- *Corresponding Author:
- Gang Li
Department of Pharmacy, Central Hospital Affiliated To Shandong First Medical University, Jinan, Shandong 250014, China
E-mail: ligang20751@163.com
| Date of Received | 18 July 2021 |
| Date of Revision | 10 November 2022 |
| Date of Acceptance | 22 March 2023 |
| Indian J Pharm Sci 2023;85(2):445-455 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Rehmannia glutinosa extracts have profound medicinal value in the treatment of liver diseases. It contains micro ribonucleic acid molecules that have an average of 21 nucleotides. These micro ribonucleic acids are responsible for gene regulation and expression during the treatment of liver diseases. The study's main objective is to screen for active ingredients in Rehmannia glutinosa and its mechanism of action in treating liver diseases. Our study used the gene expression omnibus database to obtain datasets associated with liver diseases. The GSE167308 dataset consisted of 19 samples of ribonucleic acid profiles of micro dissected hepatocytes from the human liver in 3 groups (alcoholic hepatitis (n=7), alcoholic cirrhosis (n=7) and controls (n=5)). The GSE54350 dataset involving CD14+ cells from diabetic and non-diabetic samples. We used the GEO2R tool, ShinyGO, R software, STRING, and Network Analyst tools to analyze differentially expressed genes. We obtained 25 significantly expressed genes with an adjusted p-value of 0.05 and log(Fold Change) of -1.0. Also, we obtained seven active ingredients of Rehmannia glutinosa, their descriptions and target proteins. The differentially expressed genes were enriched in various Kyoto Encyclopedia of Genes and Genomes pathways such as nuclear factor-κB, Mitogen-activated protein kinases, glycolysis, Protein kinase B-serine/threonine kinase 1 signaling, regulation of autophagy, or Transforming growth factor-beta signaling pathways. The protein-protein interaction networks revealed 9 hub genes (Interleukin 6, Superoxide dismutase 1, Catalase, tumor necrosis factor, toll-like receptor 4, Proliferator-Activated Receptor Gamma, Nuclear Factor Kappa B Subunit 1, NOD-Like Receptor family Pyrin domain containing 3 and prostaglandin-endoperoxide synthase 2) that were significant in liver diseases. Rehmannia glutinosa exerts its anti-inflammatory response through active ingredients such as catalpol, Rehmannioside A, B, C, and Shanzhiside. These ingredients are mainly iridoid glycosides that activates the Nuclear factor kappa B and mitogen-activated protein kinase signaling pathways and reduces the production of pro-inflammatory cytokines. Rehmannia glutinosa contains several active components (for example, Catalpol, Rehmannioside A and Shanzhiside) that are crucial in regulating pro-inflammatory cytokines and hub genes associated with liver diseases.
Keywords
Rehmannia glutinosa, liver diseases, active ingredients, microRNA, differentially expressed genes, nuclear factor-κB pathway, anti-inflammatory response
Rehmannia glutinosa belongs to the Scrophulariaceae family and its extracts have profound medicinal value in treating liver diseases[1]. It is a traditional Chinese medicine, mainly produced in the Huai region and Henan provinces of mainland China[2]. Rehmannia glutinosa contains micro Ribonucleic Acid (miRNA) molecules that have an average of 21 nucleotides. These miRNAs are responsible for gene regulation and expression during the treatment of liver diseases. The sequences of miRNAs are conserved within the eukaryotes and operate through complementary stretches of mRNA sequences associated with expression levels of various genes.
The network pharmacology of Rehmannia glutinosa involves analyzing the underlying molecular mechanisms linked to its therapeutic effects. Li et al.[3] observed that the network pharmacology of Rehmannia glutinosa consists of 200 active compounds including iridoids, glycosides and phenols. These active compounds target processes such as inflammation and oxidative stress.
According to Bone et al.[4], Rehmannia glutinosa is an adrenaline tonic that is associated with autoimmune diseases in anti-inflammatory infections. It enhances the functions of adrenocortical system. Bai et al.[5] showed that Rehmannia glutinosa has anti-aging effects by ensuring a constant quiescence and lowering senescence associated with hematopoietic stem cells. Furthermore, it is capable of treating numerous diabetic infections, improving metabolism in bone osteoporosis and eliminating liver fibrosis and inflammations.
Rehmannia glutinosa has been implicated in neuroprotection, anti-fatigue and anti-depressant actions. The herb has significant effects on the blood system, nervous, cardiovascular and endocrine systems[5]. It exhibits a robust immuno-improved effect which forms its experimental basis in the treatment of liver diseases. Wu et al.[6] showed that Rehmannia glutinosa is rich in polysaccharides and has significant effects in reducing the effects liver-related injuries and chronic liver infections such as liver cirrhosis and hepatitis B virus. These infections are significant precursors and risk factors for Hepatocellular Carcinoma (HCC). Rehmannia glutinosa has active ingredients such as Catalpols, Rehmannioside A, B, C, Leonurine, Cornin and Shanzhiside. Most of these active ingredients are iridoid glycosides, alkaloids or phenolic compounds that have significant therapeutic effects such as anti-inflammations, antioxidants, hepatoprotection, immunomodulation and anti-oxidation[7,8].
These active compounds have a synergistic effect on the target tissues which increases their therapeutic effects. For instance, certain compounds target specific proteins associated with inflammatory cytokines. Additionally, these compounds target various signaling pathways such as the Phosphatidylinositol 3-Kinase/Protein Kinase B (PI3K-Akt), Nuclear Factor kappa B (NF-κB), and the Mitogen-Activated Protein Kinase (MAPK). Also, it regulates biological processes such as cell growth, differentiation and survival. Our study adopted network pharmacology to examine the complex interactions associated with therapeutic action of Rehmannia glutinosa[9,10].
We will use bioinformatics tools to analyse transcriptomic data and identify differentially expressed genes related to various factors such as synthesis of alkaloids, salt and heat stresses. These analyses would provide significant insights into the molecular and biological mechanisms of Rehmannia glutinosa. Our main objective is to screen for active ingredients in Rehmannia glutinosa and its mechanism of action in the treatment of liver diseases.
