- *Corresponding Author:
- Bhatia M.N
Department of Pharmaceutical Chemistry, Bharati Vidyapeeth College of Pharmacy, Near Chitranagri, Kolhapur-416 013, India
E-mail: neelabhatia@yahoo.co.uk
| Date of Received : | 01 October 2007 |
| Date of Revised : | 07 June 2008 |
| Date of Accepted : | 24 September 2008 |
| Indian J. Pharm. Sci., 2008, 70 (5): 603-608 |
Abstract
Rapid, precise, accurate, specific and sensitive reverse phase liquid chromatographic and absorbance ratio spectrophotometric methods have been developed for the simultaneous analysis of ambroxol hydrochloride and cetirizine hydrochloride in their tablet formulation. The chromatographic methods were standardized using a HIQ SIL-C 18 column (250×4.6 mm i.d., 10 µm particle size) with UV detection at 229 nm and mobile phase consisting of methanol-acetonitrile-water (40:40:20, v/v/v). Ambroxol hydrochloride and cetirizine hydrochloride have absorbance maxima at 243 nm and 229 nm, respectively. The isoabsorptive wavelength for both the drugs was 236 nm. For absorbance ratio method developed, wavelengths selected were 243 nm and 236 nm. The proposed methods were successfully applied to the determination of ambroxol hydrochloride and cetirizine hydrochloride in tablets, with high percentage of recovery, good accuracy and acceptable precision. Different analytical performance parameters such as linearity, precision, accuracy, limit of detection, limit of quantitation and robustness were determined according to International Conference on Harmonization ICH Q2B guidelines. Results of analysis of the developed method were compared by performing ANOVA.
Keywords
Ambroxol hydrochloride, cetirizine hydrochloride, RP-HPLC, absorbance ratio method
Introduction
Ambroxol hydrochloride (AM) [trans-4-(2-amino-3,5- dibromobenzylamino) cyclohexanol Hydrochloride] [1] is semi-synthetic derivative of vasicine obtained from Indian shrub Adhatoda vasica. It is a metabolic product of bromhexine. It is used as broncho secretolytic and expectorant drug [2]. It stimulates the transportation of the viscous secretions in the respiratory organs and reduces the stand stillness of the secretions. Several spectrophotometric methods have been reported for the qualitative and quantitative determination of AM from pharmaceutical formulations [3-6]. Various HPLC [7-10], GLC [11,12], LCMS [13] and Capillary electrophoretic [14] methods are also reported for it’s determination from biological fluids. Cetirizine hydrocloride (CE) [2-[4-(4- chlorobenzhydryl) piperazine-1-yl]ethoxyacetic acid] is the carboxylated metabolite of hydroxyzine and having high specifi c affi nity for histamine H1 receptor. It is second generation antihistaminic drug. Literature survey revels that several spectrophotometric [15-18] methods, HPLC methods [19-22], HPLC coupled to tandem mass spectroscopy [23], capillary electrophoretic [24-25] have been also reported for determination of CE from pharmaceutical formulations and biological fl uids.
The present research work describes rapid, accurate, sensitive and reproducible RP-HPLC method and absorbance ratio method for simultaneous determination of AM and CE from the tablet formulation.
Materials and Methods
AM was procured from Litaka Pharmaceuticals, Pune (India) and CE was kindly supplied as gift sample by Mediorals, Satara (India). Hydrochlorothiazide (HT) was supplied by (IPCA) Pharmaceuticals (India). All the reagents used were of analytical reagent grade. A commercial preparation (Relent tablets, Dr. Reddy’s laboratories, India, Batch No: 2073) used for analysis was procured from the local pharmacy. Each film coated tablet contains 60 mg of AM and 5 mg of CE.
HPLC method (method I)
HPLC Model–Jasco PU-2080 equipped with UV/Vis detector (Jasco UV–2075 plus), Column dimensions, HIQ SIL-C18-column (250×4.6 mm i.d., 10 μm particle size)) Kya Tech, Japan with an isocratic mode was used at a fl ow rate of 1.0 ml/min at room temperature with injection volume of 20 μl and wavelength detection at 229 nm). The mobile phase used for RP-HPLC was methanol-acetonitrile-water (40:40:20, v/v/v).
