- *Corresponding Author:
- Malathi Seshasayee Konda Ramadoss
Department of Pharmacy, Ponnaiyah Ramajayam Institute of Science and Technology University, Puducherry 605007, India
E-mail: malathi@daffodildesign.net
| Date of Submission | 08 September 2021 |
| Date of Revision | 04 January 2022 |
| Date of Acceptance | 15 April 2022 |
| Indian J Pharm Sci 2022;84(2):501-512 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In recent years, regulatory standards of traditional herbal medicines, is a topic of discussion. India is pioneered in traditional herbal medicine and has the potential to benchmark standards. Association of Southeast Asian nations region which is the second-largest consumer of herbal medicines with high market potential is explored. The present research is focused to study the product registration process by comparing the regulatory standards in India with the standards in association of Southeast Asian nations regions. To understand the process better, the regulatory requirements, concerns and sufficiency to meet up to the standards are explained using Ashwagandha tablets as a product model. The samples from the Indian market are evaluated, the scope of regulatory sufficiency to the association of Southeast Asian nation’s regulatory requirements is examined and the extent of its compliance with association of Southeast Asian nation’s standards is evaluated. Additional requirement specific to the association of Southeast Asian nations region apart from the standards already being followed is proposed to be included. This will help the companies who wish to make a mark in the association of Southeast Asian nations regions.
Keywords
Traditional herbal medicines, regulatory standards, association of southeast Asian nations regions, Ashwagandha tablets
The awareness of the regulatory implications for traditional herbal medicines is gaining importance as they are being used extensively in the prevention and treatment of various ailments. Implementation of policies, setting up of regulations is under constant focus. Under the collaboration, the National regulatory bodies, the Ministry of Health and the World Health Organisation (WHO) are working towards regulatory harmonization. In this process, WHO has emphasized promoting universal healthcare to ensure quality, safety and efficacy in its “WHO traditional medicine strategy 2014-2023”[1].
The national regulation, product classification, and registration process of herbal medicines differ from country to country, which poses a challenge to the manufacturing companies to place a standardized herbal product in the global market. Till the harmonization is in place, the interim solution for industries to register their product is to strategize their product development process considering all the regulatory aspects. This will help to identify the gaps, fine-tune compliance with the standards and set up a common platform, through good practices and an effective Quality Management System (QMS) for the benefit of the herbal industry and healthcare community[2]. All these efforts will pave way for the manufacturers to handle the challenges right from product design, product development, manufacturing and marketing of standardized traditional herbal medicinal products.
In India, herbal drug products constitute a major share among all the recognized systems of health viz. Ayurveda, Yoga and naturopathy, Unani, Siddha and Homeopathy (AYUSH). The Ministry of AYUSH was established to work towards the optimal development and propagation of AYUSH systems of health care[3]. The Association of South East Asian Nations (ASEAN) region is the second-largest consumer which makes it a desirable region to explore. It includes Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines, Singapore, Thailand and Vietnam[4].
Country-specific national regulation in the ASEAN region and providing the sample product documentation file (dossier) before product registration is a challenge. To address this, the proposal here is to provide the dossier as per the ASEAN Common Technical Document (ACTD). Even the country-specific requirement can be addressed within the scope of ACTD. To address ACTD, the regulatory requirements must be thoroughly understood and the products development must be in line with the regulatory requirements of all the ASEAN member states. A robust product development process must be chalked out and a strategic documentation approach must be followed.
To understand the regulatory requirements of AYUSH and to extend the same to the ASEAN market, a model study with Ashwagandha tablets was chosen and compliance with quality, safety, and efficacy standards is discussed. Parameters like assay, heavy metals and microbes were tested in approved laboratories taking market samples from five different manufacturers. The results were tabulated and correlated with the regionspecific requirements. The outcome of this will help to develop official monographs. With the information from the ancient classical texts and supported by evidencebased studies, AYUSH can define the standards specific to different regions. Thus, they can guide the companies to meet the requirements and position their products in the respective markets. This way AYUSH will be setting high standards and will be the guiding force for any export market entry.
In the current scenario, considering the modern lifestyle and stress involved, Ashwagandha was chosen for this study. Ashwagandha (Withania somnifera (W. somnifera)) is widely used since time immemorial for various indications, especially as an adaptogenic for its ability to relieve stress and boost energy, making it a perfect drug of choice for physical and mental wellbeing[5].
Materials and Methods
Five random market samples of Ashwagandha (W. somnifera) tablets containing 250 mg,W. somnifera root extract from the Indian market were purchased. The AYUSH and ASEAN regulations for traditional medicines were sourced and evaluated. The documentation policy and the registration requirements are referred to from the official websites of the regulatory bodies of the respective countries. Ashwagandha is available in various dosage forms. Tablets being one of the conventional dosage forms are considered here for this research paper. AYUSH standards for Ashwagandha are observed and sufficiency for ASEAN requirements is evaluated.
Microbial analysis and heavy metal analysis were carried out by Nawal analytical laboratories recognized by Bureau of Indian Standards (BIS), Ministry of Environment and Forests (MoEF) and International Organization for Standardization (ISO) 9001:2005 certified. Assay by High Performance Liquid Chromatography (HPLC) (method followed as per United States Pharmacopeia (USP)) to check the total withanolides content (analysis performed by Eurofins analytical services India private limited-National Accreditation Board for Testing and Calibration Laboratories (NABL) certified).
The standards provided by the Central Council for Research in Ayurvedic Sciences, like general guidelines for drug development[6], general guidelines for safety/toxicity[7] and general guidelines for clinical evaluation[8] are considered in this study. The regulatory pathway for product registration in ASEAN regions and its requirements sufficiency is defined with the aid of a study model.
Results and Discussion
India has taken a major initiative by establishing pharmacopoeial standards of its traditional medicines in its Ayurvedic Pharmacopoeia of India (API). API, Part I, Vol. IX comprises the most used plant drugs, their extracts along with their chromatographic fingerprint. Since Ashwagandha has potential market demand, the inclusion of the same can be prioritized. To contribute further to this continual development, this study proposes a new perspective with the assistance of a model study to help out the companies to interpret the regulatory implications for successful product registration. The parameters mentioned in the monographs are limited and in case a company intends to market the product in ASEAN countries, there are certain additional criteria to be fulfilled. The monograph should be developed robustly and include all the parameters to support regulatory standards. Monographs specific to herb, herb part, processed herb and formulation should be defined.