Materials and Methods
Gene Expression Omnibus (GEO) datasets:
To identify datasets associated with liver diseases, our study utilized the GEO database, which is accessible at https://www.ncbi.nlm.nih.gov/geo/. We identified the GSE167308 dataset based on the GPL20301 platform (Illumina HiSeq 4000 (Homo sapiens)). The dataset consisted of 19 samples of RNA profiles of micro dissected hepatocytes from human liver in 3 groups (alcoholic hepatitis (n=7), alcoholic cirrhosis (n=7), and controls (n=5)). Also, we identified the GSE54350 based on the GPL10558 platform (Illumina HumanHT-12 V4.0 expression beadchip). The dataset consisted of 12 samples of two groups (diabetic (n=6) and non-diabetic (n=6)). In all datasets, we converted the probes into homologous gene symbols.
Identification and analysis of Differentially Expressed Genes (DEGs):
To screen for DEGs related to liver diseases, we employed R software, which can be accessed at https://www.r-project.org/. We adopted distinct experimental groups and eliminates genes without symbols and genes with multiple probes. We adopted an adjusted p-value (p<0.05) to distinguish between statistically significant and non-significant DEGs at a log2 (Fold Change) (log2 (FC)) of greater or equal to 1. Moreover, we obtained Venn diagrams (https://www.ncbi.nlm.nih.gov/geo/geo2r/) to examine the overlapping DEGs before producing volcano plots and heat maps of the up and down-regulated DEGs.
Screening of active ingredients:
The compounds in Rehmannia glutinosa were obtained from the Traditional Chinese Medicine Systems Pharmacology database (TCMSP) and analysis platform (https://tcmsp-e.com/tcmspsearch.php?qs=herb_all_name&q=Qingrejiedu+Decoction&token=76d7cbb951af2e422866695d80ce7976) consisting of pharmacological properties of every compound. We set the parameter of oral bioavailability to above 30 %, with a drug-likeness score of 0.18 and a CACO-2 permeability of greater than 0.4. Our study used specialized analogous techniques in the bioinformatics analysis tool for molecular mechanism of traditional chinese medicine to extract potential targets of the compounds in Rehmannia glutinosa. During drug screening and evaluation, we obtained information from the DisGeNET Gene database (https://www.disgenet.org/) on the active ingredients in Rehmannia glutinosa. After which, we standardized the names of these compounds using PubChem CIDs (https://pubchem.ncbi.nlm.nih.gov/). All the extracted targets of Rehmannia glutinosa were subjected to the UniProt database (https://www.uniprot.org/) to verify the genes.
Identification of targets of Rehmannia glutinosa in liver diseases:
Our study used an integrated technique to determine the target proteins in the active ingredients of Rehmannia glutinosa in TCMSP (https://tcmsp-e.com/tcmsp.php) and SymMap (http://www.symmap.org/) databases. We acquired the Comparative Toxicogenomics Database (TCD) (https://ctdbase.org/) to establish genes associated with liver diseases. The inference score was set to less than 30 and the targets of the desired genes were then transformed into unique gene symbols using the UniProt knowledge database (https://www.uniprot.org/help/uniprotkb).
Once we identified the active ingredients in Rehmannia glutinosa,we used the DrugBank database (https://www.drugbank.ca/) and searched for each active ingredient. On the targets section, we listed the known target proteins associated with each active ingredient. The list of target proteins were then used to create a network of interactions using tools such as Cytoscape (https://cytoscape.org/), and NetworkX (https://networkx.github.io/).
We also used the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database and set the species as “Homo sapiens” with a confidence score of greater or equal to 0.7 (https://string-db.org/cgi/about). The Protein Protein Interaction (PPI) networks were then extracted and exported to Cytoscape (https://cytoscape.org/). The subsequent PPI networks were merged based on the interaction score to generate a single network of various degrees based on the biological functions of various nodes. Lastly, we applied the Markov Clustering Algorithm (MCL) and set the inflation parameter to 3 with the solid lines denoting the edges.
Pathway enrichment analysis:
Our study used ShinyGO version 0.76 (http://bioinformatics.sdstate.edu/go/) and the ClusterProfiler package in R software (https://bioconductor.org/packages/release/bioc/html/clusterProfiler.html) to identify the biological pathways of the genes associated with Rehmannia glutinosa in liver diseases. In ShinyGO we set our parameters such as the minimum pathway size to two and the maximum size as 2000. We removed redundancies and set the False Discovery Rate (FDR) to a cut-off value of 0.05. The Kyoto Encyclopedia of Genes and Genomes (KEGG) database (https://www.genome.jp/kegg/) was utilized in identifying the biological pathways of core targets. A p-value of less than 0.05 was considered statistically significant.
Results and Discussion
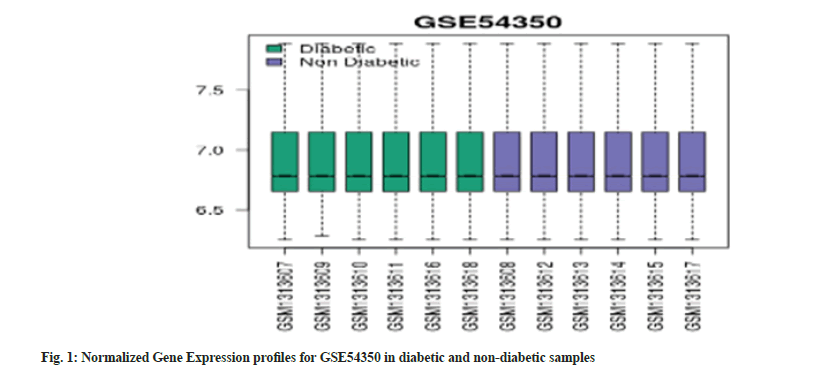
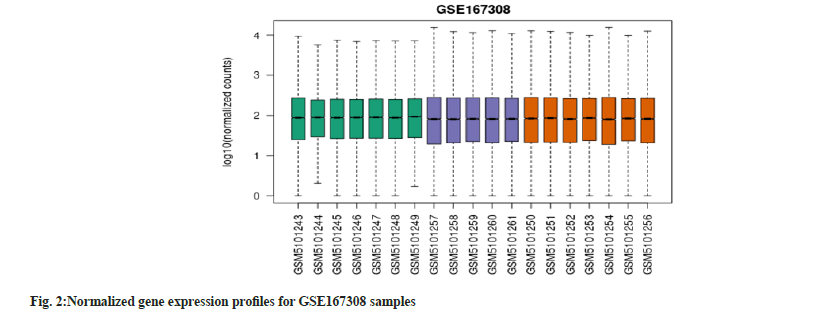
Our study acquired two datasets from the GEO database. The GSE167308 consisted of 19 samples of RNA profiles of micro dissected hepatocytes from human liver in 3 groups (alcoholic hepatitis (n=7), alcoholic cirrhosis (n=7), and controls (n=5)). The GSE54350 dataset consisted of 12 samples of diabetic (n=6) and non-diabetic groups (n=6). The data was normalized by applying a log2 transformation and adjusting for batch effects on various gene profiles. All the samples were analysed using GEO2R tool, R software and Network Analyst to identify the differentially expressed genes. We obtained 25 significantly expressed genes which were all up-regulated. We adjusted the p-value to 0.05 and log FC to -1.0. The boxplots (fig. 1 and fig. 2) show that our gene expression profiles were successfully normalized. The median-centered points show an effective normalisation and possibility of performing cross-comparisons. The samples were plotted on the x-axis while gene expression profiles were plotted on the y-axis.
