94 ng/ml. The method has shown consistent and good recoveries from parenteral formulation (100.06±0.86%) and nanoparticles (100.43±0.91%). The method was successfully employed for the analysis of in vitro release study samples of nanoparticle formulation. The method was also applied for determination of paclitaxel content in various pharmaceutical formulations.
Keywords
nanoparticles, paclitaxel, parenteral formulation, RP-HPLC
Paclitaxel (PAC) is a potent chemotherapeutic agent ((1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12- diacetoxy-15-{ [(2R,3S)-3-(benzoylamino)-2- hydroxy-3-phenylpropanoyl]oxy}-1,9-dihydroxy- 10,14,17,17-tetramethyl-11-oxo-6-oxatetracyc lo [11.3.1.0~3,10~.0~4,7~]heptadec-13-en-2-yl relbenzoate) indicated as first-line and subsequent therapy for treatment of lung, ovarian, breast, neck cancers and solid tumors [1]. PAC binds to β-subunit of tubulin and subsequently hyper-stabilizes its structure. This leads to disruption of normal microtubule dynamics and as a consequence causes cell death. Other mechanism of action has also been proposed, in which PAC induces apoptosis in tumor cells by binding to an apoptosis stopping protein called Bcl-2 (B-cell leukemia 2). PAC is a high molecular weight drug (Mw 845 Da) with very limited aqueous solubility (<30 μg/l) and high hydrophobicity (log P 3.96) [2]. In vitro studies in human liver microsomes and tissue slices showed majority of administered PAC dose was metabolized primarily by CYP2C8 and CYP3A4. Due to its poor physicochemical properties, affi nity for metabolizing enzymes (CYP2C8, CYP3A4) and effl ux transporters (P-glycoprotein), the oral bioavailability of PAC is found to be less than 10% [3]. These limitations led to the development of non-aqueous intravenous injection (Taxol®, containing 6 mg/ml of PAC), in which PAC is dissolved in a media containing (1:1, v/v) mixture of polyoxyethylated castor oil (Cremophor EL) and dehydrated alcohol. This solution is to be diluted with a suitable parenteral fl uid before intravenous infusion. However there have been serious complications associated with the use of Taxol® like development of hypersensitivity reactions, lethargy, hypotension, neurotoxicity, nephrotoxicity, vasodilation, attributed to the presence of Cremophor EL [4]. In order to develop a safe and effective formulation for controlled delivery of PAC, nanoparticles have been prepared in our laboratory.
Literature survey revealed many liquid chromatography based methods for estimation of PAC in biological fl uids [5-8]. Very few methods were reported for estimation of PAC from pharmaceutical dosage forms [9,10]. However, the developed methods used complex gradient systems and longer analysis time making them unsuitable for routine analysis. In the present work, a fast, sensitive and economic reversed phase liquid chromatographic method was developed for estimation of PAC in bulk, nanoparticles, parenteral formulations, and for in vitro release study of nanoparticle formulations. The developed method was validated as per ICH guidelines and applied successfully for determination of PAC content from commercially available parenteral formulation and in-house developed nanoparticles.
Materials and Methods
Paclitaxel (Assay 99.95%) obtained as a gift sample from Dr. Reddy’s Laboratories, Hyderabad, India. HPLC grade acetonitrile, dichloromethane were procured from Spectrochem, India. Analytical grade potassium dihydrogen phosphate was purchased from S. D. Fine Chemicals Ltd., Mumbai, India. Poly(lactic-co-glycolic acid) copolymer (PLGA) was generously gifted as a test sample by Purac, USA. Poly vinyl alcohol was procured from Sigma-Aldrich Chemicals Ltd., St. Louis MO, USA. Miglyol 810 was purchased from Sasol Chemicals, Germany. Taxol® (5 ml vial, Bristol-Myer’s Sqibb) was obtained from the local pharmacy. Ultra pure water was prepared using a Milli-Q® water purifi cation system (Millipore Co., USA) and fi ltered (0.22 μm) before use. All other chemicals used in the study were of analytical grade.
Chromatographic system and conditions
The HPLC system (Jasco, Japan) used in present study consisted of PU-1580 pump, AS-1559 auto injector and UV-1575 UV/Vis detector. Separations were carried out on Lichrocart® RP18 reversed phase column (Merck®, 120×4.6 mm; 5 μm). Chromatographic peaks were integrated using Borwin® work station (Jasco, Japan) loaded on a computer system (IBM, USA). Optimized mobile phase was prepared by mixing acetonitrile and 20 mM potassium dihydrogen phosphate buffer in 45:55 (%, v/v) ratio and degassed in an ultrasonic bath for 15 min just before chromatography. The flow rate was set to 1 ml/min. Injection volume of 50 μl was made and the column eluents were monitored at 230 nm over a run time of 10 min. All the separations were carried out at ambient conditions (25°) after baseline stabilization for at least 30 min.
