- *Corresponding Author:
- Jun Zhang
Department of Oncology, The Third People's Hospital of Chengdu, Chengdu, Sichuan Province 610031, China
E-mail: hsrlw@163.com
| Date of Received | 05 June 2021 |
| Date of Revision | 14 December 2021 |
| Date of Acceptance | 06 July 2022 |
| Indian J Pharm Sci 2022;84(4):832-837 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of shikonin on the oxidative damage of lung cancer cells and promotes cell senescence through p53/p21Waf signaling pathway. The lung adenocarcinoma A549 cells were cultured and made into cell suspension. The lung adenocarcinoma A549 cells were cultured for 12 h, 24 h and 48 h respectively with different concentrations of shikonin (0 μm, 2 μm and 4 μm) and the activity of lung adenocarcinoma A549 cells was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide method. The cell cycle of each group was measured by flow cytometry after 48 h of action of shikonin. The aging of each group of cells was determined by beta-galactosidase staining after 48 h of action of shikonin. The reactive oxygen species content in each group was determined by flow cytometry 48 h after the action of shikonin. The expression levels of cyclin D1, demethylase KDM2B, deoxyribonucleic acid damage marker protein p-H2AX, p53 and p21WAF were measured by Western blot. With the increase of shikonin concentration, the cell activity and the expression levels of cyclin D1 and KDM2B were significantly decreased, the proportion of resting phase/intermediate phase cells and senescent cells were significantly increased, the level of reactive oxygen species and the expression levels of p-H2AX, p53 and p21WAF were significantly increased (p<0.05). Shikonin can inhibit cell activity, induce oxidative damage and promote cell senescence, which may be achieved by activating p53/p21WAF signaling pathway.
Keywords
Shikonin, p53/p21waf signaling pathway, lung cancer, oxidative damage, cell aging
Lung cancer, also known as primary bronchogenic carcinoma, is one of the most common malignant tumors in the lungs. According to statistics, more than 10 million patients die from lung cancer each year and more than 1.2 million new cases have emerged every year. Whereas the 5 y survival rate of lung cancer patients is only about 16 %[1]. With the development of new effective anti-cancer drugs and the increase of new treatment regimens, the curative effect of chemotherapy has been greatly improved. At present, the comprehensive treatment of non-small cell lung cancer is mainly combined with radiotherapy and chemotherapy. However, the recurrence rate is high, the adverse reactions caused by chemotherapy are quite a lot and the prognosis is poor[2]. Traditional Chinese medicine is the traditional treasure of China. A variety of active ingredients of traditional Chinese medicine have a certain anti-tumor effect. Currently, the traditional Chinese medicine has become an important means to treat advanced lung cancer. In recent years, many studies have confirmed the advantages and status[3] of traditional Chinese medicine in the treatment of lung cancer. Shikonin is an important active ingredient of Lithospermum. Some studies have found that the shikonin could promote programmed cell necrosis in glioma cells and breast cancer, and play a role of clearing heat, detoxifying and anti-inflammatory, etc. In addition, the shikonin may also induce oxidative stress[4,5]. However, its mechanism and roles in lung cancer are not clear. The purpose of this study is to investigate the effect of shikonin on oxidative damage and cell senescence of lung cancer cells and to discuss its mechanism.
Materials and Methods
Experimental cells:
Cell line A549 of human non-small cell lung cancer (Wuhan Procell Life Science & Technology Co., Ltd.)
Major instruments and reagents:
Low-temperature high-speed centrifuge (Changsha Dongwang Experimental Instruments Co., Ltd.); ultra-low temperature refrigerator (Zhejiang Jisheng Cryogenic Equipment Co., Ltd., model: DW-86W300); paraffin slicer (Beijing Shengkexinde Technology Co., Ltd., model: RM2255); electron microscope (Beijing Jingkerida Technology Co., Ltd., model: EM208s); electronic balance (Changsha Dongwang Experimental Instruments Co., Ltd., model: TG20KR-D); fetal bovine serum (Shanghai Jianglin Biotechnology Co., Ltd.); anhydrous ethanol (Dongguan Wanxin Fine Chemical Co., Ltd.); reverse transcriptome kit (Shanghai Yucan Biotechnology Co., Ltd.) and shikonin (Chengdu Herbpurify Co., Ltd., purity over 98 %).