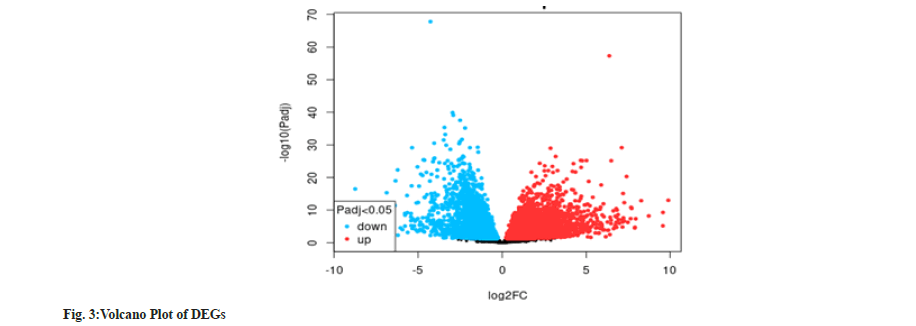
According to Table 1, we identified 7 active ingredients of Rehmannia glutinosa, their descriptions and target proteins. According to fig. 3, the differentially expressed genes are produced based adjusted p-values (y-axis) against the log2(FC) (representing the quantity of change). We defined log2(FC) as the ratio of transcript expressed values and the log of genes. In the plot, every point represents an annotated gene. Blue genes are down-regulated and the red genes are up-regulated. The horizontal dashed line represents the statistical significance depending on the adjusted p-value. We observed that up-regulated genes were overexpressed in liver diseases such as diabetes or alcoholic liver diseases. In contrast, the expression levels of down-regulated genes were limited in liver diseases.
| Active Ingredient | Description | Target Protein |
|---|---|---|
| Catalpol | An iridoid glycoside that has anti-inflammatory and neuroprotective effects | Acetylcholinesterase (AChE), Nuclear factor erythroid 2-related factor (Nrf2), Glycogen Synthase Kinase 3 Beta (GSK-3β), and Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ) |
| Rehmannioside A | An iridoid glycoside with anti-inflammatory and immunomodulatory effects | Cyclooxygenase 2 (COX-2), Tumor Necrosis Factor Alpha (TNF-α), Nuclear Factor Kappa B (NF-kB) and IL-1β |
| Rehmannioside B | An iridoid glycoside with antioxidant and anti-inflammatory effects | Cyclooxygenase 2 (COX-2), Tumour Necrosis Factor Alpha (TNF-α), Nuclear Factor Kappa B (NF-kB) and IL-1β |
| Rehmannioside C | An iridoid glycoside that has hepatoprotective and anti-inflammatory effects | Transforming growth factor beta (TGF-β), Nrf2, IL-1β, TNF-α, and Smad signaling pathways |
| Leonurine | An alkaloid that has antihypertensive and cardiovascular protective effects | Solute carrier family 12 member 1 (SLC12A1), Carbonic anhydrase 1, 2, 4, 7, 9, 12 (CA1, 2, 4, 7, 9, 12), Sodium/potassium-transporting ATPase subunit alpha-1 (ATP1A1), Calcium-activated potassium channel subunit alpha-1 (KCNMA1) |
| Shanzhiside | An iridoid glycoside that has anti-inflammatory and neuroprotective effects | B-cell lymphoma 2(Bcl-2), nitric oxide synthase, Nrf2 |
| Cornin | A phenolic compound with antioxidant and anti-inflammatory effects | Angiotensin-converting enzyme (ACE), B1 bradykinin receptor (BDKRB1) |
Table 1: List of Identified Active Ingredients in Rehmannia Glutinosa, Descriptions and Target Proteins.
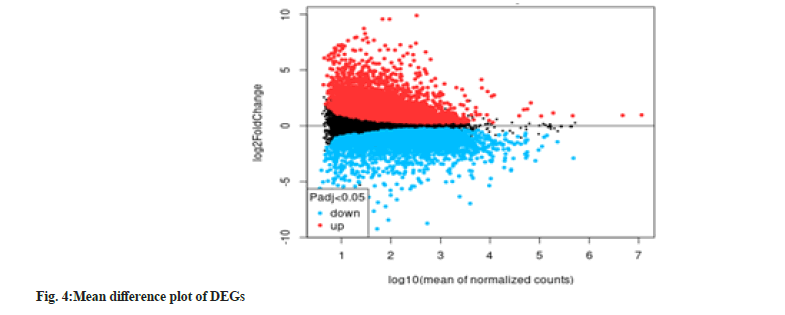
According to fig. 4, the mean difference plot represents the average log 10 (mean of normalized counts) against the log2(FC). All points on the mean difference plot represent gene annotations. The representation of up-regulated genes in red and down-regulated genes in blue is observed in the figure. Unsignificant genes are represented in black. The graph is a contrast of alcoholic liver cells and control cells. Extreme values on either side of the graph denote DEGs of higher expression levels. A greater fold change is obtained in DEGs with lower expression profiles compared to genes of a higher expression profiles. The fanning effect is observed because the differentially expressed genes moves from the right to the left.