Preparation of nanoparticles
Nanoparticles of PAC were prepared by emulsion solvent evaporation technique [11]. Briefly, 10 mg of PLGA, 35 μl of miglyol, 5 mg of PAC were dissolved in 1 ml of methylene chloride. This organic phase was dispersed in 10 ml aqueous phase containing poly vinyl alcohol (2%, w/v) as stabilizer. The resulting emulsion was stirred on magnetic stirrer over night for complete evaporation of organic phase. The preparation was centrifuged at 14 000 rpm at 25° for 30 min (Remi Compufuge, India). The nanoparticle containing fraction was washed twice with phosphate buffered saline and freeze dried (Maxi Dry Lyo, Heto, Germany) over a period of 10 h. Similarly placebo nanoparticles were also prepared without adding PAC.
Preparation of stocks and standards
Primary stock solution of 1 mg/ml of PAC was prepared in acetonitrile. Secondary stock solution of 10 μg/ml was prepared by diluting 1 ml of primary stock to 100 ml using solvent media consisting of acetonitrile: MilliQ® water (50:50, v/v). Three separate series of six calibration standards 50, 100, 250, 1000 and 2000 ng/ml were prepared by serial dilution in mobile phase. Sample standards of PAC were prepared by adding known amount of drug in blank nanoparticles and placebo mixture of parenteral formulation at five levels: 25, 50, 100, 150, 200% of the labeled claim. Similarly, placebo standards were also prepared without adding PAC. The sample standards and placebo standards were processed independently using their respective sample preparation method as described below.
Sample preparation
For parenteral formulation, 100 μl of placebo/ sample standard or test sample was added to 10 ml volumetric fl ask and the volume was made up with acetonitrile. The sample was vortex mixed and 0.83 ml of sample was added to 100 ml volumetric fl ask and diluted to volume with mobile phase.
For nanoparticles, a quantity of formulation (placebo/ sample standard or test sample) equivalent to 2 mg of PAC was weighed and transferred to a 10 ml volumetric flask. The nanoparticles were digested with 2 ml acetonitrile by ultra-sonication (10 min, 25°). The volume was made with acetonitrile and the samples were centrifuged at 10 000 rpm for 15 min. Finally, 0.25 ml of supernatant was transferred to 100 ml volumetric fl ask and the volume was made with mobile phase.
Analytical method validation
The developed reversed phase chromatographic method was validated for selectivity, linearity, range, precision, accuracy, sensitivity and system suitability as per ICH guidelines [12]. The proposed method was also applied for drug content analysis from parenteral formulation and in-house prepared nanoparticles.
Selectivity
The selectivity of the method in presence of formulation excipients was assessed by injecting the processed placebo and sample standards in three triplicates on three different days. The obtained chromatograms were compared with freshly prepared calibration standards.
Linearity and range
The linearity of the method was assessed by analyzing the calibration standards in three replicates on three different days. Average peak area was plotted against respective concentration level and subjected to least square linear regression. Calibration curve obtained from regression analysis was used to calculate the corresponding predicted concentrations. The analytical range of proposed method was obtained by analyzing residuals. One-way analysis of variance (ANOVA) was performed on each replicate obtained at six concentration levels [13].
Accuracy and precision
Placebo spiking technique was employed for establishing accuracy of the method. Sample standards containing 25, 50, 100, 150, 200% of the labeled claim of parenteral formulation and nanoparticles were processed in fi ve replicates and injected on three different days. The results were expressed as mean absolute recovery, % bias and coeffi cient of variation (%CV).
Precision of the method was expressed as repeatability (intra-batch) and intermediate precision (inter-batch). For repeatability, fi ve series of quality control (QC) samples prepared at lower (LQC, 50 ng/ml), middle (MQC, 500 ng/ml) and higher (2000 ng/ml) were freshly prepared and analyzed. For intermediate precision, fi ve series of quality control samples were prepared and analyzed on three different days. The results of precision were expressed as %CV.
Sensitivity
The sensitivity of proposed method was determined using standard deviation of intercept (σ) and slope (s) of calibration equation. Limit of detection (LOD) and limit of quantifi cation (LOQ) were calculated using 3.3 σ/s and 10 σ/s respectively.