Preparation of standard stock solutions
Standard stock solution containing AM and CE was prepared by dissolving 50 mg of AM and 5 mg CE in 40 ml of methanol. It was then sonicated for 10 min and then fi nal volume of the solution was made up to 50 ml with methanol to get stock solution containing 1000 μg/ml of AM and 100 μg/ml of CE in 50 ml volumetric fl ask. Standard stock solution of HT was prepared by dissolving 5 mg of HT in 40 ml of methanol and then sonicated for 10 min. The fi nal volume of solution was made up to 50 ml with methanol to get 100 μg/ml of HT.
Linearity study
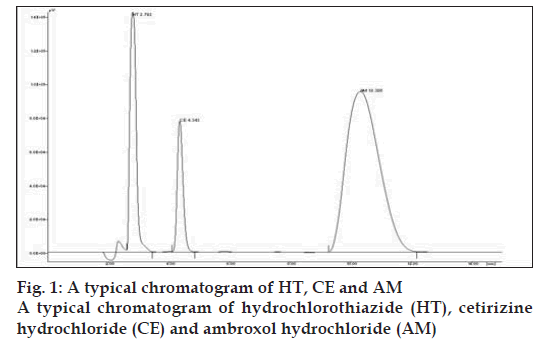
In to a series of 10 ml volumetric fl asks, 1.2 to 4.8 ml of AM and 1 to 3.5 ml of CE solution were pipetted and to each fl ask 0.2 ml of HT was added and then fi nal volume of the solutions was made up to 10 ml with methanol. A 20 μl of sample solution was injected into the injection port of chromatographic system having fixed volume loop injector. Chromatograms were noted and response factor was plotted against concentration to get calibration curve. Chromatogram of physical mixture of AM and CE is shown in fi g. 1. Overlain chromatogram of physical mixture is shown in fi g. 2. The regression equation data for the calibration curve is represented in Table 1 for AM and CE.
| Parameters | Method 1 | Method 2 | ||
|---|---|---|---|---|
| AM | CE | AM | CE | |
| Linearityrange(?g/ml) | 120?420 | 10?35 | 5-50 | 7.5-75 |
| Correlation coefficient | 0.9988 | 0.9993 | -- | -- |
| Regression equation (Y*) | ||||
| Slope(B) | 0.0235 | 0.0671 | -- | -- |
| Intercept(A) | 0.0098 | 0.0152 | -- | -- |
| Precision (%CV) | ||||
| Repeatability of sampleapplicationn=9 | 0.83 | 0.89 | 0.92 | 1.04 |
| Limit of Detection(LOD)** | 0.0328 | 0.0648 | 1.12 | 0.45 |
| Limit of Quantitation(LOQ)** | 0.1076 | 0.2160 | 3.75 | 1.45 |
| Specificity | Specific | Specific | Specific | Specific |
*Y= A+B*C, where C is the concentration in ?g/ml and Y is absorbance unit. **Data obtained by nine determinations.
Table 1: Method Validation Parameters.
Sample preparation
From the triturate of 20 tablets, an amount equivalent to 30 mg of AM and 2.5 mg of CE was weighed and dissolved in 40 ml of methanol by sonicating for 10 min. The solution was fi ltered through 0.22 μ membrane fi lter and then fi nal volume of the solution was made up to 100 ml with methanol to get stock solution containing 300 μg/ml of AM and 25 μg/ml of CE in 100 ml volumetric fl ask. After appropriate dilutions, the solutions were run on HPLC system and the concentration of each analyte was determined with the equations generated. The statistical data obtained after replicate determinations is shown in Table 2.
| Method | Sample | Label claim (mg/tablet) | Concentration estimated* | % concentration estimated* | % RSD |
|---|---|---|---|---|---|
| Method I | Laboratory samples | AM 60 | 60.31 | 100.52 | 1.02 |
| CE 5 | 4.96 | 99.27 | 0.78 | ||
| Tablets | AM 60 | 60.14 | 100.24 | 0.86 | |
| CE 5 | 5.02 | 100.43 | 0.97 | ||
| Method II | Laboratory samples | AM 60 | 60.68 | 101.14 | 0.62 |
| CE 5 | 4.96 | 99.29 | 1.11 | ||
| Tablets | AM 60 | 60.43 | 100.73 | 0.67 | |
| CE 5 | 5.06 | 101.23 | 0.92 |
*Average of nine determinations; % RSD = Relative standard deviation.
