- *Corresponding Author:
- K. B. Charuvil
Biotechnology and Bioinformatics Division,
Saraswathy Thangavelu Centre of Jawaharlal Nehru Tropical Botanic Garden and Research Institute Puthenthope,
Thiruvananthapuram,
Kerala 695586,
India
E-mail: drbijuck@gmail.com
| Date of Received | 06 April 2020 |
| Date of Revision | 19 October 2021 |
| Date of Acceptance | 25 July 2022 |
| Indian J Pharm Sci 2022;84(4):950-958 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Dengue is a vector-borne viral disease caused by Flavivirus. Current treatment and medicine are inadequate to eradicate the vector mosquitoes or to prevent the disease. Traditional healers have remedies for almost all ailments using natural products and plants. The well-known medicinal plant Euphorbia hirta L. is one of the herbs conventionally used for anti-dengue treatment even if the active compound responsible for the exact activity of the plant against the disease is not known. The plant is a rich source of various secondary metabolites. In silico screening of 76 small molecules of drug-value belongs to alkaloids, polyphenols and terpenes derived from the plant against dengue virus non-structural protein-5 protease and human inosine 5'-monophosphate dehydrogenase-II determined 16 biologically active compounds with possible activity to fight against dengue virus. Based on the therapeutic importance and drug-likeness parameters, the two best leads, 2-beta, 16-alpha, 19-trihydroxy-ent-kaurane and kaempferol were selected as promising candidates for developing anti-dengue drugs.
Keywords
Dengue, Euphorbia hirta L., flavivirus, Aedes mosquitoes, vector
Dengue fever is one of the severe health problems during the monsoon periods in India. It is a vector-borne disease transmitted by silent, female urban mosquitoes primarily of Aedes aegypti and Aedes albopictus. The disease spread to tropical and subtropical regions of the world, and 3.9 billion people inhabiting 128 countries are at risk. World Health Organization (WHO) classifies dengue as one of the 17th neglected tropical diseases. Symptoms of dengue start from the 5th d of the bite by an infected mosquito and the symptoms may last for a week or longer. It is self-limiting and characterized by high fever, headache, muscle and joint pain, skin rashes, pain behind the eyes, vomiting, and bleeding from the mouth and nose. The severe form of the disease as Dengue Hemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS) may lead to multisystem involvement and death. The etiological agent of dengue fever is a positive sense single stranded Ribonucleic Acid (RNA) virus that belongs to the family Flaviviridae and the genus Flavivirus. Every year 390 million people are infecting and not less than 96 million people develop the severe form of the disease and undergoing treatment[1]. India is one of the most vulnerable areas of dengue. An estimate of the National Vector Borne Disease Control Programme (NVBDCP) showed that 153 635 new cases with 226 deaths from India in 2017, of which 19 776 new cases and 37 deaths from God’s own land Kerala[2].
The current treatment system of dengue is highly insufficient or practically nil and is limited to vector control measures. Vaccination is one of the unsurpassed dengue prevention methods of treatment. A tetravalent dengue vaccine called Chimeric Yellow Fever Virus- Dengue Virus (DENV) Tetravalent Dengue Vaccine (CYD-TDV or Dengvaxia®) developed by “Sanofi Pasteur” is recommended by some countries. But its low level of protection against serotype-2 and the low vaccine efficacy in young seronegative candidates are of concern. In traditional medicine, plants are used as antipyretic agents to treat various fevers caused by bacteria, viruses, protozoa and other microorganisms. They are the universal manufacturers of a variety of small molecules of therapeutic value. The chemical composition and active ingredients of each plant are different. Their formation depends on the environment and the plants under various stresses. The necessity for plant-based natural medicine is getting higher as they are usually measured safer, non-toxic and less detrimental than synthetic drugs. The healing effect of herbal preparations is an independent or collective action of various small molecules present in them. Anti- dengue active compounds present in plants have not often been studied scientifically by pharmacologists. Current investigation proposed structure based virtual screening and detection of a druggable molecule that formulated the potential medicinal plant Euphorbia hirta (E. hirta) using molecular docking and other analysis tools. The significant cost and time required to separate drug candidates from a collection of natural or synthetic compounds in the early stages of drug development has been greatly reduced by the use of advanced in silico screening methods.