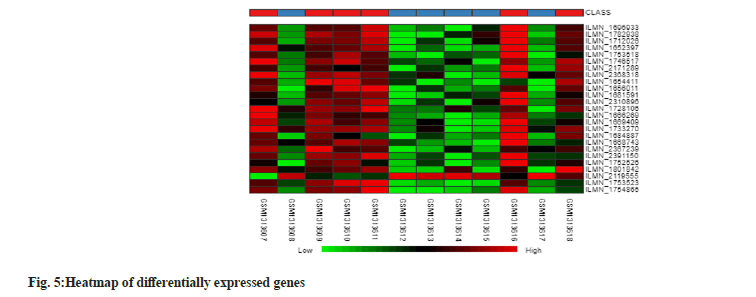
According to fig. 5, the heatmap is a representation of various expression levels of differentially expressed genes. The figure illustrates that genes with lower expression levels are denoted in green and light green, while those with higher expression levels are indicated in red. The black regions represent unsignificant genes. In the heatmap, the intersections denote genes that are annotated on both x and y axis with the third intersection producing an intensity of different color.
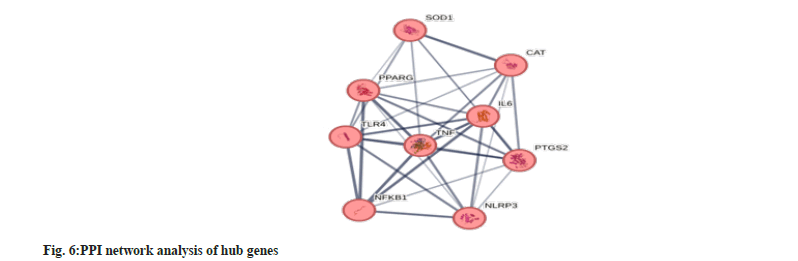
Our study established that the PPI network (fig. 6) consists of 12 nodes and 34 edges. The average node degree was 5.67, with an average clustering coefficient of 0.864. The analysis of PPI yielded an enrichment value of 6.64e-10, indicating a significant number of interactions within the network. This suggests that the proteins are biologically connected to each other, as a group, beyond what would be expected in a random set of proteins of the same size and degree distribution drawn from the genome. The PPI networks revealed 9 hub genes (Interleukin 6 (IL-6), Superoxide Dismutase (SOD-1), Catalase (CAT), Tumor Necrosis Factor (TNF), Toll-like receptor 4 (TLR4), Peroxisome Proliferator-Activated Receptor Gamma (PPARG), NFKB1, NOD-Like Receptor family, Pyrin domain containing 3 (NLRP3) and Prostaglandin-Endoperoxide Synthase 2 (PTGS2)) that were significant in liver diseases.
Our study established that the DEGs were enriched in various KEGG pathways such as NF-kB, MAPK, glycolysis, P13K-Akt signaling, regulation of autophagy or TGF-beta signaling pathways. The P13-Akt signaling pathway is an intracellular pathway that is known to be involved in various physiological processes, including cellular proliferation and cell metabolism. The NF-kB pathway was associated with the regulation of immune responses and inflammation. It is activated by pro-inflammatory cytokines, bacterial and viral pathogens, and physical and chemical stressors. The MAPK pathway was associated with cell growth and apoptosis. It is activated by growth factors, cytokines, stress signals and regulates the expression genes.
We revealed that cellular pathways were enriched in mitochondrial membrane spaces, peroxisomes, IL-6 receptor complexes, organelle envelope lumen and receptor complexes. In the representation of genes, the color red indicates a higher number, while blue indicates a lower number. According to molecular function, the molecular functions were enriched in identical protein binding, anti-oxidation, actinin binding, peroxidase enzyme, and oxidoreductase actions.
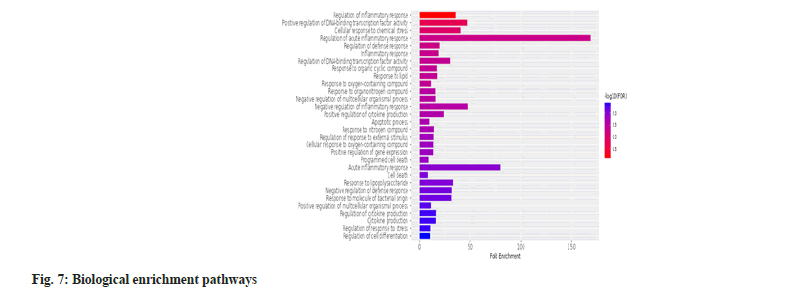
According to fig. 7, the significant genes were enriched in biological pathways such as regulation of inflammatory response, positive regulation of DNA-binding transcription factor activity, production of cytokines, response to organic cyclic compounds, response to lipids and organonitrogen compounds.
Our study established that Rehmannia glutinosa in liver diseases was associated with 9 hub genes (IL-6, SOD1, CAT, TNF, TLR4, PPARG, NFKB1, NLRP3 and PTGS2). We established that these hub genes were enriched in numerous biological pathways such as regulation of inflammatory response, positive regulation of DNA-binding transcription factor activity, production of cytokines, response to organic cyclic compounds, lipids and organonitrogen compounds. The molecular functions were enriched in identical protein binding, antioxidants, actinin binding, peroxidase activity, prostaglandin-endoperoxidase synthase activity. We revealed that cellular pathways were enriched in mitochondrial membrane spaces, peroxisomes, IL-6 receptor complexes, organelle envelope lumen, and receptor complexes.
