- *Corresponding Author:
- A. S. Bhavsar
Department of Pharmaceutical Chemistry, R. C. Patel College of Pharmacy, Karvand Naka, Shirpur-425 405, India
E-mail: gstalele@rediffmail.com
| Date of Submission | 13 June 2005 |
| Date of Revision | 1 November 2005 |
| Date of Acceptance | 4 October 2006 |
| Indian J Pharm Sci, 2006, 68 (5): 641-643 |
Abstract
A reverse phase high performance liquid chromatography method was developed for simultaneous estimation of tizanidine HCl and valdecoxib in tablet formulation. The separation was achieved by Luna C 18 column and acetonitrile: phosphate buffer pH 3.5 (50:50 v/v) as eluent, at a flow rate of 0.5 ml/min. Detection was carried out at 227 nm. Etoricoxib was used as an internal standard. The retention time of tizanidine and valdecoxib was found to be 4.43 and 16.60 min, respectively. The method was validated for linearity, accuracy and precision. Linearity for tizanidine and valdecoxib were in the range 0.4-2.0 µg/ml and 4-20 µg/ml, respectively. The developed method was found to be accurate, precise and selective for simultaneous estimation of tizanidine and valdecoxib in tablets.
Tizanidine HCl (TZ) is 5-chloro-4-{2-imidazolin-2-yl amino}-2,1,3-benzothiadiazole-4-amine [1], which is used as centrally acting muscle relaxant. Valdecoxib (VAL) is [4- (5-methyl-3-phenyl-4-isoxazolyl) benzenesulfonamide is the newest addition to the group of non-steroidal antiinflammatory drugs (NSAIDs) known as selective cox- 2 inhibitor. This drug has been recently approved by the USFDA for treatment of rheumatoid arthritis, osteoarthritis and pain [6-12]. TZ and VAL are not official in any pharmacopoeia. A tablet formulation containing 2 mg of TZ and 20 mg of VAL is available in market (Zulu-V, Altoz Life Science). A survey of literature revealed that few chromatographic and Spectrophotometric methods are reported for determination of TZ and VAL individually [8-11]. However there is no HPLC method reported for simultaneous determination of TZ and VAL from combine dosage form. The present work describes a simple, precise and accurate RP-HPLC method for simultaneous estimation of TZ and VAL in tablets.
The drug samples, TZ and VAL were obtained as gift samples from the Emcure Pharmaceuticals Pvt. Ltd., Pune. HPLC grade acetonitrile, potassium dihydrogen orthophosphate AR and phosphoric acid AR were purchased from Merck Co. Mumbai. and S. D. Fine Chemicals, Mumbai, respectively.
A gradient high performance liquid chromatography (Shimadzu HPLC class LC-10 series) with two LC-10 AT- VP pumps, variable wavelength programmable PDA detector SPD-10 AVP, SCL-10 AVP system controller (Shimadzu) and operating software Shimadzu class LC-10 data station was used. The chromatography column used was reverse phase Luna C18 column (250 mm×4.6 mm i.d, particle size 5 μ).
A mixture of acetonitrile and 10 mM phosphate buffer (adjusted to pH 3.5 using orthophosphoric acid) in the ratio 50:50 v/v was used as mobile phase and was filtered before use through 0.45 μ Millipore membrane filter. The flow rate of mobile phase was maintained at 0.5 ml/min. Detection was carried out at 227 nm at the room temperature.
Standard stock solution of VAL, TZ and etoricoxib (1 mg/ml) was prepared in mobile phase. From the standard stock solutions, mixed standard solution was prepared containing 0.8 μg/ml of TZ, 8 μg/ml of VAL and 10 μg/ml of etoricoxib. Twenty tablets, each containing 2 mg of TZ and 20 mg of VAL were weighed and finely powdered. A quantity of powder equivalent to 2 mg of TZ and 20 mg of VAL was weighed and transferred to 100 ml volumetric flask containing 50 ml mobile phase. The mixture was sonicated for 10 min. The volume was made up to 100 ml with mobile phase. Further dilutions were made to get a concentration of 0.8 μg/ml of TZ, 8 μg/ml of VAL and 10 μg/ml of etoricoxib as internal standard (theoretical value). The contents were vortexed and filtered through 0.22 μ membrane filter. Twenty microliters of the test and standard solutions were injected separately and chromatograms were recorded upto 20 min.
The present investigation was aimed at developing a simple, precise and accurate HPLC method to estimate TZ and VAL in tablet using the widely used RP-HPLC C18 column (Luna). The mobile phase was optimized with acetonitrile and 10 mM Potassium dihydrogen ortho phosphate buffer (pH 3.5) in the proportion of 50:50 v/v. With the above mentioned composition of mobile phase a good resolution between TZ and VAL was achieved. UV detection was carried out at 227 nm as both the drugs showed good absorbance at this wavelength.
