- Corresponding Author:
- S. Sharma
Faculty of Pharmaceutical Sciences, Jodhpur National University, Jodhpur - 342 001, 1Sri Balaji College of Pharmacy, Jaipur - 302 001, India
E-mail: sanjuboom_331@yahoo.co.in
| Date of Submission | 16-Nov2009 |
| Date of Revision | 27-Oct-2010 |
| Date of Acceptance | 19-Jan-2011 |
| Indian J Pharm Sci, 2011, 73 (1): 84-88 |
Abstract
A reverse phase high performance liquid chromatography method was developed for simultaneous estimation of nitazoxanide and ofloxacin in tablet formulation. The separation and quantification was achieved by Hiq Sil C18 V Size 4.6 mm Ø *250 mm column in isocratic mode, with mobile phase consisting of acetonitrile-methanol-0.4 M citric acid, (60:30:10, v/v/v). Citric acid used to stabilize nitazoxanide and ofloxacin in mobile phase. The mobile phase was pumped at a rate of 0.6 ml/min and the detection was carried out at 304 nm. The retention time of ofloxacin and nitazoxanide was found to be 3.122 and 5.902 min, respectively. The method was validated for linearity, accuracy, and precision. Linearity for ofloxacin and nitazoxanide were in the range 2-36 mg/ml and 5-90 mg/ml, respectively. The developed method was found to be accurate, precise and selective for simultaneous estimation of ofloxacin and nitazoxanide in tablets.
Keywords
HPLC, nitazoxanide, ofloxacin tablet, validation
Introduction
Nitazoxanide (NTZ), chemically, 2-acetyl- N-(5-nitro-2-thiazolyl)benzamide, is a synthetic nitrothizole derivative and it is new antiprotozal drug used in treatment of cryptosporidiosis in immunocompromised patients, including those with AIDS or HIV infection [1,2]. It is not official in any pharmacopoeia. Ofloxacin (OFX), a fluorinated carboxyquinolone, is a synthetic broad spectrum antimicrobial agent for oral administration. The chemical name of OFX is 9-fluoro-2,3-dihydro-3- methyl-10-(4-methyl-1-piperazynyl)-7-oxo-7H-pyrido- [1,2,3-de]-1,4-benzoxazine-6-carboxylic acid [3,4]. A survey of literature revealed that chromatographic and spectrophotometric methods are reported for determination of OFX and NTZ individually [5-8]. There are few references to the determination of NTZ and OFX in tablets by RP-HPLC and spectrophotometric method [9,10]. As the literature survey revels that no acid stabilized (presence of citric acid in mobile phase improves the stability of nitazoxanide) chromatographic method has been reported so far for the simultaneous determination of this OFX and NTZ in solid dosage form [11-13].
High performance liquid chromatograph instrument model-Jasco PU-1580 equipped with UV/Vis detector (Jasco UV-1570/1575), column dimensions HIQ SIL C18 column (250×4.6 mm i.d.) was used. Digital weighing balance model GR-200 (A and D Company Limited, Mumbai, India) and ultra sonicator (Enertech Electronics Ltd. Mumbai, India) was also used.
Working standard of OFX was obtained as gift sample from ONS Pharmaceuticals Ltd., Jaipur, India and NTZ was obtained as gift sample from Alembic Ltd., Vadodara, India. HPLC grade acetonitrile and methanol (Loba Chem, Mumbai) were used for the study. Citric acid AR was used for preparation of 0.4 M citric acid solution. In house produced Mili-Q water was used. Two marketed formulations with brand names, Nitazet-O (Glenmark Pharmaceutical Ltd., Mumbai, India) and Nizonide-O (Lupin Pharmaceutical Ltd., Mumbai, India) were procured from local pharmacy.
To simplify the tediousness of preparation of mobile phase, only the mixture of water and organic solvents, including 0.4 M citric acid, methanol and acetonitrile. 0.4 M citric acid were used instead of water. The mobile phase was set as (acetonitrilemethanol- 0.4 M citric acid, (60:30:10, v/v/v). Here the parent peak was separated and showed better resolution, theoretical plate count and symmetry. OFX was accurately weighed (about 10 mg) and transferred into a 100 ml volumetric flask. The final concentration of the drug solution was brought to 100 µg/ml by diluting with methanol. NTZ was accurately weighed (about 25 mg) and transferred into 100 ml volumetric flask. The final concentration of the drug solution was brought to 250 µg/ml by diluting with methanol. The solutions were filtered through the 0.45 mm Millipore filter and degassed under ultrasonic bath prior to use.