Our study proposed that the major biological pathway of Rehmannia glutinosa in liver diseases was regulation of inflammatory response. We suggest that Rehmannia glutinosa exerts its anti-inflammatory response through active ingredients such as Catalpol, Rehmannioside A, B, C and Shanzhiside. For instance, Catalpol is an iridoid glycoside that is capable of activating both the NF-kB and MAPK signaling pathways, while simultaneously reducing the production of pro-inflammatory cytokines such as IL-6, IL-1beta, and TNF-alpha[9,10]. We suggest that inhibiting the NF-kB pathway is essential in regulating tissue damage and infections[11,12]. Activation of the NF-kB pathway leads to an increase in the rate of translocation from the cytoplasm to the nucleus, where it binds to specific DNA sequences and enhances the transcription of target genes that are known to be associated with pro-inflammatory cytokines.
We established those active ingredients such as Catalpol inhibits the activity of the NF-kB pathway by preventing the reduction and degradation of inhibitory components such as nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IkB-alpha)[10]. IkB-alpha acts as an inhibitor of the translocation of NF-kB to the nucleus by binding to it in the cytoplasm[13]. However, once the NF-kB pathway is activated, IkB-alpha undergoes phosphorylation and subsequent degradation, allowing translocation of NF-kB to the nucleus. Our proposal is that Catalpol prevents the phosphorylation and subsequent degradation of IkB alpha, leading to the inhibition of translocation of NF-kB to the nucleus and thereby reducing the expression of inflammatory cytokines.
Our study proposed that the MAPK pathway plays a crucial role in regulating the expression levels of pro-inflammatory cytokines. Activation of this pathway occurs in response to the binding of specific receptors on the cell surface by inflammatory cytokines, growth factors, or stress signals. This leads to the activation of a downstream signaling cascade[14]. The kinases within the MAPK pathway undergo phosphorylation and activation sequentially, activating Activator Protein 1 (AP-1) or NF-kB. AP-1 is a dimeric protein derived from Jun and Fos families that regulates various genes associated with inflammatory responses[15]. Catalpol has been found to inhibit the activation of the MAPK pathway by preventing the phosphorylation of MAPK proteins, including AP-1, c-Jun N-terminal kinases and extracellular signal-regulated kinases. By doing so, it reduces the expression of pro-inflammatory cytokines associated with liver diseases[10].
We propose that Rehmannioside A, an iridoid glycoside found in Rehmannia glutinosa, contributes to its immunomodulatory and anti-inflammatory effects by inhibiting the production of IL-6 and TNF-alpha. This inhibitory effect is achieved by blocking the activation of NF-kB, a critical transcription factor that regulates genes associated with inflammatory and immune responses. Hence, when hepatocytes are exposed to inflammatory stimuli like bacterial infections, Rehmannioside A may help prevent the excessive production of IL-6 and TNF-alpha and potentially mitigate the inflammatory response. NF-kB is activated and translocated to the nucleus, where it enhances the expression of pro-inflammatory cytokines[14-16]. Rehmannioside A, hinders the activation of NF-kB by inhibiting the phosphorylation and subsequent degradation of IkB-alpha. This leads to the retention of NF-kB in the cytoplasm, preventing its translocation to the nucleus.
Moreover, we propose that Rehmannioside A controls the balance between anti-inflammatory and pro-inflammatory cytokines. For instance, it increases the expression levels of IL-10 while lowering the expression levels of TNF-alpha and IL-6; therefore, it has an overall effect of anti-inflammatory response.
Our findings propose that the active ingredient Rehmannioside B is an iridoid glycoside with antioxidant and anti-inflammatory responses. It has a similar effect to Catalpol by hindering the activation of MAPK and NF-kB signaling pathways[17,18]. Moreover, it is a powerful antioxidant that eliminates the effects of free radicals which can increase damage to hepatocytes and lead to liver diseases[19]. We suggest that it attains its antioxidant mechanism by scavenging on free radicals (Reactive Oxygen Species (ROS) or Reactive Nitrogen Species (RNS)) and occluding them from destroying the hepatocytes. Also, it increases the up-regulation of antioxidant enzymes that have an overall effect of neutralizing free radicals. Conversely, it inhibits the activity of enzymes such as NADPH oxidase and NO synthase that increases the production of free radicals.
Rehmannioside B has a robust effect of lowering inflammation and oxidative stress which has a beneficial effect of increasing insulin sensitivity and reducing the prevalence of diabetes and other liver diseases[17,20]. Also, it increases glucose metabolism and could be used as a significant biomarker in treating and diagnosing diabetes.
Our study suggests that Rehmannioside C is an active ingredient of Rehmannia glutinosa associated with protection of hepatocytes and anti-inflammatory responses. It is an iridoid glycoside that prevents the release of TNF-alpha, while lowering the levels of oxidative stress markers in the liver[17]. The action of Rehmannioside C is achieved through various mechanisms, for instance, it blocks NF-kB and MAPK. Once, these signaling pathways are blocked, the activation of macrophages is inhibited resulting to a decrease in ROS and RNS. Therefore, in the liver, the levels of expression of both inflammatory cytokines and oxidative stress markers are decreased. Furthermore, Rehmannioside C is associated with elevating antioxidant enzymes such as SOD and GPx which neutralizes the effect of free radicals and offers protection to the hepatocytes from oxidative stress.
Shanzhiside was an iridoid glycoside and active ingredient in Rehmannia glutinosa associated with neuroprotection and anti-inflammatory responses. Shanzhiside reduces the production of IL-6, IL-1beta, and TNF-alpha by inhibiting the translocation of NF-kB to the nucleus[21]. These inflammatory cytokines are manufactured by the dendritic cells, monocytes and macrophages when the liver tissues are exposed to infections, injuries or chronic inflammations. We suggest that Shanzhiside prevents the release of transcription factors (for example, NF-kB) that plays a significant role in controlling the expression levels of pro-inflammatory cytokines. Therefore, Shanzhiside ensures an effective balance between anti-inflammatory and pro-inflammatory cytokines.