Materials and Methods
Source plant and ligand preparation:
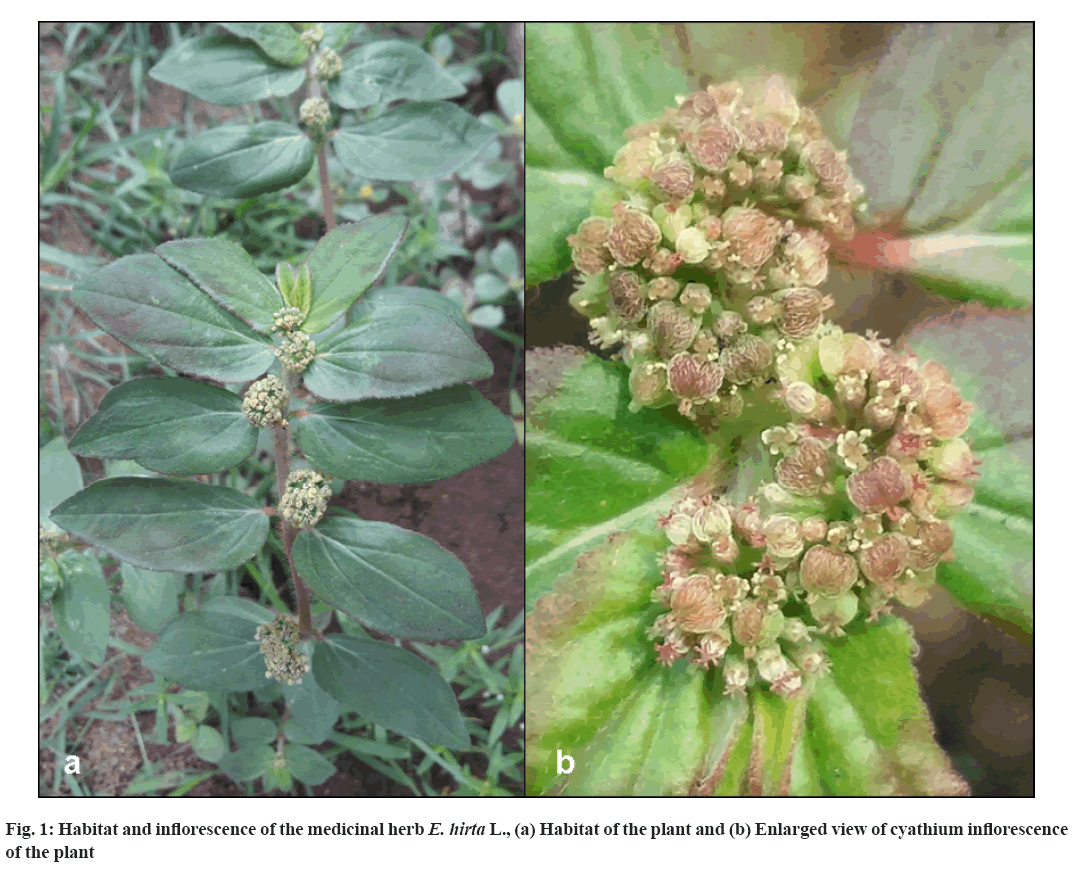
E. hirta, a popular medicinal plant commonly known as ‘asthma weed/snake weed’ in English, Chithrapala/ Nelapala in Malayalam, Dugthika/Ksheerani in Sanskrit and Tawa-Tawa in Philipino, belongs to the family Euphorbiaceae (fig. 1). It is a common herbaceous plant seen on roadsides, garden paths and grasslands; widely distributed in pan-tropic and partly subtropic areas including Australia, Queensland, New South Wales, Central America, Africa, Indomalaysia, Philippines, China and India[3]. Ethnopharmacology and the traditional use of the plant are sorted out and well documented. Parts of this plant are widely used in medicine to treat respiratory disorders, gastrointestinal diseases, wound healing, urinogenital disorders, tumors, lactation in women, viral infections, etc. The infusion of the plant is using the indigenous communities of the Philippines for the treatment of dengue fever[4]. Studies have shown that this plant has various other pharmacological properties including, antimicrobial and antiviral activities[3,5,6]. The antiviral activity of the plant is probably due to the presence of high tannin content in the plant[7].
This plant is a source of various chemicals such as flavonoids, terpenoids, phenols, essential oils, tannins, acids and other compounds such as alkaloids, saponins, amino acids and minerals[6]. Huang et al. in 2012[3] and Kausar et al. in 2016[8] reviewed the phytochemistry and pharmacology of the plant. According to Kausar et al. flavonoids in the plant include quercetin, quercitrin, quercitol and its derivatives; terpenoids include triterpenes: Alpha (α)-amyrin, beta (β)-amyrin, friedelin, taraxerol and its derivatives; tannins include the dimer rich hydrolysable dehydroellagitannins-Euphorbins A, B, C, E and terchebin; the monomeric hydrolysable tannins-Geraniin and acids include ellagic, gallic, tannic, maleic and tartaric acids[8]. Phytochemicals, their function and uses are also documented in the phytochemical database of Dr. Duke. Based on the literature, 76 small compounds with different levels of pharmacological activity, including antiviral properties, were selected for screening. Structural details of the selected compounds were obtained from the chemical database ChemSpider. The compounds, which have no structural deposits (6 out of 76) in databases and other popular sources, were drawn using an online tool, ChemSketch. Three- Dimensional (3D) structures of the compounds were generated in the CORINA 3D structure generator and subjected to docking against the target proteins.
Selection and preparation of target proteins:
A recent review on current and future flavivirus drug targets[9] provides possible therapeutic targets of DENV. Considering the crucial role of Non-Structural Protein-5 (NS5) in the genome replication and methylation and capping of RNA, they selected it as one of the targets in the current study. NS5 is the most conserved target expressed in the host during DENV infection with 900 amino acid residues (~102 kDa). It has a Methyl- Transferase (MTase) domain in the N-terminal and an RNA-dependent RNA polymerase (RdRp) domain in the C-terminal. The MTase is responsible for the capping of viral RNA and methylation of a N7 and 2’O ribose activity. On the other hand, RdRp replicates viral RNA. Moreover, it down-regulates the host immune interferon response and modulating RNA splicing within the host cell[10]. The present study chose the MTase domain of the NS5 as a receptor. Active-site of the receptor was determined based on the site where the known inhibitor molecules (S-Adenosyl-L-Homocysteine (SAH) and Guanosine-5'-Triphosphate (GTP)) were attached. The active site residues of the target include Lys14, Leu17, Asn18, Leu20, Phe25, Lys29, Ser56, Gly58, Gly81, Cys82, Gly83, Gly86, Trp87, Thr104, Lys105, His110, Glu111, Lys130, Asp131, Val132, Phe133, Asp146, Ser151, Pro152, Arg211, Ser213, Thr214, Arg499 and Lys656. The target structure available in the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) with PDB ID: 4V0Q is used here for the docking study.