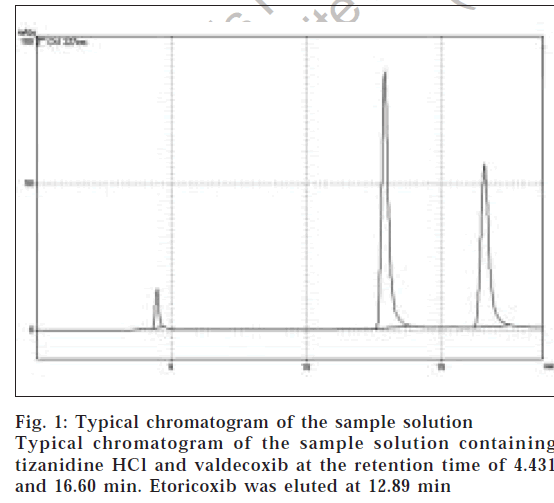
The retention time of TZ and VAL was found to be 4.43 and 16.60 min, respectively. A typical chromatogram of the test solution is shown in the fig. 1. The capacity factor of VAL was found to be 2.72. The peak shape of both drugs were symmetrical and asymmetric factor was lesser than 2.0. The response factor (peak area ratio of standard peak area and internal standard peak area) of the standard and test solution was calculated. The proposed method was validated as per ICH guidelines. Each of the samples was injected 6 times and the retention time was observed in all the cases. Precision of proposed method (RSD) was found to be 0.65% for TZ and 0.08% for VAL. The low RSD value indicated that proposed method had good precision. Linearity experiments were performed thrice for the both the compounds and the response was found to be linear in the range of 0.4-2.0 μg/ml for TZ and 4-20 μg/ml for VAL. Linearity of TZ and VAL was plotted by a graph of response factor versus concentration. The correlation coefficient (r) values (n=3) for TZ and VAL were 0.9993 and 0.9994, respectively. Accuracy of the method was calculated by recovery studies (n=3) at three levels. Amount of drug recovered at each level (n=3) was calculated. Percent recovery study at each level was calculated. Percent recovery study at each level was calculated. Percent recovery study at each level was calculated. Table 1 shows the data from the recovery Parameters Tizanidine Tailing factor 1.57 study for TZ and VAL. The average recovery of TZ and VAL were 100.5 and 100.3%, respectively. The sample recovery in the formulation was in good agreement with the label claim. High percentage recovery showed that the method was free from interferences of excipients used in the formulations. The system suitability parameters of valdecoxib and tizanidine are given in Table 2. Assay of the combination in tablet dosage form was found to be 98.5% of TZ and 98.4% of VAL. The results of study indicate that proposed method is simple, precise, highly accurate and specific.
| Drug | Amount added µg/ml | Amount recovered µg/ml (n=3) | Recovery (%) | Average recovery (%) |
|---|---|---|---|---|
| 0.64 | 0.63 | 98.6 | ||
| TZ | 0.80 | 0.81 | 101.2 | 100.5 |
| 0.96 | 0.97 | 101.7 | ||
| 6.4 | 6.39 | 99.9 | ||
| VAL | 8.0 | 8.10 | 101.3 | 100.3 |
| 9.6 | 9.57 | 99.7 |
TZ = Tizanidine; VAL = Valdecoxib
Table 1:Recovery study
| Parameters | Tizanidine | Valdecoxib |
|---|---|---|
| Tailing factor | 1.57 | 1.41 |
| Theoretical plates | 4820 | 15212 |
| Capacity factor | - | 2.74 |
| Resolution factor | - | 7.52 |
| Calibration range | 0.4-2.0 µg/ml | 4-20 µg/ml |
Table 2: System suitability parameters
References
- Reynolds, J.E.F. Eds., In; Martindale, the Extra Pharmacopoeia, 29th Edn., Pharmaceutical Press, London, 1989, 31.
- Rao, K.K., Satishkumar, M.N., Mathivanan, M., Kirankumar, N and Rao, M.E.B., Indian Drug, 2004, 41, 583.
- Malli, S. andAppalaRaju, S., Asain J. Chem., 2001, 13, 315.
- Mahadik, K.R., Paradkar, A.R., Agrwal, H. and Kaul, N., J. Pharm.Biomed. Analysis, 2003, 33, 545.
- Lee, J., Seo, H.J. and Kim, D.H., Analyst, 2002, 127, 917.
- Ormrod, D., Wellington, K. and Wagstaff, A.J., Drugs, 2002, 62, 2059.
- Gotta, A.W. Opin, Curr., Invest. Drugs, 2002, 3, 240.
- Tally, J.J., Brown, D.L., Carter, J.S., Graneto, M.J., Koboldt, C.M, Masferrer, J.L., Perkins, W.E., Rogers, R.S., Shaffer, A.F., Zang, Y.Y., Zweifel, B.S. and Seibert, K., J. Med. Chem., 2000, 43, 775.
- Sutaria, V.B., Mashru, Rajashree, Sankalia, M.G. and Parikh, Priti,Indian J. Pharm. Sci., 2004, 66, 360.
- Shrinivas, M.S., Shrinivas, L.D. and Sastry, B.S., Asian J Chem., 2004, 16, 1119.
- Ramakrishna, N.V.S., Vishwottam, K.N., Wishu, S. and Koteshwara, M., J. Chromatogr. B, 2004, 802, 271.
- Zhang, Ji.Y., Fast, D.M. and Breau, A.P., J. Pharm. Biomed. Anal., 2003, 33, 61.