Shanzhiside ensures neuroprotection by scavenging for ROS and RNS released by pro-inflammatory cytokines and is associated with destroying the neurons[22]. Moreover, it can regulate the gene expression levels associated with the survival and repair of neurons. Our study established that the action of Rehmannia glutinosa was enriched in KEGG pathways such as NF-kB, MAPK, glycolysis, P13K-Akt signaling, regulation of autophagy or transforming growth factor-beta (TGF-Beta) signaling pathway. The glycolysis pathway is associated with the break down of glucose to release ATP[23]. The process is regulated by active ingredients in Rehmannia glutinosa that controls the levels of glucose metabolism and is significant in treating liver diseases. A study by Bao et al.[24] found that extracts of Rehmannia glutinosa were essential in improving glucose tolerance and resistance to insulin in mice models with Non-Alcoholic Fatty Liver Disease (NAFLD). Particularly, it increased the activity of glycolytic kinases (hexokinase or pyruvate kinase) and reduced the activity of gluconeogenic kinases (for example, phosphoenolpyruvate carboxykinase) in the liver. Wu et al.[25] established that extracts of Rehmannia glutinosa protect against hepatic fibrosis by preventing the overexpression of glycolytic enzymes and lowering the buildup of lactate in the liver tissues. The P13-Akt signaling pathway is an intracellular pathway linked with physiological processes such as cellular proliferation. An aberrant activation of this pathway increases liver cancer, diabetes, and cardiovascular infections. Wu et al.[25] suggested that extracts of Rehmannia glutinosa and its active ingredients such as Catalpol regulates cell proliferation and increases chances of survival in liver diseases. In contrast, Rahman et al.[26] suggested that extracts of Rehmannia glutinosa are capable of inhibiting the P13-Akt pathway and inducing apoptosis in liver cancer cells. Rehmannioside A has been reported to suppress the PI3K-Akt signaling pathway in chondrocytes and osteoclasts, leading to the inhibition of bone resorption and the prevention of osteoporosis.
The PPI networks revealed 9 hub genes (IL-6, SOD1, CAT, TNF, TLR4, PPARG, NFKB1, NLRP3 and PTGS2) that were associated with Rehmannia glutinosa in liver diseases. We established that IL-6 was an essential cytokine regulating inflammation and immune responses. In liver infections, IL-6 undergoes upregulation and enhances the pathogenesis of various infections such as cancer, diabetes, or hepatitis. Rehmannia glutinosa modulates IL-6 and inhibits its expression in the liver. Several studies[27-29] have proposed that active ingredients of Rehmannia glutinosa lower the levels of IL-6. For example, administration of Catalpol in a rat model of liver fibrosis resulted in a significant reduction in the levels of IL-6. Likewise, in a mouse model of acute liver injury, treatment with Rehmannioside A led to a decrease in IL-6 levels.
SOD1 is an antioxidant enzyme that protects the liver from oxidative damage caused by ROS[30]. Rehmannia glutinosa and its active compounds, such as Catalpol and Rehmannioside B, can increase the expression and activity of SOD1 in the liver. This upregulation of SOD1 can help to reduce oxidative stress and protect liver cells from damage.
CAT is an antioxidant enzyme in the liver. It catalyzes the breakdown of hydrogen peroxide (H2O2) into water and oxygen, thus protecting liver cells from ROS[31]. Rehmannia glutinosa and its active compounds can up-regulate CAT gene expression in liver cells and improve the antioxidant defense system. For example, Sun et al.[32] found that treatment with Rehmannia glutinosa extract increased the expression of CAT in rats with liver injury induced by carbon tetrachloride (CCl4). Catalpol increased the expression of CAT and other antioxidant enzymes in liver cells exposed to oxidative stress.
TNF is a cytokine with pro-inflammatory properties that has been implicated in the pathogenesis of various liver diseases, including, alcoholic liver disease, non-alcoholic fatty liver disease, and viral hepatitis[33]. TNF activates immune cells, including Kupffer cells, which release pro-inflammatory cytokines, leading to liver inflammation and injury.
Rehmannia glutinosa possess anti-inflammatory effects, including the inhibition of TNF production, which can help alleviate liver inflammation and injury. Some studies have shown that treatment with Rehmannia glutinosa can reduce serum TNF levels in animal models of liver injury[34,35]. Additionally, Catalpol and Rehmannioside B, have been shown to inhibit the activation of NF-κB, associated with TNF and other pro-inflammatory cytokines.
The inhibition of TNF production, suggest that it may have potential therapeutic benefits for liver diseases characterized by inflammation and immune cell activation. More research is required to fully understand the mechanisms underlying the effects of Rehmannia glutinosa as a potential treatment for liver diseases. Additionally, optimal dosage and treatment duration also need to be determined. TLR4 are proteins associated with immune responses and enhance the development of liver infections such as NAFLD, Alcoholic Liver Disease (ALD), and hepatitis[36]. The activation of TLR4 with corresponding ligands increases the levels of pro-inflammatory cytokines which increases the damage to liver cells. Rehmannia glutinosa possess anti-inflammatory effects and may therefore has a protective effect against liver diseases that involve TLR4-mediated inflammation.
We propose that treatment with Rehmannia glutinosa can reduce TLR4 expression in the liver and decrease the production of pro-inflammatory cytokines, including TNF-α and IL-6. Rehmannia glutinosa has been shown to have hepatoprotective effects in animal models of liver injury induced by TLR4 ligands such as Lipopolysaccharide (LPS).
Our study established that the PPARG is a gene that encodes a transcription factor critical in regulating lipid metabolism and glucose homeostasis[37]. PPARG is expressed in various tissues, including the liver, regulating lipid storage and glucose metabolismAberrations in PPARG signaling have been associated with the emergence of metabolic disorders such as NAFLD and type 2 diabetes. Treatment with Rehmannia glutinosa extract can increase the expression of PPARG in liver cells, leading to improved lipid metabolism and glucose homeostasis.
In a study on Rehmannia glutinosa on NAFLD, it was found that treatment with Rehmannia glutinosa extract improved lipid metabolism and reduced fat accumulation in the liver, in part through upregulation of PPARG expression[38]. Another study showed that Rehmannia glutinosa extract improved insulin sensitivity and glucose metabolism in obese rats by up-regulating PPARG expression in liver cells.
Our study established that NLRP3 is a gene that encodes a protein involved in the activation of the inflammasome complex, and release of IL-1β and IL-18. Dysregulation of NLRP3 has been implicated in the pathogenesis NAFLD, ALD, and liver fibrosis[39]. Catalpol hinders the activation of NLRP3 inflammasome and reduces the release of IL-1β in a mouse model of ALD. Similarly, Rehmannia polysaccharide attenuates liver injury and fibrosis in a mouse model of NAFLD by inhibiting the action of NLRP3 inflammasome.