Table 2: Results of Analysis
Absorbance ratio method (Method II)
A PC based Jasco V-530 recording spectrophotometer with spectral bandwidth of 2 nm and wavelength accuracy±0.5 nm (with automatic wavelength correction) was employed for all measurements using a matched pair of 10 mm quartz cell. Shimadzu AY 120 analytical balance was used for weighing. Methanol HPLC grade was purchased from Merck Pharmaceuticals (India). Glass distilled water was prepared in the laboratory.
Preparation of standard stock solution
Standard stock solution containing AM and CE was prepared by dissolving 10 mg of AM and CE separately in 20 ml of methanol and then fi nal volume of both the solutions was made up to 100 ml with glass distilled water to get stock solution containing 100 μg/ml of each AM and CE in two different 100 ml volumetric flasks.
Determination of absorptivity
By appropriate dilution of standard drug solutions with glass distilled water, six working standard solutions containing 10, 15, 20, 25, 30 and 35 μg/ml of AM and CE were prepared separately and scanned in the range of 200-350 nm. The absorbance were recorded at the selected wavelengths and the absorptivity and molar absorptivity values were determined for AM and CE. Molar absorptivity values determined for AM at 236 and 243 nm were 8455.76 and 10370.8 cm-1 mol-1 l-1, while molar absorptivity values determined for CE at 236 and 243 nm were 9951.63 and 2217.92 cm-1 mol-1 l-1.
Preparation of mixed standard solutions
Each marketed tablet formulation of the two drug contains AM 60 mg and CE 5 mg. The standard stock solution of AM and CE was used to prepare seven mixed standards containing 30-48 μg/ml of AM and 7.5-12 μg/ml of CE.
Framing Equations
From the molar absorptivity values determined for AM and CE the simultaneous equation is derived for determination of AM and CE in pure drug mixed standards and in its pharmaceutical formulation. The equations framed are C1= [(Q0–Q2)/(Q1-Q2)]×[(A)/ (a1)]-----(1) and C2= [(Q0–Q1)/(Q2-Q1)]×[(A)/(a2)]---- -(2), where C1 is concentration of AM in g dm-3, C2 is concentration of CE in g dm-3, a1 is absorptivity of AM at 236 nm, a2 is absorptivity of CE at 236 nm, Q0 is ratio of absorbance of sample at 243 nm and 236 nm, Q1 is ratio of absorptivity of AM at 243 nm and 236 nm, Q2 is ratio of absorptivity of CE at 243 nm and 236 nm and A is absorbance of sample at isoabsorptive point.
Sample preparation
Marketed tablet formulations containing 60 mg of AM and 5 mg of CE were analyzed by this method. From the triturate of 20 tablets, an amount equivalent to 30 mg of AM and 2.5 mg of CE was weighed and transferred to 100 ml volumetric fl ask. A 5 mg of pure CE was added to the volumetric flask. The contents of the fl ask were dissolved in the 60 ml of the solvent with the aid of ultrasonication for 10 min. The solution was filtered through whatmann filter paper no. 41 and then final volume of the solution was made up to 100 ml with glass double distilled water to get a stock solution containing 300 μg/ml of AM and 75 μg/ml of CE. After appropriate dilutions, the absorbances were measured and the concentration of each analyte was determined with the equations generated. The statistical data obtained after replicate determinations (n = 9) is shown in Table 2.
Results and Discussion
The primary target in developing this liquid chromatographic method was to achieve simultaneous estimation of AM and CE in the pharmaceutical formulation under common conditions that are applicable for routine quality control of this product in laboratories. In RP-HPLC method, mobile phase containing of a mixture of methanol-acetonitrile-water (40:40:20) was selected which produces satisfactory resolution, reasonable retention and acceptable peak shape for both the drugs. The optimization of wavelength was done at different wavelengths for detection by UV detector. In the present investigation, drug solutions of 10 μg/ml of AM and 10 μg/ml of CE were prepared in methanol. AM and CE showed wavelength maxima at 243 nm and 229 nm, respectively. After observing UV spectra of both the drugs, wavelength of 229 nm was selected for further study. Keeping in view the physicochemical properties of both the drugs in the formulation, few drug molecules were tested for use as an internal standard with respect to resolution suitability. HT was found to give good resolution and accurate and precise quantitative results. A fl ow rate of 1 ml/min resulted in optimum retention times with good resolution and with all the drug components and internal standards eluting within 15 min.