Human Inosine 5’-Monophosphate Dehydrogenase-II (IMPDH-II) is the enzyme in the biosynthesis of purine nucleotides is the second target. It is the enzyme that catalyzes the biosynthesis of guanine nucleotides from Inosine Monophosphate (IMP). Human IMPDH has two isoforms, type I and type II having 84 % sequence similarity. They exist in tetramers consisting of 55 805 Diacetylene (DA) Da monomers with 514 amino acid residues. In normal host cells, IMPDH-I is the prevailing type. IMPDH-II is predominant and up-regulated in the rapidly replicating cells (neoplastic) and pathogenic virus multiplication sites[11]. Selective catalyzing property articulate type two IMPDH which is a promising target for antiviral drug development. Inhibition of the enzyme could interfere with or even terminate its activity by altering or blocking the natural substrate’s (IMP, Nicotinamide Adenine Dinucleotide (NAD+)) binding site[12]. Nair et al. detailed the active site region of the enzyme. The IMP binding site is a cavity formed by 36 residues including Ser68, Pro69, Met70, Asp71, Thr72, Val73, Thr74, Ser276, Gln277, Asn303, Val304, Arg322, Val323, Ser327, Gly328, Ser329, Ile330, Cys331, Ile332, Thr333, Gln334, Glu335, Val336, Asp364, Gly365, Gly366, Ile367, Gln368, Met385, Met386, Gly387, Ser388, Leu389, Leu390, Tyr412 and Arg413. The structure of the IMPDH complexed with Ribavirin Monophosphate (RVP) in the RCSB PDB with PDB ID: 1NF7 is used here for the docking study.
Validation of docking protocol:
The reliability of the docking protocols needs to be validated before the actual docking study begins. The Root Mean Square Deviation (RMSD) has been used to measure the quality of reproduction of a known binding pose by a computational method, such as docking. To perform docking validation, the ligand that is bound to the crystallographic structure of the target is separated from their active site and re-docks to the same binding site using a computational docking tool and superimposes them in PyMol to understand the RMSD of the docked molecules. RMSD less than 1.5 Å or even less than 1 Å is ideal and represents better reproduction of the correct pose. Here, the RMSD between the crystallographic and docked structure of SAH is 0.056 Å, and the RMSD between the crystallographic and docked structure of RVP is 0.953 Å. It indicates the reliability of the docking method in reproducing the experimentally observed binding pose.
Molecular docking studies and drug-likeness assessment:
Docking calculations with both enzyme targets and the phytochemicals were carried out in AutoDock 4.2.6 with the AutoDock Tool (ADT) 1.5.6 using standard procedures. A 3D grid cavity is large enough to accommodate and center around the binding site of NS5 and that of IMPDH-II respectively, were positioned and set the grid point spacing of 0.375 Å. Before docking, remove water/solvent molecules and the co-crystallized compounds from the targets. Targets with rigid residues in the active sites were used and set default parameters in the AutoDock for performing docking analysis and scrutinized hit molecules with the least free energy of binding and inhibition constant was further evaluated. Top hit compounds were tested for their compliance with the Lipinski’s rule of five and analyzed molecular properties such as logP, polar surface area, and the number of molecules capable of donating and accepting hydrogen bonds for their suitability for drug development. The bioactivity and drug-likeness is measured using Molinspiration, Molsoft and Swiss Absorption, Distribution, Metabolism and Excretion (SwissADME) online tools. The presence of toxic substructures in the hits and their toxicity were studied using the MCULE toxicity checker.
Results and Discussion
The phytochemical profile of E. hirta revealed that the outstanding medicinal property of the plant is due to the presence of high potential secondary metabolites. 76 such compounds were subjected here for the in silico screening trial against both the MTase domain of NS5-protease and human IMPDH-II-oxidoreductase enzymes. Compounds with molecular weight greater than 500 g/mol (14 molecules) were kept away from the present phytochemical scrutiny.