Cell culture:
The A549 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM). The cells in good growth condition and in logarithmic phase were made into cell suspension. The cell suspension was taken and centrifuged repeatedly for 3 times, cultured in an incubator containing 10 % fetal bovine serum, 5 % carbon dioxide at 37°. The culture medium was changed daily. The trypsin was added for digestion when the cells grow to about 80 %. The culture was terminated when the cells became round, then gently blown until the cells fell off completely. The cell suspension was repacked into a new culture flask and cultured in an incubator containing 10 % fetal bovine serum, 5 % carbon dioxide at 37°. The cells were counted and inoculated into cryopreservation tube with the concentration of 10 000 cells/ml. It was slowly frozen at 4° first, then placed in an ultra-low temperature refrigerator at -80° for 1 h and finally put into the liquid nitrogen standby for testing.
Observation indicators:
Determination of cellular activity: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method was used for the detection of the change of A549 cell activity. Cell suspension was taken and the A549 cells were cultured with different concentrations (0 µM, 2 µM and 4 µM) of shikonin for 12 h, 24 h and 48 h. Then it was cultured for 2 h at 37° after adding 5 mg/ml of MTT solution. The absorbance value at 470 nm was determined by enzyme-linked immunosorbent assay (ELISA).
Cell cycle measurement: The change of cell cycle was measured by flow cytometry in each group 48 h after the action of shikonin. The cell suspension was taken and cultured for 48 h with different concentrations (0 µM, 2 µM and 4 µM) of shikonin, washed by phosphate buffer and centrifuged. The supernatant was removed and 1 ml of phosphate buffer was added and suspended again. Then 600 μl Propidium Iodide (PI) was added in, gently vortexing and blending. The cells were incubated at 37° away from light for 0.5 h and fully mixing for computer testing.
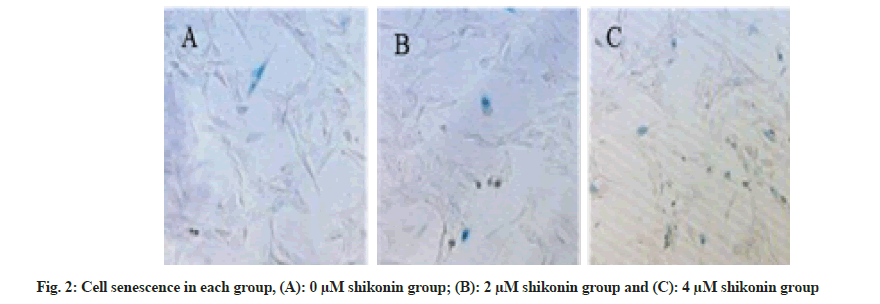
Determination of cellular senescence: The cell senescence of each group was determined by beta (β)-galactosidase staining 48 h after the action of shikonin. The cell suspension was taken and A549 cells were cultured for 48 h with different concentrations (0 µM, 2 µM and 4 µM) of shikonin. The fixed solution was added at room temperature for 10 min. It was washed by phosphate buffer solution and stained by X-gal staining solution. b-galactosidase-positive cells were observed by microscope and the percentage of positive cells was calculated, namely, the proportion of senescent cells (positive cell ratio=positive cell number/total cell number×100 %).
Determination of Reactive Oxygen Species (ROS): The content of ROS in each group was determined by flow cytometry 48 h after the action of shikonin. The cell suspension was taken and A549 cells were cultured for 48 h with different concentrations (0 µM, 2 µM and 4 µM) of shikonin. The trypsin was added for digestion. It was washed by phosphate buffer solution. The cells were re-suspended by adding 1 ml of phosphate buffer and then adding 1 μl of stain. It was incubated at 37° for 0.5 h and centrifuged. The supernatant was removed and 1 ml of the phosphate buffer solution was added for computer testing.