The tablet matrix was also estimated to check interference if any, from the excipients used in the tablet matrix. No signifi cant peaks from tablet matrix were observed in the developed chromatograms indicated there is no interference from the excipients of the tablet matrix. The retention time is 2.792 min for HT, 4.155 min for CE and 10.495 min for AM, respectively. The run time is less than 15 min.
In absorbance ratio method, methanol: water (1:4) was selected as common solvent. Drug solutions of concentration 10 μg/ml of AM and CE separately were prepared by appropriate dilution of stock solutions. These drug solutions were scanned in the range of 200 to 350 nm to determine wavelength of maximum absorption and isoabsorptive point. AM shows characteristic peaks at 207, 243 and 306 nm while absorbance maxima for CE is 229 nm, respectively. The isoabsorptive point was obtained at 236 nm. The wavelengths selected were 243 nm (λmax of AM) and 236 nm (isoabsorptive point). The overlain spectra of AM and CE is shown in fi g. 3.
The linearity of responses of the method I for both drugs was verified at six concentration levels 120 to 480 μg/ml for AM and 10-35 μg/ml for CE, respectively. The calibration curve was constructed by plotting response against concentration of the drug. The results showed that an excellent correlation existed between peak area and concentration of each drug within the concentration range tested by this method. The quantitation limit of an individual analyte is the lowest concentration of analyte in a sample, which can be established at a signal to noise ratio of 10. The LOQ of AM and CE was found to be 0.1076 and 0.2160 μg/ml, respectively. The detection limit of an individual analyte is the lowest amount of analyte in a sample, which can be detected but not necessarily quantitated as an exact value. The LOD of AM and CE was found to be 0.0328 and 0.0648 μg/ml, respectively. The data for LOD and LOQ is given in Table 1.
Accuracy was determined by applying developed method to synthetic mixtures of excipients to which known amounts of each drug corresponding to 50, 100 and 150 percent of label claim had been added.
The accuracy was then calculated as the percentage of analyte recovered from the tablet matrix. Mean recovery (mean percentage±standard deviation) values were 101.26±1.18 and 99.14±0.25 for AM and CE, respectively. The data for accuracy is given in Table 3.
| Method | Label claim (mg/tablet) | Amount added (%) | Total amount added (mg) | concentration recovered* | % Recovery ±SD | % RSD |
|---|---|---|---|---|---|---|
| (mg)±SD | ||||||
| Method I | AM 60 | 50 | 90 | 90.42 | 100.46±0.93 | 0.92 |
| 100 | 120 | 120.89 | 100.74±0.55 | 0.56 | ||
| 150 | 150 | 149.23 | 99.48±0.89 | 0.88 | ||
| CE 5 | 50 | 7.5 | 7.61 | 101.46±0.62 | 0.63 | |
| 100 | 10 | 10.11 | 101.1±0.95 | 0.96 | ||
| 150 | 12.5 | 12.69 | 101.52±1.03 | 1.04 | ||
| Method II | AM 60 | 50 | 90 | 90.79 | 100.87±1.02 | 1.01 |
| 100 | 120 | 119.20 | 99.33±1.11 | 1.12 | ||
| 150 | 150 | 148.57 | 99.04±0.82 | 0.83 | ||
| CE 5 | 50 | 7.5 | 7.40 | 98.66±1.06 | 1.06 | |
| 100 | 10 | 10.09 | 100.90±1.10 | 1.09 | ||
| 150 | 12.5 | 12.74 | 101.92±0.76 | 0.77 |
*indicates that each value is a mean±standard deviation of three determinations;%RSD = Relative standard deviation
Table 3: Recovery Studies.
The linearity of responses of the method II for both the drugs was verified by preparing different concentration levels 1 to 50 μg/ml and 2 to 80 μg/ ml for AM and CE, respectively. Beer’s law was obeyed in the concentration range of 5 to 50 μg/ml and 7.5 to 75 μg/ml for AM and CE, respectively. The LOQ of AM and CE were found to be 3.75 and 1.45 μg/ml, respectively. The LOD of AM and CE was found to be 1.12 and 0.45 μg/ml, respectively. The data for LOD and LOQ is given in Table 1.