The formulation chosen for our study is Ashwagandha tablets, which constitutes-Ashwagandha (W. somnifera root dry extract) 250 mg as the active ingredient. The documentation process for market authorization should be addressed in the following order. Drug substance: Ashwagandha (W. somnifera) herb-root form; Drug preparation: Ashwagandha (W. somnifera root dry extract); Drug product: Ashwagandha tablets; Actives: W. somnifera root dry extract 250 mg; Inactives: excipients. Drug substance or herbal substance comprises mainly of whole, fragmented or cut plants, plant parts, certain exudates without any treatment for microbial control, it is usually unprocessed dried form or sometimes fresh. The nomenclature, source and criteria of selection of the herb, should be provided. The permitted or restricted list of substances should be considered[9]. The herb should be chosen based on its availability in the plant database[10-12].
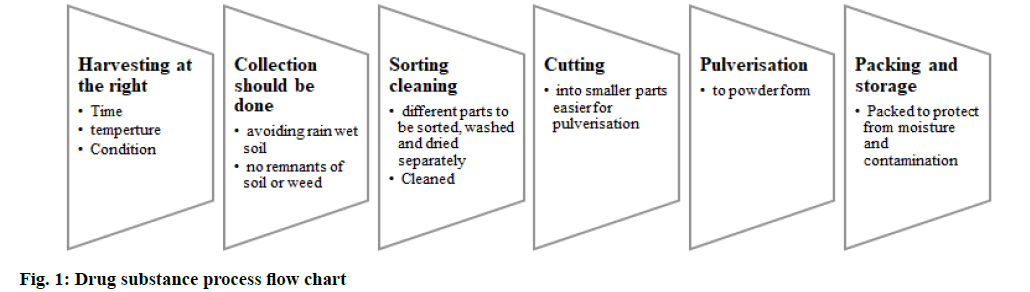
The herb selected for the model study-Ashwagandha is listed in the database, it is accepted in the ASEAN region. Part used is Ashwagandha root and it must be procured from the approved supplier as root or root powder. All the related documents and agreements on quality must be as per the national/regional regulations. The standards of control should be as per good agricultural and collection practices Good Agricultural and Collection Practices (GACP) guidelines[13]. Information of the sourcing of the herb (root), its harvesting and collection information along with the processing details like drying of herb, cutting, storage (fig. 1) should be included. The supplier should provide the details of the description of the plant, cultivation, harvesting, drying and storage conditions as per GACP, followed by information on batch size, process flow and process control, and the container closure system.
Herb authentication certificate from an authorized body is a must with the report of the analytical evaluation. The intention is to rule out any adulteration and to procure an authentic, hygienic herbal substance that is handled throughout the process with care and control. In this study-Ashwagandha, must be done under the right conditions. The harvested herbs should be collected without any remnants of soil and free from weeds. The part used is Ashwagandha root-should be sorted, cleaned and then dried, the drying conditions, duration and quality of the air used for drying should be monitored, the unwanted materials or foreign bodies if any must be removed. Before being subjected to the cutting process, dried medicinal plants/herbal preparation/herbal substances ought to be stored in recommended conditions to ensure quality throughout storage.
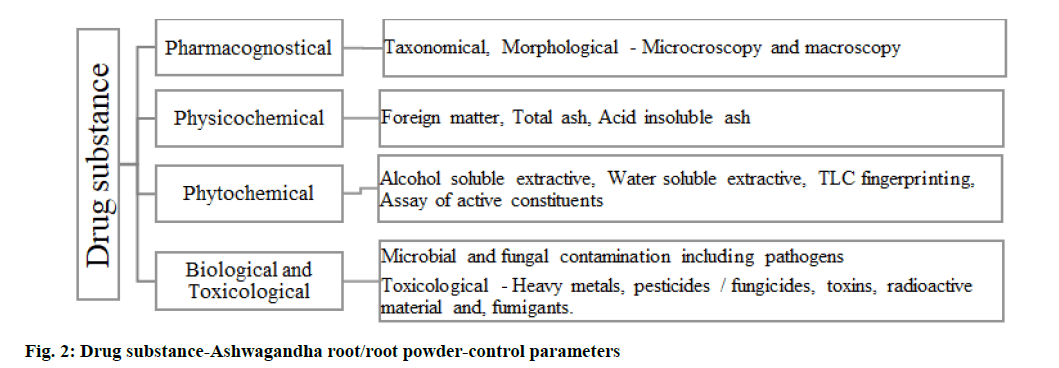
Further, the cut root parts are pulverized, care is taken so that the powder is not heated up or loses its quality. The dry root powder should be packed and transported in clean and humidity-controlled conditions, with protection from degradation and attack from pests. Samples of Ashwagandha roots should be drawn and tested for the parameters (fig. 2) using validated analytical methods and reports provided accordingly by the supplier. Foreign matter not more than 2 %, total ash not more than 7 %, acid insoluble ash should not be more than 1 %, alcohol soluble extractive (25 %) not less than 15 % and water-soluble extractive should be nil. Assay for active constituents-total alkaloids, the limit provided for Ashwagandha when assayed gravimetrically is not less than 0.2 % of total alkaloids[14].
Biological parameters are as follows. Microbial and fungal contamination should comply as per permissible limits and pathogens like Escherichia coli (E. coli), Salmonella, Pseudomonas and Staphylococcus should be absent. Toxicological parameters are as follows. Heavy metals like lead, mercury, arsenic and cadmium should comply with the permissible limits. Pesticide residue limits, absence of toxins, radioactive material, fumigants should be confirmed with analytical evaluation and should comply as specified. Control of aflatoxins is a requirement where aflatoxin B1 should not be more than 2 parts per billion (ppb), aflatoxin B1+B2+G1+G2 should be within 5 ppb.