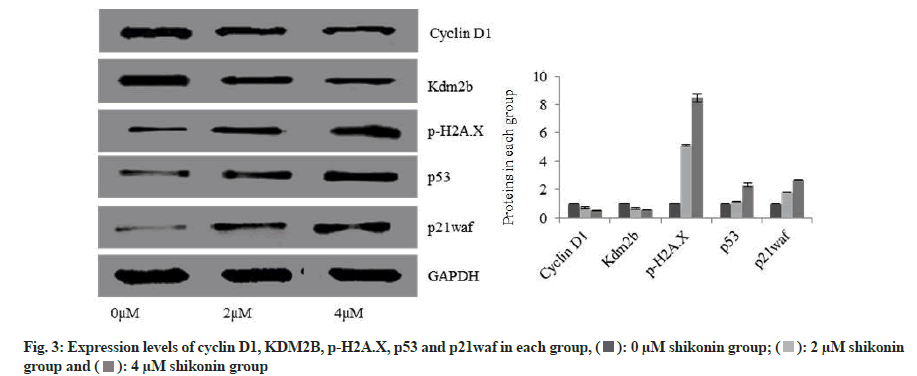
The expression levels of cyclin D1, demethylase KDM2B and Deoxyribonucleic Acid (DNA) injury marker protein p-H2AX, p53 and p21waf in each group were determined by Western blotting. The cell suspension was taken and cultured with different concentrations (0 µM, 2 µM and 4 µM) of shikonin for 48 h and then the cell lysis buffer was added for full pyrolysis. The supernatant was taken after being centrifuged at 4°, namely, the total cell protein. The sample protein was taken to calculate the sample volume. It was heated for protein denaturation. The separation gel and spacer gel were put in to go through loading, electrophoresis, trarsmembrane and sealing. Primary antibodies were added and incubated at 4°. The horseradish peroxide-cohered goat polyclonal secondary antibodies to rabbit Immunoglobulin G (IgG) was added and incubated at room temperature. The chemiluminescence solution was added. It was placed in a luminous machine for exposure and storage of strips. The image grayscale analysis was carried out using Quantity One.
Statistical methods:
The comparison of measurement data was accordance with normal distribution, including cell activity, cell proportion of Resting phase/Intermediate phase (G0/G1), percentage of senescent cells, ROS content, and expression levels of cyclin D1, KDM2B, p-H2AX, p53 and p21waf in each group of this study. The comparison among multiple groups was based on univariate multi-sample mean. The independent sample t test was used for pairwise comparison and all expressed as (x̄±s). The Statistical Package for the Social Sciences (SPSS) 22.0 software package was used to analyze the statistical data in this study and the statistical results of p<0.05 was considered that the difference was statistically significant.
Results and Discussion
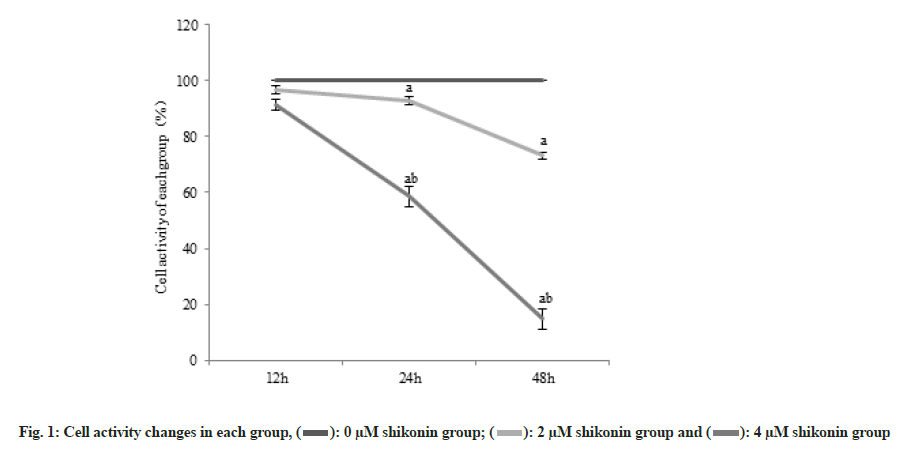
The cell activity decreased significantly (p<0.05) in the 2 µM shikonin group compared with the 0 µM shikonin group and the cell activity decreased significantly (p<0.05) in the 4 µM shikonin groups compared with the 2 µM shikonin group. Cell activity in each group decreased significantly (p<0.05) with the increase of shikonin concentration and extension of time as shown in Table 1 and fig. 1.