System suitability and sample solution stability
System precision was carried out by analyzing six replicates of calibration standards. Various chromatographic parameters like capacity factor (k), tailing factor (Tf) and number of theoretical plates (N) were recorded. Further, the stability of PAC in mobile phase was determined by injecting calibration standard 1000 ng/ml at 0, 6, 12, 24, 36, and 48 h in five replicates.
Formulation analysis
As an application, the proposed method was used for determination of drug content from marketed formulation Taxol® and in-house prepared nanoparticles. For Taxol®, 100 μl of sample was taken and processed as in sample preparation section. For nanoparticles, amount equivalent to 2 mg of PAC was accurately weighed and processed as described in sample preparation section. Finally, 50 μl of resulting solution was injected in triplicates and analyzed.
In vitro release study of nanoparticles
The in vitro release study of nanoparticles was carried out in triplicates using dialysis bag diffusion method using phosphate buffer saline (pH 7.4) with Tween 80 (2%, w/v) as dissolution media [14]. Briefl y, 5 mg of freeze dried nanoparticles were dispersed in 2 ml of MilliQ® water and placed in a dialysis membrane bag (Molecular weight cutoff 12 KDa) and sealed. Release studies were carried out using modifi ed USP Type II apparatus (Electrolab, India) with 50 ml of dissolution media, set at 50 rpm, 37±2°. Samples of 2 ml were withdrawn at specifi ed time points over a period of 48 h and same amount of blank dissolution media was added. The obtained samples were centrifuged and analyzed by proposed HPLC method. The obtained in vitro release data was fi tted into various mathematical models like zero order, fi rst order, Higuchi model and reciprocal powered time (RPT) model [15].
Results and Discussion
The aim of the present study was to develop a simple, accurate and precise reversed phase HPLC method for quantifi cation of PAC in bulk and in pharmaceutical formulations. Based on the UV-profile of PAC, the wavelength was optimized at 230 nm for better sensitivity and selectivity from formulation excipients. Initial optimization of mobile phase was carried out to select a suitable organic modifi er and buffering agent. Initial trials with acetonitrile and MilliQ water (50:50, v/v) resulted in poor peak parameters with inadequate retention (Rt ≈ 3.5 min). Trials with acetonitrile and 20 mM potassium dihydrogen phosphate buffer (50:50, v/v) showed better peak parameters with improved retention (Rt ≈ 4.7 min). Based on above results, the fi nal mobile phase was set to acetonitrile and 20 mM potassium dihydrogen phosphate buffer (45:55, v/v). This combination of mobile phase showed excellent chromatographic peak parameters with good retention (Rt 7.7±0.2 min).
Placebo standards showed no interference in the vicinity of PAC peak, when compared with freshly prepared calibration standards indicating the selectivity of developed method for PAC in presence of formulation excipients. Overlay of calibration standards is shown in fig. 1 and overlay of placebo standards and sample standards (100%) is shown in fig. 2.
Calibration data of PAC is shown in Table 1. The calibration curve obtained by least square analysis showed linear relationship with regression coeffi cient (R2) of 0.9999. The best fit equation obtained was mean peak area= 137.58×concentration (ng/ml) + 1765.94. At all concentration levels, the standard deviation was low and %CV did not exceed 2%. The predicted concentrations were in close agreement with the theoretical concentrations. The linearity range was found to be 50-2000 ng/ml. Analysis of residuals showed that the residuals were normally distributed with uniform variance across studied concentration levels, indicating homoscedastic nature of the data. The standard error of slope and intercept were found to be 0.150 and 140.883, respectively. The obtained slope and intercept values were well within the 95% confidence intervals (confidence interval for slope: 137.16-137.99, confidence interval for intercept: 1374.78-2157.09). The goodness of fit of linear regression equation was supported by low standard error or estimate (203.95, with respect to mean peak area and 1.48 with respect to concentration). The one-way ANOVA performed on peak area at each concentration level indicated that calculated F-value (0.22×10-3) was less than the critical F-value (2.152) at 5% signifi cance level.