Drug preparation and Ashwagandha dry extract of root is as follows. To obtain drug preparation or herbal preparations, drug substances or herbal substances are subjected to treatments like extraction, distillation, expression, purification, concentration or fermentation. The production and primary processing of the medicinal plant/herbal substance has a direct influence on the quality of active raw material. Hence care should be taken to define the process flow and monitor process control. The herbal preparation for our product is Ashwagandha dry root extract. For herbal preparation, in addition to the information of the herb, it should also include information on solvents used for extraction[15].
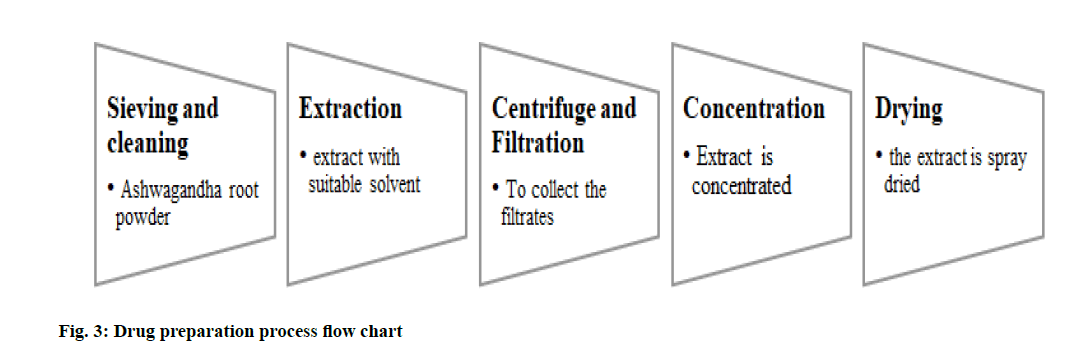
The detailed process of extraction and drying process, here being Ashwagandha dry root extract, the extraction procedure and the drying process should be detailed, along with personnel, premises, sanitation, hygiene, in-process quality control, packing, documentation and record management. The manufacturing process should comply with the ASEAN guidelines for Good Manufacturing Practices (ASEAN GMP)[16]. In case the extraction process is not carried out in-house (i.e., by the product manufacturing company), the dry extract of Ashwagandha can be procured directly from the approved supplier and confirmation on whether the standards are in line with the ASEAN GMP must be checked.
For which the manufacturer/supplier needs to have an approved plant for the extraction process. The Ashwagandha powder should be cleaned and sieved and subjected to extraction with solvent. Upon completion of extraction, the extract should be centrifuged to separate the residues from the filtrates the process is repeated till all the alkaloids are extracted. The extract is tested periodically to check if any alkaloids are still left out. The extract is concentrated and spray dried to obtain dry root extract of Ashwagandha. The dried extract should be packed in moisture-resistant containers and should be stored properly. Stability studies should be conducted to establish the shelf life of the extract and to specify the storage conditions. Document support should be provided with corresponding reports of the same (fig. 3).
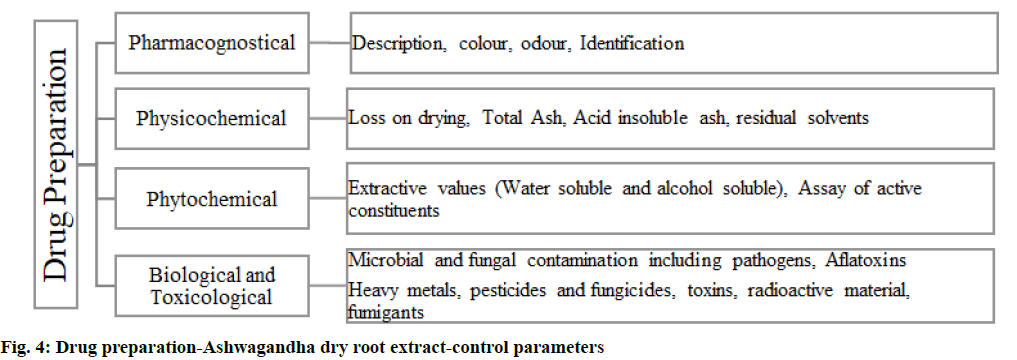
Certificate of authenticity and certificate of analysis should be provided along with the parameters. Marker principles are to be recognized and the analytical methods validated as per WHO and International Council on Harmonisation (ICH) guidelines[17-19]. Control parameters and drug preparation is described below. The control parameters should include a description for the preparation, like color and odor; the identification is done by thin-layer chromatography; extractive values for water-soluble extractive and alcohol soluble extractive; active constituent alkaloids should be tested. Potential contaminants arising from the dry root extraction process should be addressed in the specification. The certificate of analysis should also carry the same. Heavy metals, mycotoxins, radioactive contamination and microbial contamination, aflatoxins should comply, it is important to address the residual solvents to rule out any remnant of the solvent used during extraction, potential adulterants should be discussed either as compendial requirements or information as received from the supplier[20,21] (fig. 4).
The source and specification of the Ashwagandha root extract with acceptance criteria can be procured by either the manufacturer or from the supplier. Details of the finalized specification based on three validated batches should be available in the document repository,along with the validation protocol and validation report[22]. The container used for packing should be food-grade and the specification and grade certificates should be obtained from the vendor. Stability studies should be conducted as per zone IV B, for both the herbal substance and herbal preparation in its final pack, the long-term study have to be carried out at 30°±2°/75 % Relative Humidity (RH)±5 % RH and accelerated stability study for 6 mo at 40°±2°/75 % RH±5 % RH. If the study reports are submitted for three production batches commitment should be provided to continue the study during the re-test period. If the study reports are submitted for less than 3 batches then commitment should be provided to continue the studies for the next immediate batch[23].