We established that the PTGS2 hub gene encodes for the COX-2 enzyme, and has been implicated in inflammatory response. COX-2 produces prostaglandins, which are potent mediators of inflammation[40]. Rehmannia glutinosa downregulates the levels of PTGS2/COX-2 in liver cells and animal models of liver injury[40,41]. This suggests that Rehmannia glutinosa may exert its protective effects on the liver, at least in part, by inhibiting the COX-2-mediated inflammatory response. The hepatoprotective effects of Rehmannia glutinosa against liver fibrosis, hepatitis, and HCC may also involve the inhibition of COX-2.
Our study established that Rehmannia glutinosa contains several active components (for example, Catalpol, Rehmannioside A, and Shanzhiside) crucial in regulating pro-inflammatory cytokines and hub genes associated with liver diseases. We established 9 hub genes (IL-6, SOD1, CAT, TNF, TLR4, PPARG, NFKB1, NLRP3 and PTGS2) that were associated with Rehmannia glutinosa and could be used as significant biomarkers in the diagnosis and treatment of liver diseases. Our study revealed that Rehmannia glutinosa targets several biological pathways involved in the regulation of inflammatory response, DNA-binding transcription factor activity, and cellular response to chemical stress. Additionally, KEGG pathway analysis demonstrated that Rehmannia glutinosa modulates pathways such as NF-kB, MAPK, glycolysis, and p13K-Akt signaling, which are essential in liver diseases. Overall, our findings suggest that Rehmannia glutinosa exerts its hepatoprotective effects by inhibiting the release of inflammatory cytokines.
Conflict of interests:
The authors declared no conflict of interests.
References
- Yang Y, Chen X, Chen J, Xu H, Li J, Zhang Z. Differential miRNA expression in Rehmannia glutinosa plants subjected to continuous cropping. BMC Plant Biol 2011;11(1):1-53.
[Crossref] [Google Scholar] [PubMed]
- Wen XS, Yang SL, Wei JH, Zheng JH. Textual research on planting history of Rehmannia glutinosa and its cultivated varieties. Chin Tradit Herbal Drug 2002;33(10):946-9.
- Li XE, Chen SL, Wei SQ, Wei JH, Lan J. Analysis on adaptive area of Rehmannia glutinosa L. and it’s class partition. Chin J Chin Materia Med 2006;31(4):344-6.
- Bone K, Mills S. Principles and practice of phytotherapy: Modern herbal medicine. Elsevier Health Sciences; 2012.
- Bai L, Shi GY, Yang YJ, Chen W, Zhang LF, Qin C. Rehmannia glutinosa exhibits anti?aging effect through maintaining the quiescence and decreasing the senescence of hematopoietic stem cells. Animal Model Exp Med 2018;1(3):194-202.
[Crossref] [Google Scholar] [PubMed]
- Wu PS, Wu SJ, Tsai YH, Lin YH, Chao JC. Hot water extracted Lycium barbarum and Rehmannia glutinosa inhibit liver inflammation and fibrosis in rats. Am J Chin Med 2011;39(06):1173-91.
[Crossref] [Google Scholar] [PubMed]
- Kim SH, Yook TH, Kim JU. Rehmanniae radix, an effective treatment for patients with various inflammatory and metabolic diseases: Results from a review of Korean publications. J Pharmacopuncture 2017;20(2):81-8.
[Crossref] [Google Scholar] [PubMed]
- Zhang RX, Li MX, Jia ZP. Rehmannia glutinosa: Review of botany, chemistry and pharmacology. J Ethnopharmacol 2008;117(2):199-214.
[Crossref] [Google Scholar] [PubMed]
- El-Hanboshy SM, Helmy MW, Abd-Alhaseeb MM. Catalpol synergistically potentiates the anti-tumour effects of regorafenib against hepatocellular carcinoma via dual inhibition of PI3K/Akt/mTOR/NF-κB and VEGF/VEGFR2 signaling pathways. Mol Biol Rep 202;48(11):7233-42.
[Crossref] [Google Scholar] [PubMed]
- Zhao J, Tan Y, Feng Z, Zhou Y, Wang F, Zhou G, et al. Catalpol attenuates polycystic ovarian syndrome by regulating sirtuin 1 mediated NF-κB signaling pathway. Reprod Biol 2022;22(3):100671.
[Crossref] [Google Scholar] [PubMed]
- Lee DS, Kim KS, Ko W, Bae GS, Park SJ, Jang JH, et al. A fraction from Dojuksan 30 % ethanol extract exerts its anti-inflammatory effects through Nrf2-dependent heme oxygenase-1 expression. Int J Mol Med 2016;37(2):475-84.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Yue Y, Zhang Q, Liang L, Li C, Chen Y, et al. Structural characterization and anti-inflammatory effects of an arabinan isolated from Rehmannia glutinosa Libosch. Carbohyd Polym 2023;303:120441.
- Li Q, Li L, Yang JN, Wei Q. Identification, functional analysis and preliminary validation of differentially expressed genes in hyperacute cerebral infarction patients. 2021.
- Kim SH, Smith CJ, Van Eldik LJ. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1β production. Neurobiol Aging 2004;25(4):431-9.
[Crossref] [Google Scholar] [PubMed]
- Kyriakis JM. Activation of the AP-1 transcription factor by inflammatory cytokines of the TNF family. Gene Expr 1999;7(4-5):217-31.
[Google Scholar] [PubMed]
- Zhou J, Xu G, Yan J, Li K, Bai Z, Cheng W, et al. Rehmannia glutinosa (Gaertn.) DC. polysaccharide ameliorates hyperglycemia, hyperlipemia and vascular inflammation in streptozotocin-induced diabetic mice. J Ethnopharmacol 2015;164:229-38.
[Crossref] [Google Scholar] [PubMed]
- Li M, Jiang H, Hao Y, Du K, Du H, Ma C, et al. A systematic review on botany, processing, application, phytochemistry and pharmacological action of Radix Rehmnniae. J Ethnopharmacol 2022;285:114820.