| Concentration (ng/ml) | Average peak areaa (±SD) | %CV | Predicted concentration (ng/ml) |
|---|---|---|---|
| 50 | 8498.00 ± 62.11 | 0.73 | 48.93 |
| 100 | 15456.67 ± 190.50 | 1.23 | 99.51 |
| 250 | 36462.67 ± 282.83 | 0.78 | 252.20 |
| 500 | 70308.89 ± 518.60 | 0.74 | 498.22 |
| 1000 | 139592.56 ± 1515.72 | 1.09 | 1001.82 |
| 2000 | 276822.67 ± 2618.24 | 0.95 | 1999.31 |
Table 1: Calibration Data for Paclitaxel
The developed method showed high and consistent absolute recoveries at all studied levels for both parenteral formulation and nanoparticles. The recovery data from parenteral formulation and nanoparticles is shown in Table 2. For parenteral formulation, the mean absolute recovery ranged from 99.61 to 100.60% and for nanoparticles, it ranged from 100.23 to 100.75%. At all studied concentration levels, the standard deviation was low (< 1.40) representing the accuracy of the proposed method. Additionally, the obtained recoveries were found to be normally distributed with low and uniform %CV at all concentration levels. Moreover, the %bias values were low (< 1) at all studied levels indicating that there was no signifi cant interference of formulation excipients. Hence, the recovery study demonstrated the suitability of proposed method for determination of PAC from parenteral formulation and nanoparticles.
| Product | Amount of drug added (% of label claim)a | Mean absolute recovery (%) | %CV | % Bias |
|---|---|---|---|---|
| Parenteral | 25 | 100.27 ± 0.67 | 0.66 | 0.27 |
| Formulation | 50 | 100.60 ± 1.31 | 1.30 | 0.60 |
| 100 | 99.85 ± 0.50 | 0.50 | 0.15 | |
| 150 | 99.61 ± 0.82 | 0.82 | 0.39 | |
| 200 | 99.96 ± 0.57 | 0.57 | 0.04 | |
| Overall recovery = 100.06 ± 0.86 | ||||
| Nanoparticles | 25 | 100.54 ± 0.71 | 0.71 | 0.71 |
| 50 | 100.41 ± 1.15 | 1.15 | 1.15 | |
| 100 | 100.23 ± 1.01 | 1.01 | 1.01 | |
| 150 | 100.24 ± 0.82 | 0.82 | 0.82 | |
| 200 | 100.75 ± 0.91 | 0.91 | 0.91 | |
| Overall recovery = 100.43 ± 0.91 | ||||
Table 2: Recovery Study by Placebo Spiking Technique
The results for precision study are shown in Table 3. In repeatability, the %CV ranged from 0.16 to 2.06. At all QC levels, the variation was insignificant indicating the repeatability of the method. Similarly, low inter-batch %CV (< 1.83) was observed for intermediate precision. The %CV values were very well within the acceptable range indicating the repeatability and intermediate precision of the developed method.
| QC Level | Repeatability (intra-batch) | Intermediate precision (inter-batch) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Batch-I | Batch-II | Batch-III | |||||||
| Meana | %CV | Meana | %CV | Meana | %CV | Mean | %CV | ||
| LQC | 49.91 | 1.82 | 50.27 | 1.87 | 50.38 | 2.06 | 50.19 | 1.83 | |
| MQC | 501.62 | 0.97 | 499.28 | 1.04 | 499.90 | 1.13 | 500.27 | 0.99 | |
| HQC | 2022.48 | 0.23 | 2014.32 | 0.27 | 2011.28 | 0.16 | 2016.03 | 0.32 | |
Table 3: Results For Repeatability and Intermediate Precision
The LOD and LOQ of the method were found to be 7.57 and 22.94, ng/ml respectively. The method has demonstrated high value of slope with minimal standard error. Insignificant change in chromatographic peak properties (retention time and peak area) were observed upon re-injection at quantifi cation limit. Hence the method was found to be highly sensitive for determination of PAC.
The method has shown excellent chromatographic peak parameters such as capacity factor (k ≥ 3.25), number of theoretical plates (N≥3700) and tailing factor (Tf ≈1.065). The obtained peak parameters were well within the acceptable limits indicating the suitability of the method for PAC determination. Low variability in peak area and retention time were observed upon re-injection indicating that the developed method was specific, precise and stable for estimation of PAC. In addition, the drug peak exhibited no response and chromatographic change for 48 h when compared against freshly prepared standards. The results demonstrated the stability of drug in mobile phase over a period of 48 h with variation less than 0.80%.
The developed method was used to estimate the PAC content from marketed formulation Taxol® and in-house prepared nanoparticles. The mean recoveries for each formulation were in good agreement with the labeled claim indicating the accuracy of method for determination of PAC. The mean absolute recovery for Taxol® and nanoparticle formulation were found to be 100.79 and 99.52% respectively. The %CV was found to be 0.81 and 1.85% for Taxol® and nanoparticles, respectively. Thus, the proposed method was found to be suitable for determination of PAC from both the formulations.