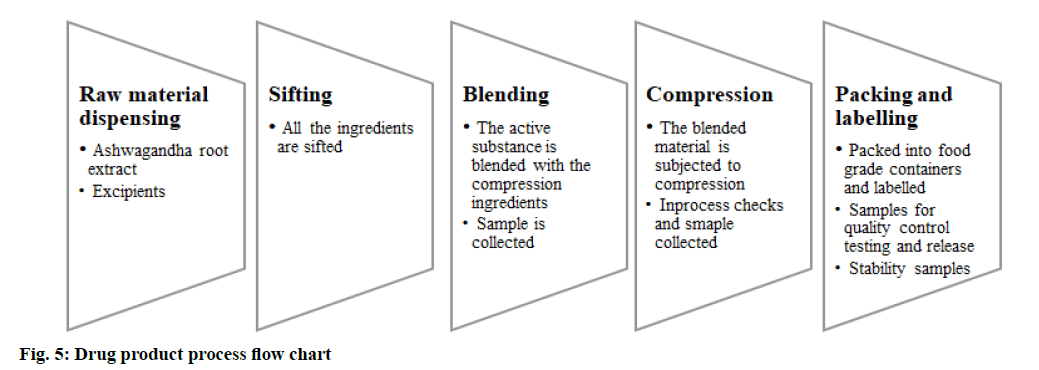
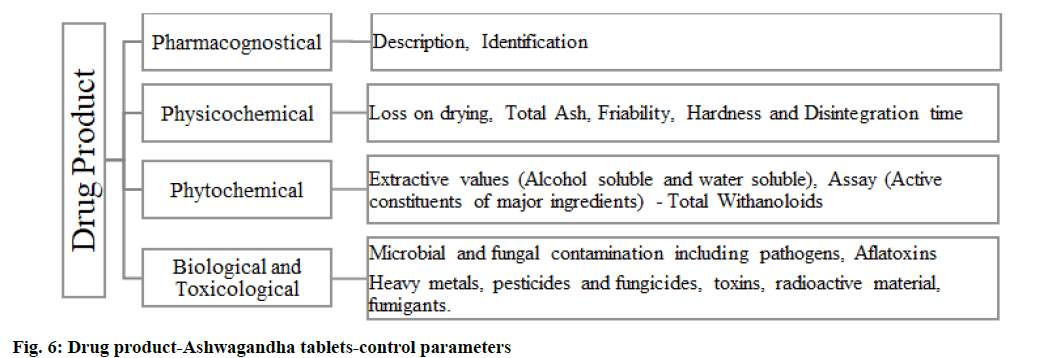
A herbal drug product is defined as a medicinal product exclusively containing one or more herbal substances/ herbal preparation or herbal substances in combination with one or more herbal preparation, mostly presented with therapeutic or prophylactic claims. Ashwagandha tablets are used in our study, the formulation includes the active constituent-Ashwagandha (W. somnifera root dry extract) 250 mg. The information on product profile, qualitative and quantitative composition, dosage form, excipients used should be addressed in the product development report. The focus should be on the rationale behind the formulations, sufficiently supported by bibliographic evidence. The active raw material should be sifted and blended with the excipients and then compressed into tablets, periodical in-process checks on uniformity in blending, followed by the tablet hardness and disintegration time. The compressed tablets should be packed in their final container and labeled appropriately. Product compatibility with the packaging must be studied and supported by stability studies. The product composition should specify the actives, here Ashwagandha (W. somnifera) root extract 250 mg and also indicate the inactives used for tablet compression. Quality standards should be evaluatedfig. 5 and fig. 6.
Details of the packing material, its grade, specification of the primary and secondary packing material and their certificate of analysis must be collected from the vendor. The scaled-up batch size must be in place along with the list of equipment used, the manufacturing process must be specified with in-process quality checks and control. Other requirements include process validation and its reports, excipients controls, finished product specification and certificate of analysis and method validation reports and batch analysis report for 3 batches as per the finished product specification. Method validation of impurity profiling and assay must accompany the specification of the finished product[24-26]. The finished product specification should also mention the pharmacopoeial reference like Ashwagandha API, followed by parameters like disintegration test[27], uniformity of dosage forms tests[28], friability[29] and contamination profile biological-microbial and fungal contamination including pathogens, aflatoxins and toxicological-heavy metals, pesticides and fungicides, toxins, radioactive material, fumigants.
All these parameters should be incorporated into the product specification and in case of any constraints, a proper justification stating the same can be submitted. Evaluation of study model is described in detail. To understand the controls better Ashwagandha tablets samples from five different manufacturers from the Indian market were picked up and coded as sample AA, sample AB, sample AC, sample AD and sample AE, respectively. Product description can range from light brown colored to dark brown colored tablets. The HPLC chromatographic fingerprinting can be used for identification conformity, uniformity of weight should not deviate by more than ±5 % of the average weight of the tablet. Tablet hardness must be greater than 2 kg/ cm2 and friability percent should not be more than 1 %. The disintegration time for uncoated tablets must be less than 35 min[6].
Analytical comparisons were conducted for three selected parameters (Table 1), these were tested by approved laboratories and the results were used for discussion purposes. The results obtained were compared with the AYUSH and ASEAN requirements. Assay for total withanolides-as per HPLC; Heavy metal content where lead, mercury, arsenic and cadmium were evaluated; microbial contamination tested specifically for total aerobic count, yeast and mold and pathogens. Perspective on microbial contamination is as follows. Here all the five samples-sample AA, sample AB, sample AC, sample AD and sample AE comply with the limits of both AYUSH as well as ASEAN requirements. The limits for total aerobic microbial count in Singapore[30] and the Philippines[31] is not more than 105 colony-forming units (cfu)/g, while in Malaysia[32] and ASEAN[33] the limit is not more than 5105 cfu/g, the value considered for standards in the table is National Microbiological Target (NMT) 105 cfu/g which is the more stringent one. Similarly for yeast and mold the limit of not more than 5×102 is considered in the table, while the limits specific to Singapore and the Philippines is not more than NMT 5×102 cfu/g and in Malaysia and ASEAN the limit is not more than 5×104 cfu/g.
