- *Corresponding Author:
- S. D. Rajendran
J. S. S. College of Pharmacy, Ootacamund - 643 001, India
E-mail: sdrajendran@rediffmail.com
| Date of Submission | 6 September 2006 |
| Date of Revision | 24 August 2007 |
| Date of Acceptance | 23 November 2007 |
| Indian J. Pharm. Sci., 2007, 69 (6): 796-799 |
Abstract
A high performance liquid chromatographic method is described for estimation of glibenclamide in human serum. After precipitation with methanol, the separation of glibenclamide and internal standard was accomplished using reversed phase chromatography. The mobile phase, a combination of acetonitrile and 25 mM phosphate buffer (pH 3.5) at 3:2 ratio was run isocraticaly through a C18 analytical column. The UV detection was done at 253 nm for glibenclamide. Analytical run time was less than 12 min. Mean recovery was 92% for 0.5 µg/ml concentrations. The assay exhibited good linear relationship between peak area ratios and serum concentration. Quantification limit was at least 25 ng/ml of glibenclamide and accuracy and precision were over the concentration range of 50-500 ng/ml. Assay was successfully applied to the measurement of glibenclamide in serum for therapeutic drug monitoring.
Keywords
Glibenclamide, human serum, TDM, RPHPLC
Glibenclamide is an effective, long-acting, second generation sulfonylurea used in the treatment of non-insulin-dependent diabetes mellitus (NIDDM) [1]. It occurs as a white or almost white, odourless crystalline powder. Solubility of the drug in water is approximately 4 μg/ml at pH 4 and 600 μg/ml at pH 9 and 3 mg/ml in alcohol. The drug has a pka of 6.8. It decreases blood glucose levels by stimulating insulin release from functioning beta cells in the pancreas. After prolonged administration, the hypoglycemic effects of the drug appear to be related to extra-pancreatic effects possibly including reduction of basal hepatic glucose production and enhanced peripheral sensitivity to insulin, the later may result either from an increase in the number of insulin receptors or from changes in events subsequent to insulin binding [2].
Studies conducted in western population reveal that the terminal elimination half-life of the drug is 1.4-1.8 h (0.7–3 h) [3]. The usual initial adult dosage is 2.5-5 mg daily. The adult maintenance dose for type II diabetes ranges from 1.25-20 mg daily [4]. The maximum recommended dosage is 20 mg daily. Following oral administration of a single 5 mg dose, the drug appears in the plasma or serum within 15–60 min and average peak plasma or serum concentration of approximately 140-350 ng/ml usually are attained with in 2-4 h [5,6]. The glibenclamide at a concentration of 0.05-10 μg/ml in vitro, is more than 99% bound to serum proteins and its metabolite 4-trans-hydroxyglibenclamide, is more than 97% bound to serum proteins [7]. A number of assay methods for use in pharmacokinetic studies are available for determination of glibenclamide in biological specimen [8-14] (Table 1). Here we describe a high performance liquid chromatographic method for the estimation of glibenclamide in human serum. The method can be used in pharmacokinetic studies of glibenclamide where multiple, serial blood sample collection is desired in individuals.
| Species | Matrix | Volume (ml) | Validated LLQ (ng/ml) | Reference |
|---|---|---|---|---|
| Human | Serum | 1 | 5 | [8] |
| Human | Plasma | 0.2 | 5-10 | [9] |
| Human | Plasma | 0.5 | 10-400 | [10] |
| Human | Serum | 0.5 | NS | [11] |
| Human | Serum | 0.5 | 20-400 | [13] |
| Dog | Serum | 1 | 20 | [14] |
The above table provides the references of the previously published studies on the development of high-performance liquid chromatographic method for the analysis of glibenclamide from blood of animals and human. NS: Not Stated
Table 1: Previously published assay methods for glibenclamide in blood fluids
Materials and Methods
Glibenclamide (Zydus Cadila, India) and glimepride (Micro Laboratories, India) were obtained as gift samples. Methanol, acetonitrile, potassium dihydrogen ortho-phosphate and ortho-phosphoric acid were purchased from Qualigens Fine chemicals (Mumbai, India). Water for analytical purpose was obtained from Milli-Q R-O system.