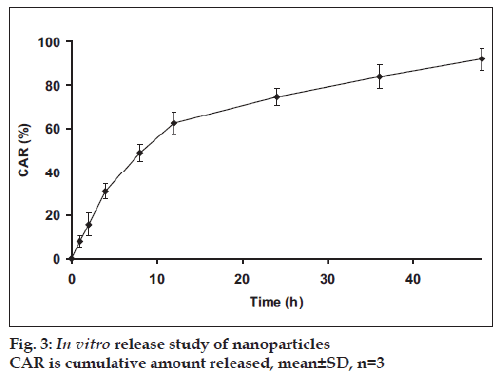
The developed method was applied successfully to determine amount PAC from in vitro release study. The in vitro release profile of PAC from nanoparticles is shown in fig. 3. The release of PAC from nanoparticles showed initial burst till 12 h followed by continuous and slow release till 48 h. The release profi le was also evaluated by fi tting into different mathematical models. The release profi le was described better by RPT model (R2=0.9913) compared to zero order (R2=0.8228), first order (R2=0.9790) and Higuchi model (R2=0.9597). The time for 50% dissolution (t50%) was found to be 8.02 h.
In summary, the proposed method was found to be simple, sensitive, accurate, and precise for estimation of PAC from parenteral formulation and nanoparticles. The analysis of parenteral and in-house prepared nanoparticle formulations showed good agreement with labeled claims indicating insignificant interference of formulation excipients in the estimation. The method was also successfully applied for determination of amount of PAC release from nanoparticles. Thus, the method can be used for routine analysis of PAC from bulk and pharmaceutical formulations.
Acknowledgements
The authors are thankful to Dr. Reddy’s laboratories for providing paclitaxel gift sample and also grateful to Purac, USA for providing test sample of PLGA.
References
- Rowinsky EK, Donehower RC. Paclitaxel (Taxol). N Engl J Med 1995;332:1004-14.
- Rowinsky EK, Cazenave LA, Donehower RC. Taxol: A novel investigational antimicrotubule agent. J Natl Cancer Inst 1990:82; 1247-59.
- Nakajima M, Fujiki Y, Kyo S, Kanaya T, Nakamura M, Maida Y, et al. Pharmacokinetics of paclitaxel in ovarian cancer patients and genetic polymorphisms of CYP2C8, CYP3A4, and MDR1. J Clin Pharmacol 2005;45:674-82.
- Friedland D, Gorman G, Treat J. Hypersensitivity reactions from taxol and etoposide. J Natl Cancer Inst 1993;85:2036.
- Kim SC, YU J, Lee JW, Park ES, Chi SC. Sensitive HPLC method for quantitation of paclitaxel (Genexol®) in biological samples with application to preclinical pharmacokinetics and biodistribution. J Pharm Biomed Anal 2005;39:170-6.
- Panchagnula R, Babu DA, Kaur KJ, Singh I, Kaul CL. Determination of paclitaxel in human plasma by HPLC. Pharm Pharmacol Commun 1999;5:587-9.
- Caporossi L, Rosa MD, Pera A, Papaleo B. Simple analytical method for the determination of paclitaxel (Taxol®) levels in human plasma. Chromatographia 2007;66:921-4.
- Suno M, Ono T, Iida S, Umetsu N, Ohtaki K, Yamada T, et al. Improved high-performance liquid chromatographic detection of paclitaxel in patient's plasma using solid-phase extraction, and semi-micro-bore C18 separation and UV detection. J Chromatogr B AnalytTechnol Biomed Life Sci 2007;860:141-4.
- Badea I, Ciutaru D, Lazar L, Nicolescu D, Tudose A. Rapid HPLC method for the determination of paclitaxel in pharmaceutical forms without separation. J Pharm Biomed Anal 2004;34:501-7.
- Anupama M, Deepak C, Neeraj K. HPLC method for the determination of carboplatin and paclitaxel with cremophorEL in an amphiphilic polymer matrix. J Chromatogr B Analyt Technol Biomed Life Sci 2007;855:211-9.
- Guerrero DQ, Allemann E, Fessi H, Doelker E. Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers. Drug Dev Ind Pharm 1998;24:1113-28.
- The Eurpoean Agency for the Evaluation of Medical Products. ICH Topic Q2B: Note for Guidance on Validation of Analytical Procedures: Methodology GPMP/ICH/281/95, 1996.
- Bolton S. Pharmaceutical statistics: practical and clinical applications. New York: Marcel Dekker; 1997.
- Levy MY, Benita S. Drug release from submicronized o/w emulsion: A new in vitro kinetic evaluation model. Int J Pharm 1990;66:29-37.
- Barzegar-Jalali M, Adibkia K, Valizadeh H, Shadbad MR, Nokhodchi A, Omidi Y, et al. Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci 2008;11:167-77.