| S. No. | Parameters | ASEAN Limits | AYUSH Limits | Sample values | ||||
|---|---|---|---|---|---|---|---|---|
| Sample AA | Sample AB | Sample AC | Sample AD | Sample AE | ||||
| 1 | Microbial | |||||||
| Total aerobic microbial count | NMT 105 cfu/g | NMT 105 cfu/g | <10 | 500 | <10 | 1300 | 63 000 | |
| Yeast and mold | NMT 5×102 cfu/g | NMT 103 cfu/g | <10 | <10 | <10 | <10 | <10 | |
| Pathogens | ||||||||
| E. coli | Absent | Absent | Absent | Absent | Absent | Absent | Absent | |
| Salmonella | Absent | Absent | Absent | Absent | Absent | Absent | Absent | |
| Pseudomonas | Absent | Absent | Absent | Absent | Absent | Absent | Absent | |
| S. aureus | Absent | Absent | Absent | Absent | Absent | Absent | Absent | |
| 2 | Heavy metals | |||||||
| Lead | NMT 10 ppm | NMT 10 ppm | 0.317 | 1.114 | <0.25 | 1.137 | <0.25 | |
| Mercury | NMT 0.5 ppm | NMT 1 ppm | <0.05 | 1.036 | <0.05 | 6.286 | <0.05 | |
| Cadmium | NMT 0.3 ppm | NMT 0.3 ppm | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | |
| Arsenic | NMT 5 ppm (0.3 ppm Philippines) | NMT 3 ppm | <0.05 | 1.01 | 0.066 | 0.541 | <0.05 | |
| 3 | Assay | |||||||
| HPLC | Total Withanolides (per 100 g) | 0.71 g | 0.48 g | 1.37 g | 0.24 g | 0.025 g | ||
Note: NMT: Not more than; BLQ: Below Limit of Quantification
Table 1: Comparative Table
Bile-tolerant gram-negative bacteria as per ASEAN requirement should not be more than 104 cfu/g. Care should be taken to keep the values under check throughout the shelf-life period. It should also be asserted to the manufacturer that at the time of usage of active raw material, the aflatoxin limits of aflatoxin B1 (not more than 2 ppb) and aflatoxin B1+B2+G1+G2 (not more than 5 ppb) are verified and complied. Perspective on heavy metals is as follows. As observed in the table the results of sample AA, sample AB, sample