- *Corresponding Author:
- C. N. Nalini
Department of Pharmaceutical Analysis, C. L. Baid Metha College of Pharmacy, Thoraipakkam, Chennai-600 096, India
E-mail: nalini_cn@yahoo.co.in
| Date of Submission | 16 January 2004 |
| Date of Revision | 4 April 2005 |
| Date of Acceptance | 17 February 2006 |
| Indian J Pharm Sci, 2006, 68 (1):95-97 |
Abstract
A reversed phase high performance liquid chromatographic method has been developed using Shimadzu HPLC-VP series, LC-10 ATV pump, SPD10 AVP and C8 column, for simultaneous determination of pseudoephedrine hydrochloride and cetrizine hydrochloride in three marketed tablet formulations (extended release). The mobile phase consists of phosphate buffer of pH 7.0 and acetonitrile HPLC grade in the ratio of 1:1. The flow rate was maintained at 1 ml/min and the ultraviolet detection was done at 242 nm, which is the isosbestic point. Linearity coefficients, assay values, recovery studies and repeatability studies showed that the method is accurate and precise.
Pseudoephedrine hydrochloride (PEH) is official in IP [1]. Cetrizine hydrochloride (CEH) is official in BP [2]. Fixed dose combinations of PEH and CEH are widely used for the symptomatic treatment of allergic rhinitis. Many methods have been reported in the literature for the determination of similar formulation with various other drugs using HPLC [3-5], HPTLC [6] and spectrophotometry [7]. However, a method for the simultaneous determination of PEH and CEH in tablets by HPLC has not been reported. In this present work, efforts have been made to develop an isocratic method using a simple mobile phase and UV detection for the simultaneous determination of the above drugs.
A high performance liquid chromatographic system from Shimadzu VP series, consisting of LC10 ATV Pump and SPD 10 AVP UV detector, was used for the analysis. C8 (150×4.6 mm, 5 μ) column was used in the analysis with a flow rate of 1 ml/min. A Rheodyne injector with a 20 μl loop was used for injecting the samples. Ammonium hydrogen phosphate (AR grade) and acetonitrile (HPLC grade) were used for mobile phase preparation. Working reference standards of PEH and CEH were obtained from Dr. Ceeal Analytical Lab, Chennai. Three marketed tablet formulations were selected for the study. The brand names of the tablets are Alerid (batch no. T10593, Cipla Ltd.), Cetrizet (batch no. SK 10893, Sun Pharmaceuticals) and Zyncet (batch no. 14086, Noble Medicure Pvt. Ltd.). Phosphate buffer (20 mM), pH 7.0 and acetonitrile in the ratio 1:1 was used as the mobile phase with a flow rate of 1 ml/min. The HPLC procedure was carried out at an ambient temperature.
A stock solution containing 6 mg/ml of PEH and 250 μg/ ml of CEH was prepared. Standard solution containing PEH and CEH was prepared from stock solution by suitable dilution to get a concentration of 3.6 mg/ml and 150 μg/ml respectively.
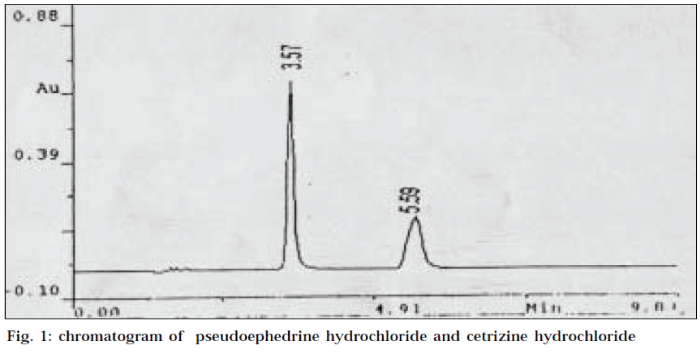
Twenty tablets were weighed and crushed to a fine powder. A powder quantity equivalent to 7.5 mg of CEH was weighed and transferred to a 50 ml volumetric flask. The powder was dissolved in water by shaking for 15 min, filtered and then made up to the mark with mobile phase. Dilutions were made to get the concentration of 3.6 mg/ml of PEH and 150 μg/ml of CEH. A volume of 20 μl each of standard and sample solutions was injected into the stabilized HPLC system. Detection was kept at 242 nm, which is the isobestic point. The retention times of PEH and CEH were found to be 5.59 min and 3.57 min respectively. The chromatogram of PEH and CEH is given in Fig. 1. The respective peak areas of standard and sample for each ingredient were used for quantification.