Hit molecules obtained against DENV NS5-protease are shown as follow. Out of 62 phytochemicals docked with the DENV NS5 protease, 41 molecules shown strongly to moderate binding affinity with the target and those ligands with the best docking (11 molecules), based on the least binding free energy (ΔGbind≤-8 kcal/mol), inhibition constant, number of H-bonds, bond strength and hydrophobic interactions, were screened as top hits (Table 1). All the hits showed ideal therapeutic properties. Betulin (ΔGbind=-8.07) is one of hits and a pentacyclic triterpene that inhibit the activity of the target. It has antitumor activities when combined with cholesterol[13]. Cholesterol is another moderately binding molecule present in the plant. Derivatives of betulin are good anti-microbial agents[14]. Antiviral properties of betulinic acid derived from the precursor, betulin, emphasized its efficacy against Herpes simplex virus type 1 and 2, and Enteric Cytopathic Human Orphan (ECHO)-6 virus[15,16]. Ent-kaurane- diterpenoids are natural compounds displaying a broad spectrum of potential therapeutic effects on anticancer activity. 2-beta (β), 16-alpha (α), 19-trihydroxy-ent- kaurane (ΔGbind=-8.38) and 2-β, 16-α-dihydroxy-ent- kaurane (ΔGbind=-8.62) are two such recently isolated compounds[17]. They firmly bind the active site of the target by 4 and 5 H-bonds (bond length between 2.4 to 3.17 Å) and hydrophobic interaction of 7-8 residues. Triterpenoid cycloartenol (ΔGbind=-8.61) is a phytosterol that showed a moderate binding affinity with the target. It forms one of the precursors for the biosynthesis of various sterols and has various pharmacological activities including, anti-inflammatory, anti-tumour, antioxidant, antibiotic and anti-Alzheimer’s disease activities[18]. 24-methylenecycloartenol (ΔG =-8.89) is the other triterpenoid that showed moderate activity against the target. Taraxerone (ΔGbind=-9.03) and taraxerol (ΔGbind=-9.41) are the two triterpenes showing considerable binding affinity with the target. Taraxerone can inhibit cancer cell colony formation and induce apoptosis[19]. It also exhibited weak antiviral activity against herpes simplex virus type 1 and 2[20]. Friedelin (ΔGbind=-9.44) is another terpenoid having anti-microbial properties against Mycobacterium tuberculosis, Candida spp., etc. Moreover, it has anti-inflammatory and antipyretic properties besides gastro-protective, antioxidant and hepatoprotective activities[21]. α-amyrin (ΔGbind=-9.61) and β-amyrin (ΔGbind=-9.58) are two bioactive triterpenes that showed a strong binding affinity with the target. They have anti-inflammatory, anti-microbial, anti-fungal and antiviral activities[22]. It also protects from gastric ulcers and tumor formation. Stigmasterol (ΔGbind=-10.56), an unsaturated sterol, is strongly bound with the least free energy of binding to the target. It has antiosteoarthritic, antihypercholesterolemic, antitumor, hypoglycaemic, anti-mutagenic, antioxidant, anti-inflammatory and Central Nervous System (CNS) activities[23].
| S. No. | Hit molecules | NS5 (Target-1) | ||
|---|---|---|---|---|
| ΔGbind (kcal/mol) | Inhibition constant (Ki) | Residues with H-Bonds (bond length in Å) | ||
| 1 | Betulin | -8.07 | 1.22 | Asp146 (3.23), Lys180 (3.23), Tyr218 (3.05) |