| Group | Number of cases | Cell activity (%) | ||
|---|---|---|---|---|
| 12 h | 24 h | 48 h | ||
| 0 µM shikonin group | 6 | 100.00±0.01 | 100.00±0.01 | 100.00±0.01 |
| 2 µM shikonin group | 6 | 96.65±1.42a | 92.71±1.55a | 73.11±1.26a |
| 4 µM shikonin group | 6 | 91.44±1.79ab | 49.58±3.73ab | 14.77±3.62ab |
| F | 64.15 | 819.25 | 2325.9 | |
| p | <0.001 | <0.001 | <0.001 | |
Note: Compared with 0 µM shikonin group, ap<0.05 and compared with 1 µM shikonin group, bp<0.05
Table 1: Changes of Cell Activity (x̄±s).
The percentage of cells in G0/G1 was significantly increased (p<0.05) in the 2 µM shikonin group 48 h after the action of shikonin compared with the 0 µM shikonin group and the percentage of cells in G0/G1 was significantly increased (p<0.05) in the 4 µM shikonin group compared with the 2 µM shikonin group. With the increase of shikonin concentration, the percentage of cells in G0/G1 was increased significantly (p<0.05) in each group as shown in Table 2.
| Group | Number of cases | Percentage of cells in G0/G1 |
|---|---|---|
| 0 µM shikonin group | 6 | 42.69±1.63 |
| 2 µM shikonin group | 6 | 50.86±1.38a |
| 4 µM shikonin group | 6 | 57.23±0.22ab |
| F | 207.43 | |
| p | <0.001 | |
Note: Compared with 0 µM shikonin group, ap<0.05 and compared with 1 µM shikonin group, bp<0.05
Table 2: Changes of Cell Percentage in G0/G1 of each Group (x̄±s).
The percentage of senescent cells was significantly increased (p<0.05) in the 2 µM shikonin group 48 h after the action of shikonin compared with that in the 0 µM shikonin group and the percentage of senescent cells in the 4 µM shikonin group was significantly increased (p<0.05) compared with the 2 µM shikonin group. With the increase of shikonin concentration, the percentage of senescent cells was increased significantly (p<0.05) in each group as shown in Table 3 and fig. 2.
| Group | Number of cases | Percentage of senescent cells |
|---|---|---|
| 0 µM shikonin group | 6 | 10.04±0.09 |
| 2 µM shikonin group | 6 | 49.74±2.68a |
| 4 µM shikonin group | 6 | 81.67±2.36ab |
| F | 1816.55 | |
| p | <0.001 | |
Note: Compared with 0 µM shikonin group, ap<0.05 and compared with 1 µM shikonin group, bp<0.05
Table 3: Cell Senescence in each Groups (x̄±s).
The ROS content was significantly increased (p<0.05) in the 2 µM shikonin group 48 h after the action of shikonin compared with the 0 µM shikonin group and the percentage of ROS content in the 4 µM shikonin group was significantly increased (p<0.05) compared with the 2 µM shikonin group. With the increase of shikonin concentration, the ROS content was decreased significantly (p<0.05) in each group as shown in Table 4.
| Group | Number of cases | ROS content (%) |
|---|---|---|
| 0 µM shikonin group | 6 | 8.63±0.74 |
| 2 µM shikonin group | 6 | 11.85±0.81a |
| 4 µM shikonin group | 6 | 28.97±2.67ab |
| F | 258.21 | |
| p | <0.001 | |
Note: Compared with 0 µM shikonin group, ap<0.05 and compared with 1 µM shikonin group, bp<0.05
Table 4: ROS Expression Levels in each Group (x̄±s).
Expression levels of cyclin D1 and KDM2B were significantly decreased in the 2 µM shikonin group 48 h after the action of shikonin compared with the 0 µM shikonin group while the expression levels of p-H2AX, p53 and p21waf were significantly increased (p<0.05); and the expression levels of cyclin D1 and KDM2B were significantly decreased in the 4 µM shikonin group compared with the 2 µM shikonin group while the expression levels of p-H2AX, p53 and p21waf were significantly increased (p<0.05). With the increase of shikonin concentration, the expression levels of cyclin D1 and KDM2B were decreased significantly in each group while the expression levels of p-H2AX, p53 and p21waf were increased significantly (p<0.05) as shown in fig. 3.