The assays were carried out by the proposed method, and the results are tabulated in Table 1. Accuracy of the method was established by performing recovery studies. The study was carried out by spiking the known concentration of the samples with active ingredients at three levels (20, 40, 60 μg/ml for PEH and 2, 4, 6 μg/ml for CEH), and the results are tabulated in Table 2. Linearity and range of the method was carried out by injecting five mixed standard solutions containing 1.2 to 6 mg/ml of PEH and 50 to 250 μg/ml of CEH. The calibration curves were plotted using peak area vs concentration. Linearity coefficients obtained are given in Table 3. Precision of the method was demonstrated by repeatability studies [8]. This was assessed by using nine determinations for each tablet (3 concentrations/3 replicates each) covering the specified range for the procedure. Percentage RSD was calculated and given in Table 3. The system suitability studies9 were carried out to determine resolution factor, symmetry factor and precision of the instrument. Results are tabulated in Table 3. This systematic study revealed that the proposed method for the simultaneous determination of PEH and CEH is simple, sensitive, and with good precision and accuracy. This method can be used for the routine determination of pseudoephedrine hydrochloride and cetrizine hydrochloride simultaneously.
| Contents | Label claim mg/tablet | Brand A | Brand B | Brand C | |||||
|---|---|---|---|---|---|---|---|---|---|
| Amount found* mg/tablet | % Label claim | Amount found* mg/tablet | % Label claim | Amount found* mg/tablet | % Label claim | ||||
| PEH | 120 | 120.01 | 100.01 | 119.71 | 99.76 | 119.69 | 99.74 | ||
| CEH | 5 | 4.97 | 99.52 | 5.02 | 100.4 | 5.08 | 101.64 | ||
*Values are mean of six determinations; PEH is pseudo ephedrine hydrochloride and CEH is cetrizine hydrochloride. Brand A - Alerid, Brand B – Cetrizet, Brand C – Zyncet.
Table 1: Results of quantitative estimation in tablets
| Contents | Label claim mg/tablet | Amount Added µg/ml | % Recovery* ± RSD | ||
|---|---|---|---|---|---|
| Brand A | Brand B | Brand C | |||
| PEH | 120 | 20 | 100.72 ± 0.69 | 100.18 ± 0.51 | 99.84 ± 0.05 |
| 40 | 100.75 ± 0.72 | 100.20 ± 0.60 | 100.41 ± 0.25 | ||
| 60 | 100.70 ± 0.51 | 99.95 ± 0.72 | 100.20 ± 0.32 | ||
| CEH | 5 | 2 | 100.28 ± 0.63 | 100.05 ± 0.51 | 100.25 ± 0.43 |
| 4 | 100.30 ± 0.52 | 99.98 ± 0.42 | 100.10 ± 0.21 | ||
| 6 | 100.21 ± 0.70 | 100.10 ± 0.32 | 100.21 ± 0.52 | ||
*Values are mean of three determinations; PEH is pseudo ephedrine hydrochloride and CEH is cetrizine hydrochloride. Brand A - Alerid, Brand B – Cetrizet, Brand C – Zyncet.
Table 2: Recovery studies
| Parameters | PEH | CEH |
|---|---|---|
| Linearity range | 1.2-6 mg/ml | 50-250 µg/ml |
| Correlation Coefficient (r) | 0.9990 | 0.9982 |
| Quantitative estimation (% C.V.) | 0.0173 | 0.3078 |
| Repeatability Intra-day* (% C.V.) | 0.3405-0.525 0.1850-0.4150 | |
| Resolution factor (RS) | 5.15 | 5.15 |
| Symmetry factor | 0.92 | 1.0 |
| No. of theoretical plates | 1896.06 | 3155.68 |
* n = 9; PEH is pseudo ephedrine hydrochloride and CEH is cetrizine hydrochloride.
Table 3: Method validation and system suitability parameters
Acknowledgements
The authors wish to thank the Management of C. L. Baid Metha College of Pharmacy, Chennai, for providing the facilities to carry out the work. We wish to extend our thanks to Dr. Ceeal Analytical Lab, Chennai.References
- Indian Pharmacopoeia, 4th Edn., Vol. I, The Controller of Publications, New Delhi, 1996, 641.
- British Pharmacopoeia, Vol. I, Her Majesty’s Stationery Office, London, 2002.
- Raman, R., Yadgire, S.K. and Bhanu, R., Indian Drugs, 2001, 38, 307.
- Shindae, V.M., Desai, B.S. and Tendolkar, N.M., J. Pharm. Sci.,1995, 57, 35.
- Vasudevan, M., Ravishankar, S., Sathyanarayanan, A. and Chandan, R.S., Indian Drugs, 2001, 38, 276.
- Sane, R.T., Francis, M., Khedkar, S., Pawar, S. and Moghe, A., Indian Drugs, 2001, 38, 436.
- Mohana, R., Kawathekar, N. and Chaturvedi, S.C., J. Pharm. Sci., 1996, 58, 93.
- ICH Harmonized Tripartite Guideline Q2B, Validation of Analytical Procedures: Methodology, step 4, ICH steering committee, European Union, Japan and USA,1994.
- The United States Pharmacopoeia XXIV, NF XIX, United States Pharmacopoeial Convention, Inc., Rockville, MD, 2000, 1924.