| 2 | 2-β,16-α,19-trihydroxy-ent- kaurane | -8.38 | 0.72 | Glu149 (2.90), Val132 (3.17), Lys105 (2.65), Thr104 (2.85) |
| 3 | Cycloartenol | -8.61 | 0.49 | Val132 (3.04), Asp131 (2.75) |
| 4 | 2-β-16-α-dihydroxy-ent- kaurane | -8.62 | 0.45 | His110 (2.92), Glu111 (2.67), Arg84 (2.9), Gly85 (3.01), Cys82 (2.40) |
| 5 | 24-methylenecycloartenol | -8.89 | 0.3 | Asp131 (2.50), Val132 (2.95) |
| 6 | Taraxerone | -9.03 | 0.24 | Trp87 (2.68) |
| 7 | Taraxerol | -9.41 | 0.13 | Cys82 (2.70), Arg84 (3.07), Gly85 (2.87), Gly86 (3.19) |
| 8 | Friedelin | -9.44 | 0.12 | Trp87 (2.76) |
| 9 | β-amyrin | -9.58 | 0.09 | Cys82 (3.10), Trp87 (3.24) |
| 10 | α-amyrin | -9.61 | 0.09 | Nil |
| 11 | Stigmasterol | -10.56 | 0.02 | Cys82 (2.85) |
Note: Top hits obtained against the target NS5 with their free energy of binding (ΔGbind), Inhibition constant (Ki) and the target residues established H-bonds with bond length
Table 1: Docking Result of HIT Molecules Against NS5 Protease
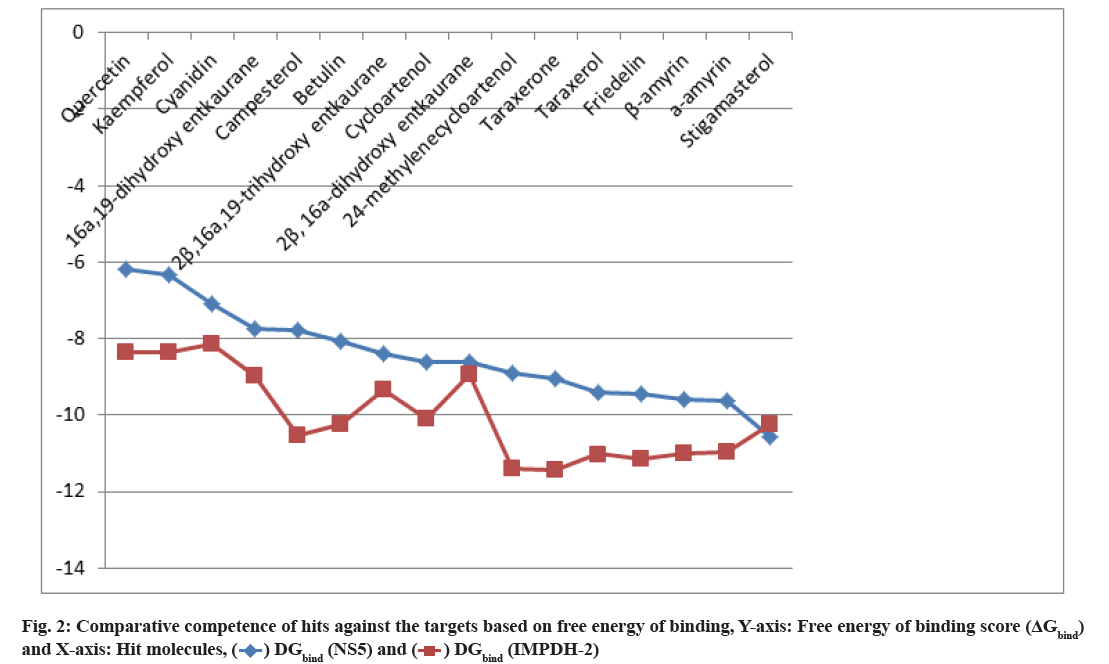
Hit molecules obtained against Human IMPDH-II are shown as follow. The in silico screening of the phytochemicals with the target IMPDH-II protease resulted in 44 active compounds. Out of them, eleven top hits (ΔGbind≤-8 kcal/mol with at least one H-bond) have been identified (Table 2) following similar standards used for the first target NS5. Comparing the docking results of NS5 and IMPDH-II, betulin, 2-β, 16- α, 19-trihydroxy ent-kaurane, 2-β, 16-α-dihydroxy-ent- kaurane, β-amyrin α-amyrin and stigmasterol was found to be similar hits (fig. 2). The remaining five active hit compounds include three flavonoids, a diterpenoid and a phytosterol. Quercetin, kaempferol and cyanidin are the flavonoids that are water-soluble polyphenolic molecules and are generally antioxidants. Quercetin (ΔGbind=-8.37) is the most abundant flavonoid that offers a variety of potential therapeutic uses. It prevents allergies, asthma, hay fever, rheumatoid arthritis and cancer cell growth. It exerts antibacterial activity and exhibit anti-infective and anti-replicative effect on virus[24]. Kaempferol (ΔGbind=-8.35) has antioxidant, anti-inflammatory, anti-microbial, anticancer, antidiabetic, anti-allergic and analgesic activities[25]. Besides, it has potent anti-Human Immunodeficiency Virus (HIV)-1[26] and anti-Japanese encephalitis virus activities[27]. Cyanidin (ΔG =-8.14), a water-soluble plant pigment anthocyanidin, is another antioxidant that protects against various types of cancer, heart disease and brain disorders. It prevents arthritis, fatty liver and eye diseases. It induces the immune system and supports the intestine, bone and joints[28,29]. All the three flavonoids are firmly bound to the target with seven to eight hydrogen bonds with a bond length between 2.5 to 3.29 Å. 16-α, 19-dihydroxy ent-kaurane (ΔGbind=-8.97) is one of the three ent-kaurane compounds in the plant that interact firmly with the target. Campesterol (ΔGbind=-10.53) is the simplest plant-derived steroid that helps to reduce cholesterol absorption in the intestine and prevent cancer[30].
| S. No. | Hit molecules | IMPDH-II (Target-2) | ||
|---|---|---|---|---|
| ΔGbind (kcal/mol) | Inhibition constant (Ki) | Residues with H-Bonds (bond length in Å) | ||
| 1 | Quercetin | -8.37 | 0.73 | Gly324 (2.95), Gly326 (2.62), Ser68 (2.92), Ser68 (2.99), Ile367 (3.00), Ile367 (2.79), Ser388 (2.65), Leu389 (3.08) |