Chromatographic conditions
The HPLC system consisted of a Shimadzu LC-10AT liquid chromatographic pump, SIL-10a manual injector and SPD-10A UV/Vis UV absorbance detector (Shimadzu, Kyoto, Japan). Data collection, integration and calibration were accomplished using Class VP Chromatography Data System Version 4.2 computer software (Shimadzu, Kyoto, Japan). The chromatographic separations of glibenclamide and internal standard (glimepride) were accomplished using a 250 mm×4.6 mm ID Luna phenomenex 5u C18 analytical column (Phenomenex, USA). A Guard-Pak precolumn module (Phenomenex, USA) containing an ODS cartridge insert was placed serially just before the analytical column. The mobile phase consisted of acetonitrile:25 mM phosphate buffer (pH: 3.5) in a combination of 60:40 v/v. Before use the mobile phase was degassed by passing it through a 0.22 μm filter.
The mobile phase was pumped at an isocratic flow rate of 1 ml/min at room temperature. The UV detection wave length was set at 253 nm. The wavelength of 253 nm represented the UV maximum of glibenclamide in acetonitrile: water in 1:1 ratio.
Assay procedure
A stock solution representing 100 µg/ml of glibenclamide was prepared in acetonitrile: water in 1:1 ratio. These solutions were stored at -20° until use. Glibenclamide was found to remain stable for at least 3 mo. under these conditions, based on our preliminary findings. The working standard solutions were prepared prior to use from the stock solution by sequential dilution with a combination of acetonitrile: water in 90:10 ratio to yield final concentrations of 50, 100, 200, 400, and 500 ng/ml of glibenclamide. The internal standard stock solution was prepared by dissolving 1 mg of glimepride in 100 ml of acetonitrile: water in 1:1 ratio. This solution was stored at -20° until use.
In a 2 ml microcentrifuge tube, 500 μl of serum was added along with 500 μl of internal standard solution. The serum was precipitated by the addition of 500 μl of methanol, and then the tubes were vortexed for 30 s and centrifuged at 5000 g for 8 min. The supernatant was transferred to a clean, similarly labeled tube and was subsequently re-centrifuged for 2 min. The resulted solution was injected in to the HPLC.
Assay parameters
The extraction efficiency was determined by comparing the peak area of known amounts glibenclamide (unextracted) in mobile phase directly injected to peak area of samples containing the same amounts of glibenclamide in plasma after extraction.
Quantification was based on calibration curves constructed using peak area ratios of drug to internal standard vs. nominal concentration. Intra-day reproducibility was tested by using four different concentrations (50, 100, 200, 400, 500 ng/ml). The procedure was repeated on three separate days to allow determination of inter-day precision and accuracy. Intra-day accuracy was estimated based on the mean percentage error, and the inter-day accuracy was calculated as the mean of the intra-day accuracy determinations. The precision, expressed as a percentage, was evaluated by calculating the intra- and inter-day relative standard deviations. The standard drug solutions in varying concentrations ranging from 50 ng/ml to 500 ng/ml were examined by the assay procedure. The peak area was calculated. The calibration curve was plotted using peak area vs concentration of the standard solutions.
Patient samples
Patient serum samples were obtained from patients who were on long term and short term oral glibenclamide therapy. The protocol was approved by the institutional ethics committee. Five blood samples were collected i.e 0.5-2 h, 2-5 h, 5-10 h and 24 h post dose of glibenclamide administration. The samples were placed on ice immediately after collection. Subsequently, serum samples were kept frozen at -70° until analysis. Blank serum samples were collected from volunteers for method validation and were stored at -70°.