The accuracy was calculated as the percentage of analyte recovered from the tablet matrix. Mean recoveries (mean percentage±standard deviation) values were 99.82±0.62 and 100.35±1.26 for AM and CE, respectively. Results of recovery study are close to the 100 percentage indicating non-interference of common excipients used in the tablet formulation. The data for recovery studies and precision is given in Tables 3 and 4, respectively.
| Label claim (mg/tablet) | Concentration μg/ml |
Intra-day precision % concentration* |
Inet-day precision % concentration |
|
|---|---|---|---|---|
| ±% RSD | ±% RSD | |||
| Method I | AM 60 | 10 | 100.41±0.68 | 100.62±1.14 |
| 20 | 99.32±0.99 | 100.87±1.05 | ||
| 30 | 100.13±0.72 | 100.45±1.13 | ||
| CE 5 | 06 | 99.31±1.06 | 98.89±0.87 | |
| 12 | 102.19±0.47 | 101.87±0.41 | ||
| 18 | 99.58±1.02 | 100.29±1.05 | ||
| Method II | AM 60 | 40 | 99.78±1.98 | 99.01±1.12 |
| 80 | 100.01±0.84 | 100.10±1.58 | ||
| 120 | 101.27±1.25 | 101.00±1.57 | ||
| CE 5 | 25 | 99.72±0.80 | 100.91±0.90 | |
| 50 | 100.93±1.06 | 100.85±1.18 | ||
| 75 | 100.53±0.92 | 98.97±0.71 |
The results of analysis of methods developed were compared with the reported HPTLC method by performing two way anova studies. Anova studies were performed by using software Graphpad Prism 5.0. F test value at confidence interval 99% was found to be less than table F value. Since the F test values is less than the table F value, the difference in the results of analysis between the developed methods is not signifi cant. Results of analysis of anova studies are given in Table 5.
| Source of variation | Degree of freedom | Sum of squares | Mean square | F |
|---|---|---|---|---|
| Interaction | 2 | 1.095 | 0.5475 | 1.064 |
| Column | 2 | 0.2740 | 0.1370 | 0.2662 |
| Row Factor | 1 | 9.783 | 9.783 | 19.01 |
| Residual | 12 | 6.176 | 0.5146 | -- |
| Total | 17 | 17.328 | 10.9821 | -- |
Table 5: Classification For Medical Devices By Australian Register For Therapeutic Goods 7.
AM and CE was simultaneously determined in tablet matrix using two different analytical methods. The methods developed are simple, accurate, rapid, sensitive and specifi c. RP-HPLC and absorbance ratio spectrophotometry may be recommended for routine and quality control analysis of investigated drugs in two component pharmaceutical preparations.
Acknowledgements
The authors are thankful to Litaka Pharmaceuticals Pvt. Ltd., Mediorals Ltd., Mumbai for supplying gift samples of AM and CE to carry out this research work. The team acknowledges Bharati Vidyapeeth College of Pharmacy, Kolhapur for providing the necessary facilities to carry out this work.
References
- Reynolds JE. Martindale; The Extra Pharmacopoeia. 31st ed. London: Published By Direction of The Council of The Royal Pharmaceutical Society of Great Britain and Prepared in The Society?s Publication Department; 1996. p. 1062.
- Budavari S. The Merck Index: An Encyclopedia of Chemicals, Drugs and Biologicals, 12th ed. New Jersey: Published By Merck Research Laboratories Division of Merck and Co., Inc. Whitehouse Station: 1996. p. 67.
- Kuchekar BS, Shinde GS, Naikwadi IT, Todkar KJ, Kharade SV. Spectrophotometric Estimation of Ambroxol Hydrochloride in Tablets. Indian J Pharm Sci 2003;30:193-5.
- Reddy MN, Rao KV, Swapna M, Sankar DG. Spectrophotometric Determination of Ambroxol. East Pharmacist 1998;125-26.
- Zafer D, Hasan B, Nilgun GG. Quantitative Determination of Ambroxol in Tablets by Derivative UV Spectrophotometric Method and HPLC. J Pharm Biomed Anal 2003;31:867-72.
- Gunawan I, Ratna H. Quantitative Determination of Ambroxol Hydrochloride in Tablets. J Pharm Biomed Anal 1993;11:781-4.