| 2 | Kaempferol | -8.35 | 0.76 | Met70 (3.14), Ser68 (2.99), Ser68 (3.09), Gly324 (2.89), Gly326 (2.68), Ser388 (3.00), Leu389 (3.20), Ile367 (2.74) |
| 3 | Cyanidin | -8.14 | 1.08 | Ser68 (3.29), Met70 (3.16), Gly326 (2.83), Ile367 (2.98), Ile367 (2.51), Leu389 (3.27), Ser388 (2.70) |
| 4 | 16-α,19- dihydroxy-ent- kaurane | -8.97 | 0.27 | Ser68 (2.79), Arg322 (3.19), Gly324 (2.89), Ser276 (2.94) |
| 5 | Campesterol | -10.53 | 0.019 | Met385 (3.14), Gly365 (2.78), Asp364 (2.56) |
| 6 | Betulin | -10.23 | 0.032 | Asp364 (2.78), Met420 (3.01) |
| 7 | 2-β,16-α,19- trihydroxy- ent-kaurane | -9.33 | 0.145 | Asp364 (3.31), Ser68 (2.45), Ser388 (2.69), Ile367 (3.16) |
| 8 | 2-β-16-α- dihydroxy-ent- kaurane | -8.95 | 0.275 | Asp274 (3.07), Asn303 (2.87), Arg322 (2.80), Asp364 (2.47), Gly365 (3.06) |
| 9 | β-amyrin | -11.01 | 0.008 | Ala419 (2.66) |
| 10 | α-amyrin | -10.95 | 0.009 | Asp364 (2.85) |
| 11 | Stigmasterol | -10.25 | 0.031 | Tyr411 (2.61), Met70 (2.89) |
Note: Top hits obtained against the target IMPDH-II with their free energy of binding (ΔGbind), Inhibition constant (Ki) and the target residues established H-bonds with bond length
Table 2: Docking Result of HIT Molecules Against Human IMPDH-II
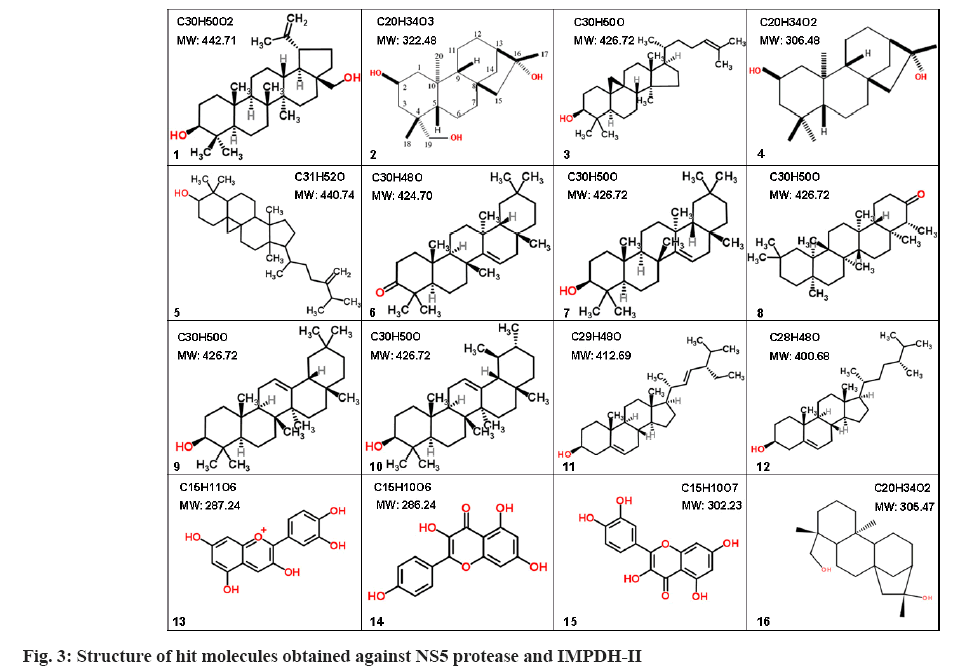
Structure of hit molecules obtained against NS5 protease and IMPDH-II are shown in fig. 3. The following molecules are represented as (1) Betulin; (2) 2-β-16-α-19- trihydroxy-ent-kaurane; (3) Cycloartenol; (4) 2-β-16-α dihydroxy-ent-kaurane; (5) 24-methylenecycloartenol; (6) Taraxerone; (7) Taraxerol; (8) Friedelin; (9) β-amyrin; (10) α-amyrin; (11) Stigmasterol; (12) Campesterol; (13) Cyanidin; (14) Kaempferol; (15) Quercetin and (16) 16-α-19-dihydroxy-ent-kaurane.
Top hit compounds (fig. 3) that showed firmly to moderate proximity on both targets were subjected to pharmacokinetic and toxicity analysis. A comparative description of them in Table 3 shows 2-β, 16-α, 19-trihydroxy-ent-kaurane and kaempferol isolated from the whole plant were agreed with drug-likeness and ADME scores. It further evidenced the absence of a toxic substructure. Gibbs free energy level of 2-β, 16- α, 19-trihydroxy-ent-kaurane with both NS5 (-8.38 kcal/ mol) and IMPDH-II (-9.33 kcal/mol) underlined the potential to formulate it as a drug. Kaempferol is also equally prospective though its free energy of binding level with NS5 (-6.33 kcal/mol) is comparatively lower than with IMPDH-II (-8.35 kcal/mol). The bioactivity of the biological compounds are either significantly active (score>0.00), moderately active (score between -0.50 and 0.00), or inactive (score<-0.50)[31]. The bioactivity of the hits towards Glycoprotein-Coupled Receptor Ligand (GPCRL), Ion Channel Modulator (ICM), Nuclear Receptor Ligand (NRL), Protease Inhibitor (PI), Kinase Inhibitor (KI) and Enzyme Inhibitor (EI) were observed. Based on the observations 2-β, 16-α, 19-trihydroxy-ent- kaurane is significantly active (>0.00) towards GPCRL, ICM, NRL, PI and EI while moderately active (<0.00) to KI. Kaempferol is moderately active to all except KI, NRL and EI. The structure of the leads, molecular interaction between the active site of targets and leads, and the Molsoft graph showing the drug-likeness of leads are depicted in fig. 4.