Lung cancer is one of the most common respiratory malignancies clinically. In China, its morbidity and mortality rank first in malignant tumors. Among them, non-small cell lung cancer accounts for about 80 % of lung cancer and it is a common type of lung cancer. At present, surgical resection is still the main method for the treatment of lung cancer. However, the clinical manifestations of lung cancer are often complex and diverse. In the early stage of lung cancer, because the clinical symptoms of most patients are not obvious, it is difficult to attract the attention of patients. Therefore, most of the patients have already been in the middle and late stage at the time of diagnosis. Some patients even have experienced distant metastasis and missed the best treatment time. Moreover, the prognosis of the patients is poor and the 5 y survival rate is low. Therefore, it is the main goal of the current treatment to find out a therapy to alleviate the patients’ condition and improve their quality of life. As one of the effective ingredients of Lithospermum, shikonin has been used for a long time in the treatment of burns, carbuncle, measles, yellow macula and sore throat, etc. In recent years, some scholars have found that the shikonin can inhibit the growth of tumor cells and induce cell death[6]. The purpose of this study is to investigate the related roles and mechanisms of shikonin in lung cancer.
Cell senescence is a comprehensive manifestation of the decline and disorder of physiological function in the degenerative period and it is an irreversible process. Some studies have found that initiating cell senescence procedure will significantly improve the efficacy of anti-tumor drugs, which may be an important mechanism[7] to inhibit tumors. The main feature of tumor cells is that the cells proliferate indefinitely without differentiation, with some characteristics of the immortalization. More and more studies have found that the cell immortalization has an important relationship with tumorigenesis and development, which may be an important prerequisite for tumorigenesis, while the cell senescence is a process[8,9] against cell immortalization. It can be seen that inducing tumor cell senescence may be an important way to inhibit tumors. Furthermore, some studies have found that cell cycle G0/G1 arrest is also a prerequisite[10] for the cell senescence. Cell cycle is the basic process of life activity between the cells and organism. The cell cycle plays an important role in cell proliferation, differentiation, senescence and death. When the cell cycle is abnormal, the abnormal expression of cell division genes will make the cells enter the state of transformation and carcinogenesis, and the cell will proliferate indefinitely, resulting in the occurrence[11] of cancer. Cyclin D1 is a cyclin that regulates cells from G0/G1 to Synthesis (S) phase. The study has found that the cyclin D1 is expressed significantly in tumor cells and promotes the cell proliferation[12]. The results of this study reveal that shikonin could significantly promote cell senescence, suppress cell activity, inhibit the expression of cyclin D1 and block cell cycle in G0/G1. It is concentration dependent.
According to domestic and foreign research reports, the imbalance of expression of proto-oncogene and anti-oncogene, oxidative stress and shortening of telomerase may be the factor[13] affecting cell senescence. Among them, ROS can be produced by redox reactions or external stimuli. Under normal circumstances, the ROS maintains a relatively dynamic balance. When it is stimulated, the ROS homeostasis imbalance causes DNA damage and activates downstream p53 and p21waf, thereby inducing cell senescence[14]. p53 is a tumor suppressor, which can remove diseased cells caused by DNA injury or abnormal regulation of cell cycle, induce phenotypic changes of cell senescence, suppress cell proliferation and inhibit the possibility[15] carcinogenesis. P21 is a product of waf while the waf is a target gene of the p53, which can block cell cycle and prevent cells from entering S stage. KDM2B is a histone demethylase. It has been found that KDM2B can participate in the redox reaction of cells and play a role of directly inhibiting the generation[16] of ROS. The results of this study show that the shikonin could induce cells producing ROS, lead to DNA damage, induce p53 and p21waf expression and activate signal pathways.
Above all, shikonin can inhibit cell activity, induce oxidative damage in cells and promote cell senescence, which may be achieved by activating p53/p21waf signaling pathway.
Authors’ contributions:
Liwen Rong and Yuxin He have contributed equally to this work.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Erren TC, Morfeld P, Glende CB, Piekarski C. Silica and lung cancer. Epidemiology 2007;18(4):521.