The commercial tablet formulation of OFX and NTZ are available in the strength of 1:2.5. Based on this ratio mixed standard solution were prepared containing 100 µg/ml of OFX and 250 µg/ml of NTZ. The working standard solutions of both drugs were used to prepare different mixed standard by serial dilution technique.
Twenty tablets of two pharmaceutical companies were accurately weighed, finely powdered and triturated well. The powder sample equivalent to 100 mg of OFX and 250 mg of NTZ was weighed and transferred to a 100 ml volumetric flask and about 80 ml of HPLC grade methanol was added. The content was sonicated for 15 min to dissolve. The volume was made up to the mark with HPLC grade methanol. This solution was filtered through 0.45 mm membrane filter and further diluted so as to obtain concentration 16 mg/ml of OFX and 40 mg/ml of NTZ and sonicated to degas. The prepared solution was injected five replicates into the HPLC system and the observations were recorded.
The specificity of the RP-HPLC method was studied by injecting the placebo (lactose 50 mg, magnesium Stearate 2 mg, microcrystalline cellulose 50 mg, Starch 55 mg and Talc 5 mg) of the tablets prepared in the diluents as per the procedure applied to sample solution. No peak was detected at the retention time of NTZ and OFX hence proving the specificity of the method. Linearity for both drugs was checked by preparing standard solutions at 18 different concentration levels ranging from 5-90 mg/ml and 2-36 mg/ml for NTZ and OFX, respectively. From the mean of standard peak area observed and the respective concentration values, the response ratios (response factor) were found out by dividing the peak area with the respective concentration. The response curve was plotted between standard (peak area/ concentration) v/s concentration. To study validity and reproducibility of proposed method, recovery studies were carried out by applying the standard addition method. A known amount of drug corresponding to 80, 100 and 120% of the label claim was added to preanalysed sample. The percentage recoveries were calculated six times, at each level of recovery. Repeatability was performed by inter-day and intraday precision. The intra-day precision was determined by analyzed the three different concentration of drug for three times within one day. An interday precision was determined by analyzed the three different concentration of drug for three days in a week. To determine the robustness of the developed method, experimental conditions were deliberately altered and performed the assay of NTZ and OFX bulk samples against its working standard. Various chromatographic conditions used were, 0.5 ml/min and 0.7 ml/min flow rate and 56:36:08 and 66:26:08 v/v/v ratio of acetonitrile:methanol:0.4M citric acid [14,15].
The solution stability of NTZ and OFX in the assay method was carried out by leaving both the test solution of sample and reference standard in tightly capped volumetric flask at room temperature for 8 h. The same solutions were assayed for every one hr interval up to the study period. System suitability testing is an integral part of many analytical procedures. The tests are based on the concept that the equipment, electronics, analytical operations and samples to be analyzed constitute an integral system that can be evaluated as such.
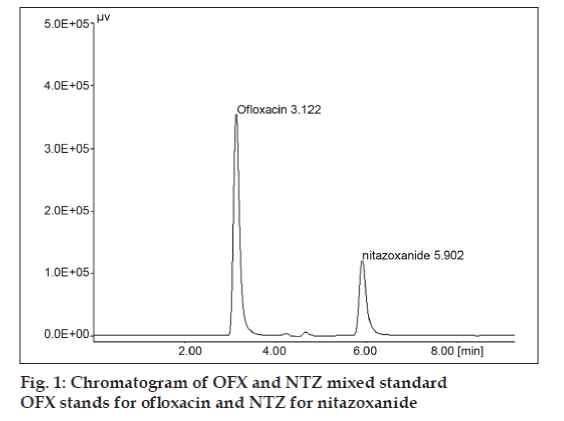
The development of an analytical method for the determination of drugs by HPLC has received considerable attention in recent years because of their importance in quality control of drug products. The objective of this study was to develop a rapid and sensitive HPLC method for estimation of NTZ and OFX in tablet formulations using the most commonly employed RP C-18 column with UV detection. The mobile phase was optimized with acetonitrile-methanol-0.4M citric acid, (60:30:10, v/v/v). From the overlain spectrum of nitazoxanide and ofloxacin, wavelength was selected, at 304 nm, isoabsorptive point for both the drugs. Good resolution was carried out at 304 nm and both drugs showed good absorbance at this wavelength with minimum interference of the other drug. The chromatogram obtained is shown in fig. 1.