AC, sample AD and sample AE comply as per ASEAN and AYUSH for lead (not more than 10 ppm) and cadmium (not more than 0.3 ppm), here AYUSH requirements match the ASEAN requirements. As far as mercury is concerned, sample AB and AD do not comply, both as per AYUSH as well as ASEAN standards. For mercury, the ASEAN limit is stringent by 0.5 ppm, here adherence to the limits should be strictly enforced. The limits of AYUSH being 1 ppm and that of ASEAN being 0.5 ppm, necessitates a change to comply with the ASEAN prerequisite.
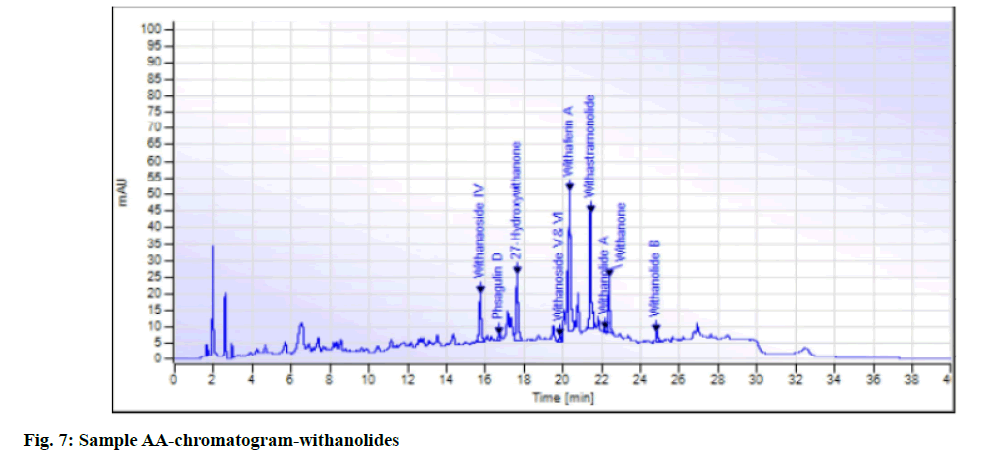
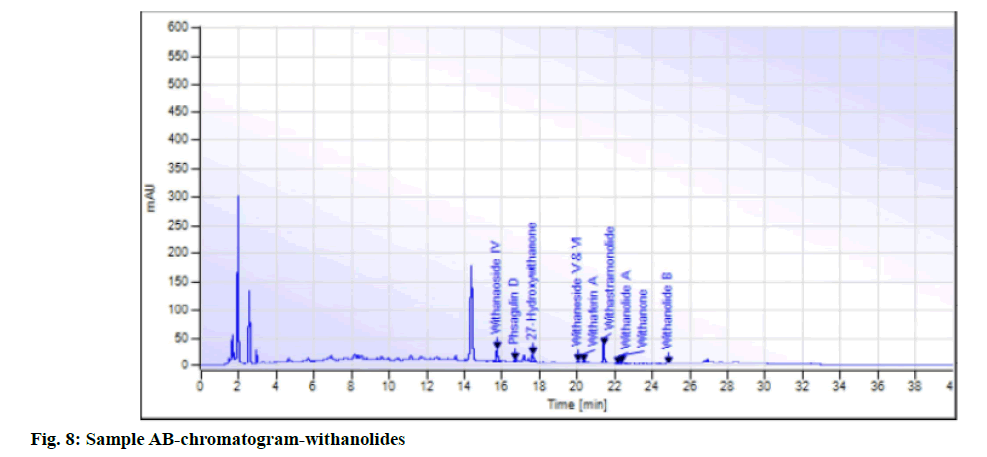
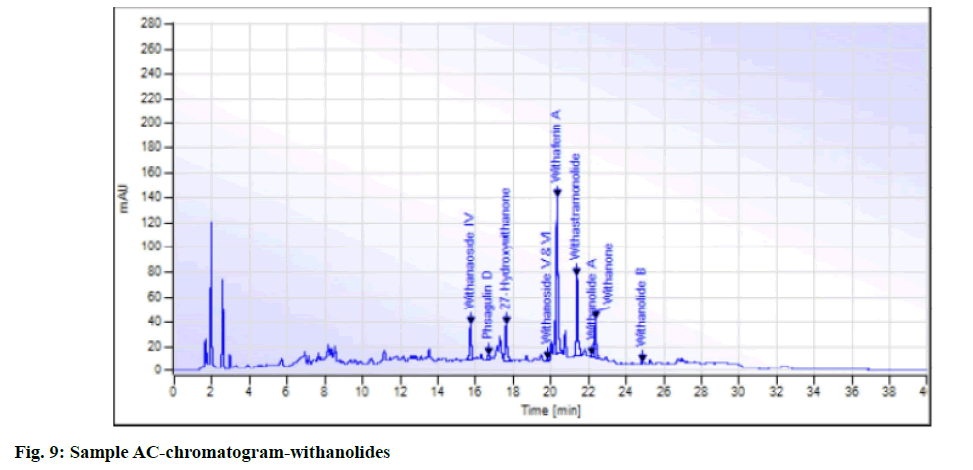
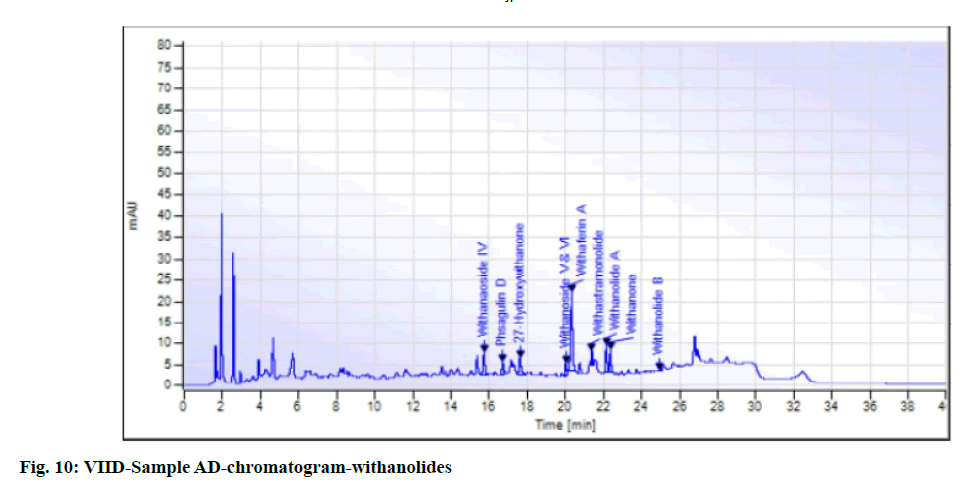
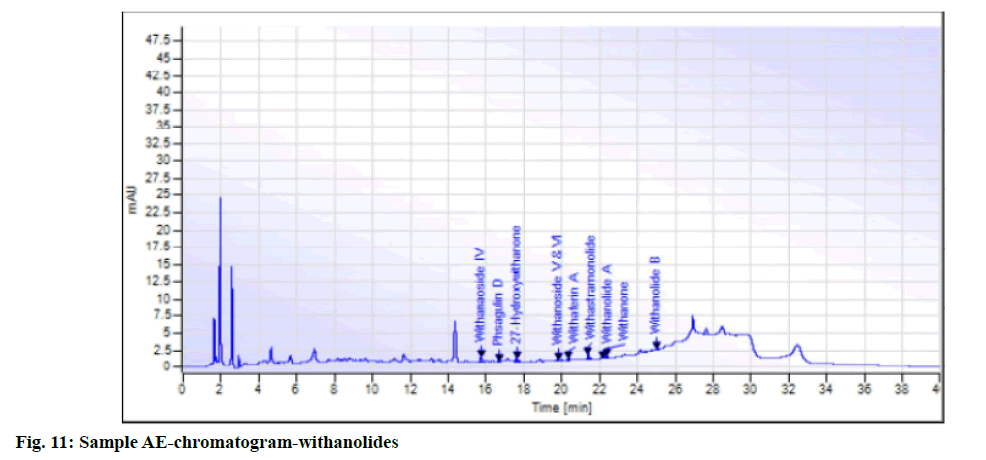
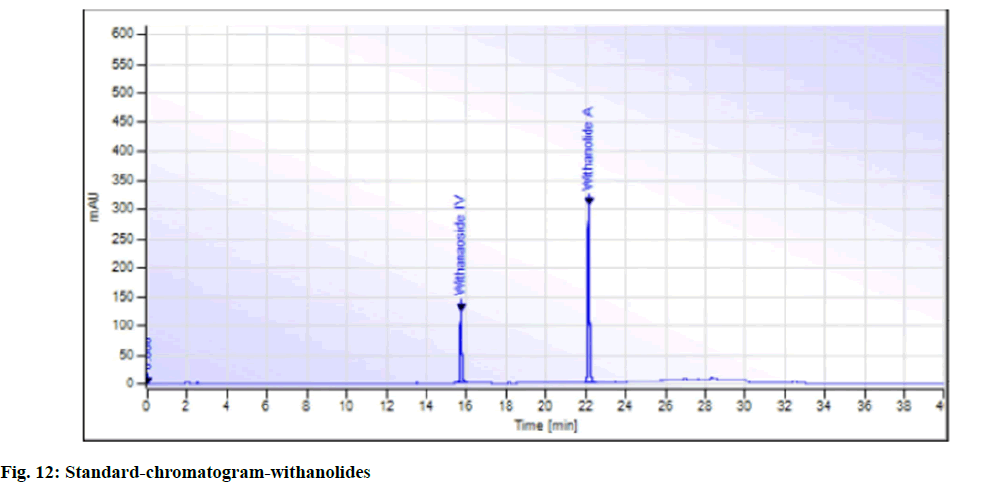
While in the case of arsenic it complies with the AYUSH as well as ASEAN standards (not more than 5 ppm and 3 ppm respectively), but for the Philippines, the limit is not more than 0.3 ppm. In case a manufacturer plans to market in the Philippines, precise compliance to the limit of not more than 0.3 ppm is required. Perspective on assay-total withanolides is as follows. API Part I Volume 9, consists of HPLC procedures for the herbal ingredients and is in the process of continual development. HPLC for Ashwagandha is yet to be included in the pharmacopoeia. Sample AA, sample AB, sample AC, sample AD and sample AE of Ashwagandha tablets which contain root extract was subjected to Assay by HPLC (method followed as per USP) to check the total withanolides content and their chromatograms for chromatographic fingerprinting,(fig. 7 for sample AA, fig. 8 for sample AB, fig. 9 for sample AC, fig. 10 for sample AD, fig. 11 for sample AE and fig. 12 for standard). The withanolides content of the samples varied from 0.025 g/100 g to 1.37 g/100 g (0.025 % to 1.37 %).