Results and Discussion
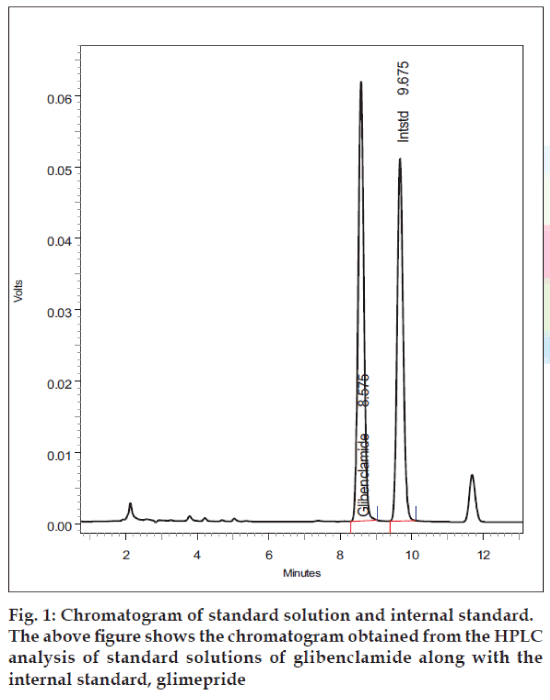
Peaks corresponding to internal standard, and glibenclamide eluted free of interfering substances, at 9.6 and 8.5 min, respectively (fig.1). The analytical run time was 12 min for each serum sample. The mean extraction efficiencies of glibenclamide from 500 μl of serum at a concentration of 50-500 ng/ml were 90-93%. Good linear relationships were obtained between peak area ratios and the corresponding plasma concentration over a range of 50-500 ng/ml of glibenclamide. The calibration curve shows a linear response over the range of concentrations used in the assay procedure. The calibration curve passes through the origin, which justifies the use of single point calibration.
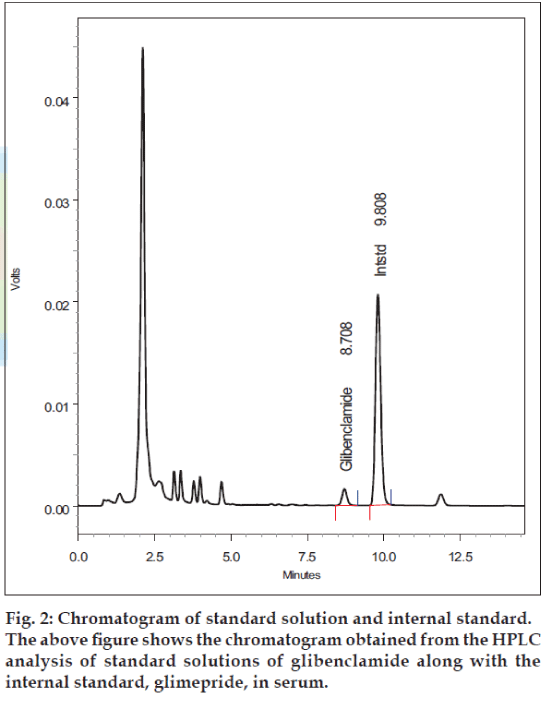
The inter-day and intra-day coefficients of variation were less than 2% (Table 2). Over the range of concentrations from 50-500 ng/ml, the intraday accuracies ranged from 95.1% to 98.9%, and average interday accuracy ranged from 94.9% to 98.8%. Based on this data, the validated lower limit of quantification of the method was 25 ng/ml based on 500 μl of human plasma. In patients taking a dose of 5-15 mg/ day of glibenclamide, serum samples were analyzed using the described HPLC method (fig. 2).
| Drug | Added Concentration (ng/ml) | Intraday (n=5) | Interday (n=5) | ||||
|---|---|---|---|---|---|---|---|
| Observed concentration (ng/ml) | CV % | Accuracy % | Observed concentration (ng/ml) | CV % | Accuracy % | ||
| Glibenclamide | 50 | 47.56 | 1.9728 | 95.13 | 47.46 | 1.1634 | 94.92 |
| 100 | 97.65 | 0.9963 | 97.64 | 97.48 | 0.8689 | 97.48 | |
| 200 | 196.92 | 0.4442 | 98.45 | 196.55 | 0.3853 | 98.27 | |
| 400 | 395.99 | 0.1673 | 98.99 | 395.63 | 0.2476 | 98.90 | |
| 500 | 494.55 | 0.1054 | 98.90 | 494.47 | 0.1697 | 98.89 | |
The above table provides information regarding the intra-day and inter-day precision and accuracy data for the analysis of glibenclamide from human serum samples
Table 2: Intraday and interday precision and accuracy data
The HPLC method described here was accurate and precise, and capable of determining concentrations of glibenclamide in small volumes of human serum. The extraction procedure was simple and the procedure used a commercially available internal standard (glimepride). The method performed well with respect to reproducibility and accuracy over the range of concentrations studied (Table 2). This assay method was rapid; preparation of 110 samples took 8 h from initial protein precipitation to final placements of samples in the HPLC. The chromatographic run time was less than 12 min. The lower limits of quantification and the small plasma necessary for this assay make the assay suitable for studying glibenclamide pharmacokinetics.