- Maarit H, Coral B. Validation of an HPLC Method for The Quantification of Ambroxol Hydrochloride and Benzoic Acid in A Syrup as Pharmaceutical Form Stress Test for Stability Evaluation. J Pharm Biomed Anal 2001;24:1005-10.
- Botterbolm MHA. Rapid and Sensitive Determination of Ambroxol in Human Plasma and Urine by High-Performance Liquid Chromatography. J Chrom B 1987;421:211-5.
- Flores-Murrieta FJ, Hoyo-Vadillo C., Hong E. Castaneda-Hernandez G., Assay of Ambroxol in Human Plasma by High-Performance Liquid Chromatography with Amperometric Detection. J Chrom B 1989;490:464-9.
- Koundrourellis JE., Eleftheria TM, Theodora AB. High Performance Liquid Chromatographic Determination of Ambroxol in the Presence of Different Preservatives in Pharmaceutical Formulations. J Pharm Biomed Anal 2000;23:469-75.
- Colombo L, Marcucci F, Marini MG, Pierfederici P, Mussini E. Determination of Ambroxol in Biological Material by Gas Chromatography with Electron Capture Detection. J Chrom B 1990;530:141-7.
- Schmid J. Assay of Ambroxol in Biological Fluids by Capillary Gas-Liquid Chromatography. J Chrom B 1987;414:65-75.
- Hohyun K., Jeong-Yeon Y, Sang BH, Hee JL, Kyung RL. Determination of Ambroxol in Human Plasma by LC-MS/MS. J Pharm Biomed Anal 2003;32:209-16.
- 14. Pospisilova M, Polasek M., Jokl V. Determination of Ambroxol and Bromhexine in Pharmaceuticals by Capillary Isotachophoresis. J Pharm Biomed Anal 2001;24:421-8.
- Prakash MS, Sundarapandian M, Meena S, Nagarjan MS. Spectrophotometric Determination of Cetirizine Dihydrochloride in Bulk Drug and Pharmaceutical Formulations. Indian Drugs 2000;37:211-2.
- Ramesh KC, Melwanki MB, Gowda BG, Seetharamappa J, Keshavayya J. A New Spectrophotometric Method for the Determination of Cetirizine Dihydrochloride in Pharmaceutical Preparations and Biological Samples. Indian J Pharm Sci 2002;64:455-8.
- El Walily AFM, Korany MA, El Gindy A, Bedair MF. Spectrophotometric and High Performance Liquid Chromatographic Determination of Cetirizine Dihydrochloride in Pharmaceutical Tablets. J Pharm Biomed Anal 1998;17:435-42.
- Tomas PR, Carmen ML, Antonio S, Eva B. Sensitive Method for the Determination of Ambroxol in Body Fluids by Capillary Electrophoresis and Fluorescence Detection. J Chrom B 2000;742:205-10.
- Zaater MF, Tahboub YR, Najib NM. RP-HPLC Method for Determination of Cetirizine in Serum. J Pharm Biomed Anal 2000;22:739-44.
- Jaber AMY, Al Sherife HA, El Omari MM, Badwan AA. Determination of Cetirizine Dihydrochloride, Related Impurities and Preservatives in Oral Solution and Tablet Dosage Forms Using HPLC. J Pharm Biomed Anal 2004;36:341-50.
- Sun OC, Seok HL, Hak SK, Eun JK and Hae-Young PC. Stereoselective Determination of Cetirizine and Studies on Pharmacokinetics in Rat Plasma. J Chrom B 2000;744:201-6.
- Rosseel MT, Lefebvre RA. Determination of Cetirizine In Human Urine By High-Performance Liquid Chromatography. J Chrom B 1991;565:504-10.
- 23. Rudaz S, Souverain S, Schelling C, Deleers M, Klomp A, Norris A Vu TL, et al. Development and Validation of a Heart-Cutting Liquid Chromatography-Mass Spectrometry Method for the Determination of Process-Related Substances in Cetirizine Tablets. Anal Chem Acta 2003;492:271-82.
- Ann VE, Yvette M. Chiral Separation by Using Capillary Electrophoresis. Electrophoresis 2006;27:2376-85.
- Peter M, Iva VK, Emil H. Enantioselective Analysis of Cetirizine in Pharmaceuticals by Cyclodextrin-Mediated Capillary Electrophoresis. J Sep Sci 2005;28:1278-84.