The monograph in the API is only for “Ashwagandha” and the assay is limited to evaluation of total alkaloids. The API claims “Ashwagandha consists of not less than 0.2 percent of total alkaloids”, whereas there are no defined limits as per Southeast Asian country requirements. The claim by API is very wide, hence to see how the product in the market varies, the withanolides content was considered for evaluation to make a comparison in specific terms. For this the assay defined by HPLC (method followed as per USP) was used. As expected, a large variation in the withanolide content among the marketed products was observed. Hence this study emphasizes that, to have a standardized product in place, the monograph has to be more exhaustive and specify the limits for Ashwagandha-in terms of root, root powder and root extract, and instead of just total alkaloids, it should also be for total withanolides as appropriate.
Stability should be conducted as per conditions specified under zone IV B as a long-term study data for 36 mo at 30°±75 % RH and an accelerated study data for 6 mo at 40°±75 % RH, the study report should establish the product stability through the proposed shelf life. A commitment should be provided for the continuation of stability studies at room temperature for the shelf life period[22]. Data for three production batches are to be submitted. If the batches are less than three then commitment should be given to continue the studies and to produce the additional batch.
Site Master File (SMF) and facility details with an equipment list, quality declaration for the actives and the finished product is part of the requirement. ASEAN mandatory requirements are as follows. The mandatory requirement for manufacturing and marketing of a product is product license; GMP certificate; compliance to Schedule T (GMP for Ayurvedic, Siddha and Unani Medicines) of the Drugs and Cosmetics Act 1940 and Rules 1945 for premises, equipment and personnel; trademark registration for company and brand; record maintenance of raw material; testing in an approved laboratory; labeling requirements should comply[34-36]; the manufacturing unit must be based in the marketing country or a registered distributor with quality personnel should be appointed; postmarketing surveillance and pharmacovigilance should be in place; the list of prohibited ingredients, synthetic drugs and list of endangered species should be taken into consideration; product samples in the final pack must be provided; advertisements and promotions require a valid permit before publishing; they may ask for halal certification if required.
For successful product registration, the registration requirements for all ASEAN countries including all the additional requirements can be addressed within the scope of the ACTD. The structure of the dossier is presented in four parts (Table 2). The document compilation with all the information gathered should be compiled into the ACTD format. The administrative part should carry all the administration information. The information gathered for Ashwagandha root/ powder, extract and tablets can be used to compile the quality part of the ACTD under drug substance, drug preparation and drug product respectively. This will constitute the quality part of the S (drug substance and preparation) and P (drug product) sections of the ACTD dossier.
The nonclinical part is to be addressed with written and tabulated summaries and evidence for the source of the herb Ashwagandha. Published literature can be used for support. Literature that can be used is mentioned here as an instance. For immunomodulatory activity, papers related to the study on the immunomodulatory effect of W. somnifera can be attached[37,38]. For antioxidant activity a reference published related to antioxidant and neurotransmitter activity can be included[39]. Similarly for neuroprotective effects-a paper reference shows that Ashwagandha root extract can be a drug of choice for any stress-related neurological disorders[40]. As far as the clinical studies are concerned, literature references can be attached to support claim substantiation. For a claim of relief from stress, a reference on the safety and efficacy of Ashwagandha roots extract in lowering stress and anxiety, can be incorporated[41]. For the adaptogenic and anxiolytic effects of the Ashwagandha root extract, the supportive literature can be used[42]. For support on safety, a publication with findings of the study on the safety and tolerability of Ashwagandha, with no toxic effects that can be taken into account[43]. Activity related to immunomodulation can be included as well[44].
Likewise, papers published in high-impact journals related to non-clinical and clinical studies can be further submitted for literature support, as appropriate under the non-clinical and clinical part of the ACTD. In addition, the trials conducted by the product license holder can be considered as a clinical trial report and can be submitted along with tabulated data. Addressing the absorption, distribution, metabolism, excretion, pharmacokinetic drug interactions, other pharmacokinetic studies is a challenge, the complexity of the same can be explained and a justification with a proper supporting document can be provided instead. In case there is no expected risk factor, safety pharmacokinetic studies are not required. Since Ashwagandha is used for longer usage in both the regions and if a safety profile in humans can be documented then it is not necessary to include toxicology studies like single-dose toxicity and repeat dose toxicity.
Herbal companies are in the process of registering products like Ashwagandha tablets in ASEAN countries. A set guideline is required for registration.
To meet these guidelines a comprehensive approach right from the procurement of the raw material to that of product presentation has to be followed like process flow and process control of the product, ACTD, assay of active ingredient by HPLC (withanolides % as in Ashwagandha), contamination profiling and many such parameters as discussed above. Companies can also approach AYUSH who can provide standards specific to the ASEAN market by their continual development process. The product license holder or the manufacturer can meet regulatory requirement sufficiency for product registration in ASEAN regions by following the above regulatory pathway thus finding it easy to obtain market authorization.
Conflict of interests:
The authors declared no conflict of interest.
References
- World Health Organization. WHO traditional medicine strategy: 2014-2023. World Health Organization; 2013.
- Wiesner J, Knöss W. Future visions for traditional and herbal medicinal products-A global practice for evaluation and regulation? J Ethnopharmacol 2014;158:516-8.
[Crossref] [Google Scholar] [PubMed]
- Verma N. Herbal medicines: Regulation and practice in Europe, United States and India. Int J Herb Med 2013;1(4):1-5.
- Sulaiman N, Ming LC. Guidelines for the regulation of herbal medicines in the South-East Asia Region. WHO. New Delhi; 2016.
- Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: A Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med 2011;8(5S):208-13.
[Crossref] [Google Scholar] [PubMed]
- Guidelines for drug development of Ayurvedic formulations, Volume-1. Central Council for Research in Ayurvedic Sciences. Ministry of Ayush. Government of India: New Delh; 2018.