[Crossref] [Google Scholar] [PubMed]
- Hopkin JM, Evans HJ. Cigarette smoke-induced DNA damage and lung cancer risks. Nature 1980;283(5745):388-90.
[Crossref] [Google Scholar] [PubMed]
- Cheng W, Chen X. Advances in the mechanism of action of traditional Chinese medicine against non-small cell lung cancer. Chin J Exp Med Formula 2016;26(24):234-41.
- Zhang X, Cui JH, Meng QQ, Li SS, Zhou W, Xiao S. Advance in anti-tumor mechanisms of shikonin, alkannin and their derivatives. Mini Rev Med Chem 2018;18(2):164-72.
[Crossref] [Google Scholar] [PubMed]
- Song X, Gao X, Han Q. Effects of shikonin on proliferation, apoptosis, migration and invasion of T24 human bladder cancer cells. Prog Anat Sci 2020;26(2):63-5.
- Wang J, Li Q, Wu J. Preparation of shikonin microemulsion modified with CD133 antibody and study on anti-triple-negative breast cancer. Chin Tradit Herbal Drug 2019;50(7):88-96.
- Xande JG, Dias AP, Tamura RE, Cruz MC, Brito B, Ferreira RA, et al. Bicistronic transfer of CDKN2A and p53 culminates in collaborative killing of human lung cancer cells in vitro and in vivo. Gene Ther 2020;27(1):51-61.
[Crossref] [Google Scholar] [PubMed]
- Katayama M, Kiyono T, Ohmaki H, Eitsuka T, Endoh D, Inoue‐Murayama M, et al. Extended proliferation of chicken‐and Okinawa rail‐derived fibroblasts by expression of cell cycle regulators. J Cell Physiol 2019;234(5):6709-20.
[Crossref] [Google Scholar] [PubMed]
- Maishi N, Kikuchi H, Sato M, Nagao-Kitamoto H, Annan DA, Baba S, et al. Development of immortalized human tumor endothelial cells from renal cancer. Int J Mol Sci 2019;20(18):4595.
[Crossref] [Google Scholar] [PubMed]
- Chen ZH, Jing YJ, Yu JB, Jin ZS, Li Z, He TT, et al. ESRP1 induces cervical cancer cell G1-phase arrest via regulating cyclin A2 mRNA stability. Int J Mol Sci 2019;20(15):3705.
[Crossref] [Google Scholar] [PubMed]
- Kim B, Kim SW, Lim JY, Park SJ. NCAPH is required for proliferation, migration and invasion of non-small-cell lung cancer cells. Anticancer Res 2020;40(6):3239-46.
[Crossref] [Google Scholar] [PubMed]
- Si-Yuan JI, Zi-Dan WU, Zhang TH, Zhang J, Zheng-Yi WE. In vitro antitumor effect of cucurbitacin E on human lung cancer cell line and its molecular mechanism. Chin J Nat Med 2020;18(7):483-90.
[Crossref] [Google Scholar] [PubMed]
- González‐Gualda E, Pàez‐Ribes M, Lozano‐Torres B, Macias D, Wilson III JR, González‐López C, et al. Galacto‐conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging cell 2020;19(4):e13142.
[Crossref] [Google Scholar] [PubMed]
- Chen KY, Chen CC, Chang YC, Chang MC. Resveratrol induced premature senescence and inhibited epithelial-mesenchymal transition of cancer cells via induction of tumor suppressor Rad9. PloS one 2019;14(7):e0219317.
[Crossref] [Google Scholar] [PubMed]
- Wan Q, Chen H, Li X, Yan L, Sun Y, Wang J. Artesunate inhibits fibroblasts proliferation and reduces surgery-induced epidural fibrosis via the autophagy-mediated p53/p21waf1/cip1 pathway. Eur J Pharmacol 2019;842:197-207.
[Crossref] [Google Scholar] [PubMed]
- Zacharopoulou N, Kallergi G, Alkahtani S, Tsapara A, Alarifi S, Schmid E, et al. The histone demethylase KDM2B activates FAK and PI3K that control tumor cell motility. Cancer Biol Ther 2020;21(6):533-40.
[Crossref] [Google Scholar] [PubMed]
 .
.
 .
.