References
- Hennessey JV, Bustamante MA, Teter ML. Bedtime dosing of glyburide and the treatment of type II diabetes mellitus. Amer J Med Sci 1994;308:234-8.
- Jaber LA, Antal EJ, Welshman IR. Pharmacokinetics and pharmacodynamics of glyburide in young and elderly patients with non-insulin-dependent diabetes mellitus. Ann Pharmacother 1996;30:472-5.
- McEvoy GK, editor. AHFS Drug Information 2004. Sulfonylureas. Maryland: American Society of Health-System Pharmacists; 2004.
- Rydberg T, Jonsson A, Karissson MO. Concentration-effect relations of glibenclamide and its active metabolites in man: Modelling of pharmacokinetics and pharmacodynamics. Br J ClinPharmacol 1997;43:373-81.
- Melander A, Donnelly R, Rydberg T. Is there a concentration-effect relationship for sulphonylureas? ClinPharmacokinet 1998;34:181-8.
- Feldman JM. A second-generation Sulphonylureahypoglycemic agent. J Phamacother 1985;5:43-62.
- Pearson JG. Pharmacokinetics of Glyburide. Am J Med 1985;79:67-74.
- Uiblein M, Sistovaris N. High performance liquid column and Thin layer chromatographic determination of human serum Glibenclamide at therapeutic levels. J Chromatogr 1982;227:93-101.
- Emilsson H, Sjoberg S, Svedner M. High performance liquid chromatographic determination of Glibenclamide in human plasma and urine. J Chromatogr 1986;383:93-102.
- Niopas I, Daftslos AC. High performance liquid chromatographic method for the determination of Glibenclamide in human plasma and its application to pharmacokinetic studies. J Pharm Biomed Anal 2002;28:653-7.
- Cui HD, Jiang WD, Zhu XX, Guo Y, Karras HO. Pharmacokinetics and relative bioavailability of tablet of micronized Glibenclamide in 4 chineese healthy men. Zhongguo Yao Lie XueBao 1993;14:193-7.
- El-sayd YM, Suleiman MS, Hasan MM. Comparison of the pharmacokinetics and pharmacodynamics of two commercial products containing Glibenclamide. J ClinPharmacolTherToxicol 1989;27:551-7.
- Abdel-Hamid ME, Suleiman MS. A Rapid high performance liquid chromatography assay of Glibenclamide in serum. J Clin Pharm Ther 1989;14:181-8.
- Adams WJ, Krueger DS. Specific and sensitive high performance liquid chromatographic determination of Glyburide. J Pharm Sci 1979;68:1138-40.
- Sartor G, Melander A, Schersten B. Comparative single-dose kinetics and effects of four sulfonylureas in healthy volunteers. J Acta Med Scand 1980;208:301-7.
- Garber A, Marre M, Blonde L, Allavoine T. Influence of initial hyperglycaemia, weight and age on the blood glucose lowering efficacy and incidence of hypoglycaemic symptoms with a single-tablet metformin-glibenclamide therapy (Glucovance) in type 2 diabetes. Diabetes ObestetMetab 2003;5:171-9.
- Scannapieco G, Franzoso F, Grasso M. Severe hypoglycemia caused by Sulfonylureas and Biguanides in a patient with obstructive anuria: Resolution with ureteral stent. Arch ItalUrolAndrol 2002;74:269-70.
- Holstein A, Plaschke A, Hammer C. Characteristics and time course of severe Glimepiride- versus Glibenclamide-induced hypoglycaemia. Eur J ClinPharmacol 2003;59:91-7.