Two brands of tablets were analyzed and amount of drugs was determined by proposed method; it was in good agreement with the label claim. Results of NTZ estimation in different tablet dosage form are shown in Table 1. All parameters of these proposed method was validated as per the ICH guidelines.
| Brand | Drug | Labeled amount (mg) | Amount found a (mg) | % of Labeled amount a | % RSD |
|---|---|---|---|---|---|
| Nitazet-O | OFLO | 200 | 199.98±0.704 | 99.85±0.510 | 0.511 |
| NTZ | 500 | 499.8±0.768 | 99.96±0.160 | 0.160 | |
| Nizonide-O | OFLO | 200 | 199.8±1.692 | 99.31±0.853 | 0.854 |
| NTZ | 500 | 499.8±1.101 | 99.97±0.221 | 0.220 |
a: Data represents mean±SD; n=5; OFLO is ofloxacin and NTZ is nitazoxanide; RSD: Relative standard deviation
Table 1: Summary of simultaneous estimation of ofx and ntz in tablets
No peak was detected at the retention time of NTZ and OFX hence proving the specificity of the method. A linearity experiment shows correlation coefficient for OFX and NTZ is 0.9997 and 0.9998 respectively over a range of 2-36 mg/ml for OFX and 5-90 mg/ ml for NTZ. The regression of OFX and NTZ in concentrations over its peak area were found to be y′=210746x′+31939 and y=43913x+6209.5, where y is the peak area and x is the concentrations for NTZ and y′ is the peak area and x′ is the concentrations for OFX. The regression equation was used to estimate the amount of NTZ and OFX, either in tablet formulations or in validation study (precision and accuracy).
The accuracy of the method was established using recovery technique i.e. by external addition of standard in to a preanalysed sample at three different levels. The % mean recovery of OFX was found to be 99.93% (sample) and 99.64% (Synthetic mixture) and for NTZ was found to be 99.86% (sample) and 99.27% (Synthetic mixture, Table 2). An accuracy criterion for an assay method is that the mean recovery should desirably be 100±2% at each concentration over the range of 80-120% of target concentration. Since the mean % recovery varies for both drug from 99.27 to 99.93% and are within the desirable confidence interval of 98-102%, (Table 2) it can be said that the proposed method is accurate. In precision experiment, (repeatability study) relative standard deviation for OFX is 0.451 and NTZ is 0.431. It was concluded that the analytical technique showed good repeatability. The calibration curves were repeated three times in a day at three different concentrations and the calibration curve was repeated three times on three different days at three different concentrations. These values confirmed the intraday and interday precision of the method. The intra-day and inter-day % RSD values were calculated which were found to be in the range of 0.62-0.83 for OFX and 0.26-0.57 for NTZ (Table 3). Hence, method at selected wavelength was found to be precise. For the robustness testing, the chromatographic conditions were changed and in all varied chromatographic conditions the resolution between OFX and NTZ was greater than 2.0, illustrating the robustness of the method. During solution stability testing, no significant change was observed in the content of NTZ and OFX. Hence it was concluded that NTZ and OFX solutions are stable for at least 8 h in the developed method. The system suitability parameters were also evaluated and results are summarized in Table 4.
| Recovery study of Nitazet-O | Recovery study of synthetic mixture | |||||
|---|---|---|---|---|---|---|
| OFX | NTZ | OFX | NTZ | |||
| % Recovery | ||||||
| 1 | 99.75 | 99.59 | 100.40 | 98.78 | ||
| 2 | 99.52 | 101.10 | 99.03 | 100.26 | ||
| 3 | 100.54 | 98.89 | 99.50 | 98.77 | ||
| % Mean recovery | 99.93 | 99.86 | 99.64 | 99.27 | ||
| SD | 0.53 | 1.13 | 0.70 | 0.86 | ||
| RSD (%) | 0.54 | 1.03 | 0.69 | 0.86 | ||
| RSD: Relative standard deviation | ||||||
RSD: Relative standard deviation
Table 2: Summary of recovery study
| Intraday studies | Intraday 1 | Intraday 2 | Intraday 3 | |