Fig 4: Lead molecules, their interaction and drug-likeness properties
Note: (A) 2-β,16-α,19-trihydroxy-ent-kaurane (lead-1); (B) Molsoft graph showing drug likeness of the lead-1,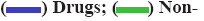
 (C) 3D view of MTase active site of NS5 with the lead-1 in discovery studio; (D) Interaction of lead-1 in LigPlot; (E) Kaempferol (lead-2); (F) Molsoft graph showing drug likeness of the lead-2,
(C) 3D view of MTase active site of NS5 with the lead-1 in discovery studio; (D) Interaction of lead-1 in LigPlot; (E) Kaempferol (lead-2); (F) Molsoft graph showing drug likeness of the lead-2, (G) 3D view of IMPDH-II active site binding with Kaempferol in discovery studio and (H) Interaction of lead-2 in LigPlot
(G) 3D view of IMPDH-II active site binding with Kaempferol in discovery studio and (H) Interaction of lead-2 in LigPlot
| S. No. | Hit molecules | Molinspiration bioactivity score | Molsoft | MCULE | ADME | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| GPCRL | ICM | KI | NRL | PI | EI | DLS | TS | LL | ||
| 1 | Betulin | 0.21 | -0.04 | -0.41 | 0.85 | 0.09 | 0.51 | -0.09 | F | No |
| 2 | 2-β,16-α,19-trihydroxy- ent-kaurane |
0.34 | 0.13 | -0.10 | 0.87 | 0.25 | 0.60 | -0.94 | NF | Yes |
| 3 | Cycloartenol | 0.21 | 0.10 | -0.40 | 0.86 | 0.14 | 0.66 | -0.31 | F | No |
| 4 | 2-β-16-α-dihydroxy-ent- kaurane |
0.29 | 0.22 | -0.15 | 0.77 | 0.13 | 0.52 | -1.22 | NF | No |
| 5 | 24-methylenecycloartenol | 0.14 | 0.11 | -0.37 | 0.90 | 0.06 | 0.60 | -0.51 | F | No |
| 6 | Taraxerone | 0.07 | -0.10 | -0.40 | 0.43 | -0.14 | 0.37 | -0.89 | NF | No |
| 7 | Taraxerol | 0.21 | 0.02 | -0.20 | 0.54 | 0.00 | 0.49 | -0.91 | NF | No |
| 8 | Friedelin | 0.02 | -0.06 | -0.39 | 0.39 | 0.02 | 0.21 | -0.48 | NF | No |
| 9 | β-amyrin | 0.22 | -0.05 | -0.31 | 0.67 | 0.11 | 0.56 | -0.23 | NF | No |
| 10 | α-amyrin | 0.22 | -0.02 | -0.41 | 0.79 | 0.19 | 0.60 | 0.09 | NF | No |
| 11 | Stigmasterol | 0.12 | -0.08 | -0.48 | 0.74 | -0.02 | 0.53 | 0.73 | FF | No |
| 12 | Campesterol | 0.11 | 0.01 | -0.48 | 0.71 | 0.01 | 0.50 | 0.71 | NF | No |
| 13 | Cyanidin | -0.13 | -0.09 | 0.02 | 0.09 | -0.30 | 0.01 | -0.1 | F | Yes |
| 14 | Kaempferol | -0.10 | -0.21 | 0.21 | 0.32 | -0.27 | 0.26 | 0.77 | NF | Yes |
| 15 | Quercetin | -0.06 | -0.19 | 0.28 | 0.36 | -0.25 | 0.28 | 0.93 | F | Yes |
| 16 | 16-α,19-dihydroxy-ent- kaurane |
0.30 | 0.18 | -0.13 | 0.75 | 0.14 | 0.49 | -0.94 | NF | No |
Note: GPCRL: G-Protein Coupled Receptor Ligand; ICM: Ion Channel Modulator; KI: Kinase Inhibitor, NRL: Nuclear Receptor Ligand; PI: Protease Inhibitor; EI: Enzyme Inhibitor; DLS: Drug Likeness Score; TS: Toxic Substructure; F: Found; NF: Not Found and LL: Lead Likeness
Table 3: Drug Likeness Assessment Using Different Tools
Overall the plant E. hirta is a valuable herb conventionally using for anti-dengue treatment. The chemistry of the plant revealed its biochemical richness and the presence of various flavonoids that make the plant an antioxidant herb and be a nutritional supplement to fight against free radicals. In silico screening of 76 small molecules derived from the plant with DENV-NS5 protease and human IMPDH-II predicted 16 biologically active compounds with possible activity to fight against the DENV. Based on the therapeutic importance and pharmacokinetic properties of selected leads, 2-β, 16-α, 19-trihydroxy-ent- kaurane and kaempferol are the two best candidates for anti-dengue drugs. Formulation of a nutraceutical with pharmaceutical-grade and standardization of nutrients from the herb as well as the development of a safe and effective medication from the leads to fighting with DENV need further in vitro and in vivo analysis based on the in silico result.
Acknowledgements:
The authors are thankful to Dr. T. Madhan Mohan, Senior advisor (Retd.), Department of Biotechnology, Government of India and Director of Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI) for providing the facilities and encouragements. We thank Kerala State Council for Science, Technology and Environment (KSCSTE), Government of Kerala for financial support.
Conflict of interests:
The authors declared no conflict of interest.
References
- WHO. Dengue and severe dengue: Fact sheet. World Health Organisation; 2017.
- National Health Mission. Dengue Cases and Deaths in the Country since 2014. National Center for Vector Borne Diseases Control (NCVBDC), Ministry of Health and Family Welfare, Government of India; 2018.
- Linfang H, Shilin C, Meihua Y. Euphorbia hirta (Feiyangcao): A review on its ethnopharmacology, phytochemistry and pharmacology. J Med Plant Res 2012;6(39):5176-85.
- de Guzman GQ, Dacanay AT, Andaya BA, Alejandro GJ. Ethnopharmacological studies on the uses of Euphorbia hirta in the treatment of dengue in selected indigenous communities in Pangasinan (Philippines). J Intercult Ethnopharmacol 2016;5(3):239-43.
[Crossref] [Google scholar] [PubMed]
- Kumar S, Malhotra R, Kumar D. Euphorbia hirta: Its chemistry, traditional and medicinal uses, and pharmacological activities. Pharmacogn Rev 2010;4(7):58-61.
[Crossref] [Google scholar] [PubMed]
- Asha S, Deevika B, Sadiq M. Euphorbia hirta Linn-A review on traditional uses, phytochemistry and pharmacology. World J Pharm Res 2014;3(4):180-205.
- Gyuris A, Szlavik L, Minarovits J, Vasas A, Molnar J, Hohmann J. Antiviral activities of extracts of Euphorbia hirta L. against HIV-1, HIV-2 and SIVmac251. In Vivo 2009;23(3):429-32.
[Google scholar] [PubMed]
- Kausar J, Muthumani D, Hedina A, Anand V. Review of the phytochemical and pharmacological activities of Euphorbia hirta Linn. Pharmacogn J 2016;8(4):310-13.