[Crossref] [Google Scholar] [PubMed]
- Gong PY, Guo YJ, Tian YS, Gu LF, Qi J, Yu BY. Reverse tracing anti-thrombotic active ingredients from dried Rehmannia Radix based on multidimensional spectrum-effect relationship analysis of steaming and drying for nine cycles. J Ethnopharmacol 2021;276:114177.
[Crossref] [Google Scholar] [PubMed]
- Morikawa T, Nakanishi Y, Inoue N, Manse Y, Matsuura H, Hamasaki S, et al. Acylated iridoid glycosides with hyaluronidase inhibitory activity from the rhizomes of Picrorhiza kurroa Royle ex Benth. Phytochemistry 2020;169:112185.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Wang J, Chan P. Treating type 2 diabetes mellitus with traditional Chinese and Indian medicinal herbs. Evid Based Complement Alternat Med 2013;2013:343594.
[Crossref] [Google Scholar] [PubMed]
- Viljoen A, Mncwangi N, Vermaak I. Anti-inflammatory iridoids of botanical origin. Curr Med Chem 2012;19(14):2104-27.
[Crossref] [Google Scholar] [PubMed]
- Dinda B, Dinda B. Pharmacology of iridoids. Pharmacology and Applications of Naturally Occurring Iridoids. 2019. p.145-254.
- Chandel NS. Glycolysis. Cold Spring Harbor Perspect Biol 2021;13(5):a040535.
- Bao Q, Shen X, Qian L, Gong C, Nie M, Dong Y. Anti-diabetic activities of catalpol in db/db mice. Korean J Physiol 2016;20(2):153-60.
[Crossref] [Google Scholar] [PubMed]
- Wu PS, Wu SJ, Tsai YH, Lin YH, Chao JC. Hot water extracted Lycium barbarum and Rehmannia glutinosa inhibit liver inflammation and fibrosis in rats. Am J Chin Med 2011;39(06):1173-91.
[Crossref] [Google Scholar] [PubMed]
- Rahman HS. Preclinical drug discovery in colorectal cancer: A focus on natural compounds. Curr Drug Targets 2021;22(9):977-97.
[Crossref] [Google Scholar] [PubMed]
- Liu CL, Cheng L, Ko CH, Wong CW, Cheng WH, Cheung DW, et al. Bioassay-guided isolation of anti-inflammatory components from the root of Rehmannia glutinosa and its underlying mechanism via inhibition of iNOS pathway. J Ethnopharmacol 2012;143(3):867-75.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Xu H, Zang Y, Liu W, Sun X. Radix Rehmannia Glutinosa inhibits the development of renal fibrosis by regulating miR-122-5p/PKM axis. Am J Transl Res 2022;14(1):103.
[Google Scholar] [PubMed]
- Kwon Y, Yu S, Choi GS, Kim JH, Baik M, Su ST, Kim W. Puffing of Rehmannia glutinosa enhances anti-oxidant capacity and down-regulates IL-6 production in RAW 264.7 cells. Food Sci Biotechnol 2019;28(4):1235-40.
[Crossref] [Google Scholar] [PubMed]
- Fukui M, Zhu BT. Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radical Biol Med 2010;48(6):821-30.
[Crossref] [Google Scholar] [PubMed]
- Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med 2018;54(4):287-93.
- Sun Z, Tan X, Xu M, Liu Q, Ye H, Zou C, et al. Effects of dietary dandelion extracts on growth performance, liver histology, immune-related gene expression and CCl4 resistance of hybrid grouper (Epinephelus lanceolatus?×Epinephelus fuscoguttatus?). Fish Shellfish Immunol 2019;88:126-34.
[Crossref] [Google Scholar] [PubMed]
- Das SK, Balakrishnan V. Role of cytokines in the pathogenesis of non-alcoholic fatty liver disease. Indian J Clin Biochem 2011;26:202-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Jia R, Wang F, Qiu G, Qiao P, Xu X, et al. Catalpol protects mice against Lipopolysaccharide/D-galactosamine-induced acute liver injury through inhibiting inflammatory and oxidative response. Oncotarget 2018;9(3):3887.
[Crossref] [Google Scholar] [PubMed]
- Yang W, Hao Y, Hou W, Fang X, Fang P, Jiang T, et al. Jieduan-Niwan formula reduces liver apoptosis in a rat model of acute-on-chronic liver failure by regulating the E2F1-mediated intrinsic apoptosis pathway. Evid Based Complement Alternat Med 2019;2019.
[Crossref] [Google Scholar] [PubMed]
- Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int 2010;4(4):659-72.
[Crossref] [Google Scholar] [PubMed]
- Houseknecht KL, Cole BM, Steele PJ. Peroxisome proliferator-activated receptor gamma (PPARγ) and its ligands: A review. Domestic Animal Endocrinol 2002;22(1):1-23.
[Crossref] [Google Scholar] [PubMed]
- Muthukumaran P, Thiyagarajan G, Babu RA, Lakshmi BS. Raffinose from Costus speciosus attenuates lipid synthesis through modulation of PPARs/SREBP1c and improves insulin sensitivity through PI3K/AKT. Chem Biol Interact 2018;284:80-9.
- Malhotra S, Sastre-Garriga J, Castillo J, Montalban X, Comabella M. NLRP3 (Nod-like receptor family, pyrin domain containing 3) inflammasome is increased in patients with primary progressive multiple sclerosis. Multiple Sclerosis J 2015;21:452.
- Bao L, Chen Y, Li H, Zhang J, Wu P, Ye K, et al. Dietary Ginkgo biloba leaf extract alters immune-related gene expression and disease resistance to Aeromonas hydrophila in common carp Cyprinus carpio. Fish Shellfish Immunol 2019;94:810-8.
[Crossref] [Google Scholar] [PubMed]
- You Y, Chen X, Chen X, Li H, Zhou R, Zhou J, et al. Jiawei Yanghe Decoction suppresses breast cancer by regulating immune responses via JAK2/STAT3 signaling pathway. J Ethnopharmacol 2023:116358.
[Crossref] [Google Scholar] [PubMed]