- General guidelines for safety/toxicity evaluation of Ayurvedic formulations, Volume-II. Central Council for Research in Ayurvedic Sciences. Ministry of Ayush. Government of India: New Delh.
- General guidelines for clinical evaluation of Ayurvedic interventions, Volume-III. Central Council for Research in Ayurvedic Sciences. Ministry of Ayush. Government of India: New Delh.
- ANNEX I-ASEAN guiding principles for inclusion into or exclusion from the negative list of substances for traditional medicines and health supplements; 2015.
- Medicinal herbs and plants database. https://www.globinmed.com/medicinal_herbs/
- Malaysian herbal monograph. https://www.globinmed.com/medicinal_herbs_category/malaysian-herbal-monograph/
- Medicinal herbs and plants monograph. https://www.globinmed.com/medicinal_herbs_category/medicinal-herbs-plants-monograph/
- Guideline on Good Agricultural and Collection Practice (GACP) for starting materials of herbal origin. Committee on Herbal Medicinal Products (HMPC). European Medicines Agency: London; 2006.
- The Ayurvedic pharmacopoeia of India. Part I, Volume I. Pharmacopoeia Commission for Indian Medicine and Homoeopathy. Ghaziabad; 2016.
- ANNEX II-ASEAN guiding principles for the use of additives and excipients in traditional medicines and health supplements; 2012.
- ANNEX VIII-ASEAN guidelines on good manufacturing practice for traditional medicines and health supplements.
- Quality control methods for herbal materials. World Health Organization. Geneva; 2011.
- Pharmaceutical quality system Q10. ICH Harmonised Tripartite Guideline. Current Step 2008;4.
- Validation of analytical procedures: Text and methodology Q2(R1). ICH Harmonised Tripartite Guideline. Current Step 1994;4.
- ANNEX III-ASEAN guidelines on limits of contaminants for traditional medicines; 2017.
- Impurities: Guideline for residual solvents Q3C(R6). ICH Harmonised Tripartite Guideline; 2016.
- ASEAN Guideline on submission of manufacturing process validation data for drug registration.
- ANNEX V-ASEAN Guidelines on stability study and shelf-life of traditional medicines; 2017.
- Guideline on quality of combination herbal medicinal products/traditional herbal medicinal products. European Medicines Agency: London; 2008.
- WHO guidelines for assessing the quality of herbal medicines with reference to contaminants and residues. World Health Organization; 2007.
- Validation of analytical procedures: Text and methodology Q2(R1). ICH Harmonised Tripartite Guideline. Current Step 1994;4.
- Evaluation and recommendation of pharmacopoeial texts for use in the ICH regions on disintegration test general chapter Q4B ANNEX 5(R1). Current Step 2010;4.
- Evaluation and recommendation of pharmacopoeial texts for use in the ICH regions on uniformity of dosage units’ general chapter Q4B ANNEX 6. Current Step 2013;4.
- Evaluation and recommendation of pharmacopoeial texts for use in the ICH regions on tablet friability general chapter Q4B ANNEX 9(R1). Current Step 2010;4.
- Regulatory overview of traditional medicines. https://www.hsa.gov.sg/traditional-medicines/regulatory-overview-of-traditional-medicines
- Drug Registration Guidance Document (DRGD). Bahagian Regulatori Farmasi Negara (NPRA). 3rd ed. 2021
- Guidelines on the registration of traditionally-used herbal products. https://www.fda.gov.ph/wp-content/uploads/2021/04/Administrative-Order-No.-184-s.-2004.pdf
- ANNEX III-ASEAN guidelines contamination for limits of traditional medicines; 2015.
- ASEAN guidelines on labelling requirements for traditional medicines and health supplements; 2014.
- ASEAN labeling requirements: Issues on country specific requirements.
- ANNEX VII-ASEAN guidelines on claims and claims substantiation for traditional medicines and health supplements.
- Kumar J, Mitra MD, Hussain A, Kaul G. Exploration of immunomodulatory and protective effect of Withania somnifera on trace metal oxide (zinc oxide nanoparticles) induced toxicity in Balb/c mice. Mol Biol Rep 2019;46(2):2447-59.
[Crossref] [Google Scholar] [PubMed]
- Ziauddin M, Phansalkar N, Patki P, Diwanay S, Patwardhan B. Studies on the immunomodulatory effects of ashwagandha. J Ethnopharmacol 1996;50(2):69-76.
[Crossref] [Google Scholar] [PubMed]
- Suganya K, Kayalvizhi E, Yuvaraj R, Chandrasekar M, Kavitha U, Suresh KK. Effect of Withania somnifera on the antioxidant and neurotransmitter status in sleep deprivation induced Wistar rats. Bioinformation 2020;16(8):631-7.
[Crossref] [Google Scholar] [PubMed]
- Bhatnagar M, Sharma D, Salvi M. Neuroprotective effects of Withania somnifera dunal.: A possible mechanism. Neurochem Res 2009;34(11):1975-83.
[Crossref] [Google Scholar] [PubMed]
- Chandrasekhar K, Kapoor J, Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults . Indian J Psychol Med 2012;34(3):255-62.
[Crossref] [Google Scholar] [PubMed]
- Salve J, Pate S, Debnath K, Langade D. Adaptogenic and anxiolytic effects of ashwagandha root extract in healthy adults: a double-blind, randomized, placebo-controlled clinical study. Cureus 2019;11(12):e6466.
[Crossref] [Google Scholar] [PubMed]
- Verma N, Gupta SK, Tiwari S, Mishra AK. Safety of ashwagandha root extract: A randomized, placebo-controlled, study in healthy volunteers. Complement Ther Med 2021;57:102642.
[Crossref] [Google Scholar] [PubMed]
- Tharakan A, Shukla H, Benny IR, Tharakan M, George L, Koshy S. Immunomodulatory effect of Withania somnifera (ashwagandha) extract-a randomized, double-blind, placebo controlled trial with an open label extension on healthy participants. J Clin Med 2021;10(16):3644.
[Crossref] [Google Scholar] [PubMed]