|---|---|---|---|---|
| OFX | Mean conc. (mg/ml) ±SD | 8.03±0.075 | 8.08±0.132 | 8.20±0.115 |
| 16.01±0.110 | 16.03±0.080 | 16.10±0.133 | ||
| 24.03±0.065 | 24.03±0.085 | 24.03±0.045 | ||
| Mean RSD (%) | 0.63 | 0.83 | 0.8 | |
| NTZ | Mean conc. (mg/ml) ±SD | 20.15±0.185 | 20.01±0.045 | 20.10±0.160 |
| 40.44±0.204 | 40.05±0.138 | 40.13±0.165 | ||
| 60.06±0.170 | 60.10±0.119 | 60.11±0.160 | ||
| Mean RSD (%) | 0.56 | 0.26 | 0.57 | |
| Interday studies | Interday 1 | Interday 2 | Interday 3 | |
| OFX | Mean conc. (mg/ml) ±SD | 8.11±0.090 | 8.10±0.113 | 8.07±0.043 |
| 16.06±0.072 | 16.14±0.085 | 16.11±0.175 | ||
| 24.03±0.076 | 23.98±0.031 | 60.14±0.190 | ||
| Mean RSD (%) | 0.62 | 0.69 | 0.79 | |
| NTZ | Mean conc. (mg/ml) ±SD | 20.10±0.070 | 20.13±0.165 | 20.19±0.133 |
| 40.14±0.170 | 40.13±0.153 | 40.09±0.184 | ||
| 60.04±0.081 | 60.23±0.105 | 60.14±0.190 | ||
| Mean RSD (%) | 0.63 | 0.46 | 0.47 |
SD: Standard deviation, RSD: Relative standard deviation
Table 3: Summary of intra-day and inter-day studies
| Parameter | Limit | Result ± S.D. |
|---|---|---|
| Resolution | Rs> 2 | 6.44±.052 |
| Asymmetry | T £ 2 | OFX 1.30±0.0098 |
| NTZ 1.27±0.024 | ||
| Theoretical plate | N > 2000 | OFX 3353±15.65 |
| NTZ 8770±6.675 | ||
| Retention time | … | OFX 3.122±.0013 |
| NTZ 5.903±.0017 | ||
| Capacity factor | 2<K<10 | 3.740.362 |
Table 4:Data evaluation of system suitability parameter
The proposed method is found to be fast, precise, accurate and selective and can be utilized as a quality control tool for simultaneous estimation of NTZ and OFX in tablet dosage form.
Acknowledgements
The authors are thankful to Alembic Pharmaceutical, Vadodara for providing the gift samples of the drug.
References
- O′Neil MJ, editor. In: The Merk Index-An Encyclopedia of chemicals. Drugs and Biologicals. 13th ed. Whitehouse Station, NJ: Merck and Co. Inc.; 2001. p. 6596.
- Sweetman SC, editor. Martindale-The Complete Drug Reference. 33rd ed. London (UK): Pharmaceutical Press; 2002. p. 598.
- Tripathi KD. Essential Medical Pharmacology. 3rd ed. New Delhi: Jaypee Brothers; 1995.
- Clarke′s Analysis of Drugs and Poisons. 3rd ed. Vol. 2. London: The Pharmaceutical Press; 2004.
- Narayan LK, Manohara YN, Gurupadayya BM. Development and Validation of Spectrophotometric method for the estimation of nitazoxanide in bulk drug and tablets. Indian J Pharm Sci 2007;69:147-9.
- Kapse GK, Prabhakar G, Raju SA. Spectrophotometric methods for the estimation of nitazoxanide in pharmaceutical formulation. Indian J Pharm Sci 2006;68:403-6.
- Narayan LK, Manohara YN, Appla RS. Development and validation of RP-HPLC method for the estimation of nitazoxanide in bulk drug and tablets. Indian Drugs 2005;43:503-6.
- Behl A, Ahuja M, Dhake S. RP-HPLC method for the quantification of ofloxacin in tables. Indian J Pharm Sci 2005;67:479-81.
- Kalta RR, Sharma R, Chaturvedi SC. Simultaneous RPHPLC determination of nitazoxanide and ofloxacin in combined tablet dosage form. Indian J Pharm Sci 2008;70:491-4.
- Pattanayak P, Chaudury YP, Sharma R, Mohapatra P, Setty DK. Simultaneous spectrophotometric estimation of nitazoxanide and ofloxacin in combined tablet dosage form. Research J Pharm and Tech 2009;2:291-3.
- Kamble NS, Venkaachalm A. High performance liquid chromatography determination of ornidazole and ofloxacin in Solid dosage form. Indian Drugs 2005;42:723-5.
- Samanidou VF, Demetrious CE. Direct determination of four Fluroquinolones, enoxacin, norfloxacin, ofloxacin, and ciprofloxacin in pharmaceutical and blood serum by HPLC. Anal BioanalChem 2003;375:623-5.
- Mohammed S, Mohsin G, Anwar S. Simultaneous Determination of ofloxacin, tetrahydrozoline hydrochloride and prednisolone acetate by HPLC. J Chromatogr Sci 2002;40:429-31.
- Beckett AH, Stenlake JB. Practical Pharmaceutical Chemistry. 4th ed. New Delhi: CBS Publishers and Distributors; 2002.
- Validation of analytical procedure: Methodology Q2B, ICH Harmonized triplicate guidelines. 1996. p. 1-8.