- Geiss BJ, Stahla H, Hannah AM, Gari HH, Keenan SM. Focus on flaviviruses: Current and future drug targets. Future Med Chem 2009;1(2):327-44.
[Crossref] [Google scholar] [PubMed]
- El Sahili A, Lescar J. Dengue virus non-structural protein 5. Viruses 2017;9(4):91.
- Franchetti P, Grifantini M. Nucleoside and non-nucleoside IMP dehydrogenase inhibitors as antitumor and antiviral agents. Future Med Chem 1999;6(7):599-614.
[Google scholar] [PubMed]
- Nair V, Shu Q. Inosine monophosphate dehydrogenase as a probe in antiviral drug discovery. Antivir Chem Chemother 2007;18(5):245-58.
[Crossref] [Google scholar] [PubMed]
- Mullauer FB, Kessler JH, Medema JP. Betulin is a potent anti-tumor agent that is enhanced by cholesterol. PLoS One 2009;4(4):1-8.
[Crossref] [Google scholar] [PubMed]
- Haque S, Nawrot DA, Alakurtti S, Ghemtio L, Yli-Kauhaluoma J, Tammela P. Screening and characterisation of antimicrobial properties of semisynthetic betulin derivatives. PLoS One 2014;9(7):1-9.
- Pavlova NI, Savinova OV, Nikolaeva SN, Boreko EI, Flekhter OB. Antiviral activity of betulin, betulinic and betulonic acids against some enveloped and non-enveloped viruses. Fitoterapia 2003;74(5):489-92.
[Crossref] [Google scholar] [PubMed]
- Visalli RJ, Ziobrowski H, Badri KR, He JJ, Zhang X, Arumugam SR, et al. Ionic derivatives of betulinic acid exhibit antiviral activity against herpes simplex virus type-2 (HSV-2), but not HIV-1 reverse transcriptase. Bioorg Med Chem Lett 2015;25(16):3168-71.
[Crossref] [Google scholar] [PubMed]
- Yan S, Ye D, Wang Y, Zhao Y, Pu J, Du X, et al. Ent-Kaurane diterpenoids from Euphorbia hirta. Rec Nat Prod 2011;5(4):247-51.
- Zhang ZL, Luo ZL, Shi HW, Zhang LX, Ma XJ. Research advance of functional plant pharmaceutical cycloartenol about pharmacological and physiological activity. Zhongguo Zhong Yao Za Zhi 2017;42(3):433-7.
[Crossref] [Google scholar] [PubMed]
- Ma XC, Dong S, Zhang SY, Jia N, Qu SL. Taraxerone triterpene inhibits cancer cell growth by inducing apoptosis in non-small cell lung cancer cells. Bangladesh J Pharmacol 2016;11(2):342-7.
- Kuljanabhagavad T, Suttisri R, Pengsuparp T, Ruangrungsi N. Chemical structure and antiviral activity of aerial part from Laggera pterodonta. Journal Health Res 2009;23(4):175-7.
- Souza-Moreira TM, Alves TB, Pinheiro KA, Felippe LG, de Lima G, Watanabe TF, et al. Friedelin synthase from Maytenus ilicifolia: Leucine 482 plays an essential role in the production of the most rearranged pentacyclic triterpene. Sci Rep 2016;6(1):1-3.
- Hernández-Vázquez L, Palazón Barandela J, Navarro-Ocaña A. The pentacyclic triterpenes α, β-amyrins: A review of sources and biological activities. In: Rao V, editor. Phytochemicals: A global perspective of their role in nutrition and health. London: IntechOpen Limited; 2012. p. 487-502.
- Kaur N, Chaudhary J, Jain A, Kishore L. Stigmasterol: A comprehensive review. Int J Pharm Sci Res 2011;2(9):2259-65.
- Lakhanpal P, Rai DK. Quercetin: A versatile flavonoid. Internet J Med Update 2007;2(2):22-37.
- Calderon-Montano JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem 2011;11(4):298-344.
[Crossref] [Google scholar] [PubMed]
- Behbahani M, Sayedipour S, Pourazar A, Shanehsazzadeh M. In vitro anti-HIV-1 activities of kaempferol and kaempferol-7-O-glucoside isolated from Securigera securidaca. Res Pharm Sci 2014;9(6):463-9.
[Google scholar] [PubMed]
- Zhang T, Wu Z, Du J, Hu Y, Liu L, Yang F, et al. Anti-Japanese-encephalitis-viral effects of kaempferol and daidzin and their RNA-binding characteristics. PLoS One 2012;7(1):e30259.
[Crossref] [Google scholar] [PubMed]
- Lucioli S. Anthocyanins: Mechanism of action and therapeutic efficacy. In: Capasso A editor. Medicinal plants as antioxidant agents: Understanding their mechanism of action and therapeutic efficacy. Kerala, India: Research Signpost; 2012. p. 27-57.
- Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients and the potential health benefits. Food Nutr Res 2017;61(1):1-21.
[Crossref] [Google scholar] [PubMed]
- Choudhary PS, Tran SL. Phytosterols: Perspectives in human nutrition and clinical therapy. Curr Med Chem 2011;18(29):4557-67.
[Crossref] [Google scholar] [PubMed]
- Khan SA, Kumar S, Maqsood AM. Virtual screening of molecular properties and bioactivity score of boswellic acid derivatives in search of potent anti-inflammatory lead molecule. Int J Interdiscip Multidiscip Stud 2013;1(1):8-12.