- Corresponding Author:
- S. D. Rajendran
J. S. S. College of Pharmacy, Ootacamund-643 001, Tamilnadu,
E-mail: sdrajendran@rediffmail.com
| Date of Submission | 21 October 2005 |
| Date of Revision | 02 March 2006 |
| Date of Acceptance | 15 November 2006 |
| Indian J Pharm Sci, 2006, 68 (6): 715-718 |
Abstract
A high performance liquid chromatographic method is described for simultaneous estimation of amiodarone and its metabolite desethylamiodarone in plasma. After precipitation with acetonitrile, the separation of amiodarone, desethylamiodarone and internal standard was accomplished using reversed phase chromatography. The mobile phase, a combination of ammonium acetate (pH 3.5 adjusted with ortho phosphoric acid) and acetonitrile was run isocratically through a C18 analytical column. The UV detection was done at 242 and 247 nm for amiodarone and desethylamiodarone respectively. Analytical run time was 10 min. Mean recovery was 84% for 0.5 µg/ml concentrations. The assay exhibited good linear relationship between peak height ratios and plasma concentration. Quantification limit was at least 0.01 µg/ml of amiodarone and desethylamiodarone. Accuracy and precision were over the concentration range of 0.01-10 µg/ml. Assay was successfully applied to the measurement of amiodarone and its metabolite desethylamiodarone in human plasma of patients who were on long-term oral therapy on amiodarone.
Amiodarone hydrochloride is an iodinated benzofuran derivative antiarrythmic agent. The drug differs structurally and pharmacologically from other currently available antiarrhythmic agents. Amiodarone hydrochloride has been an important drug for the treatment of supraventricular and ventricular arrhythmias, in short-term inpatient and long- term outpatient settings. It may also have a role in affecting outcomes in patients at risk for arrhythmic events and sudden death; its place among available therapies is being established in clinical trials [1]. The increase in amiodarone use has been prompted in large part by the realization that alternative antiarrythmic drugs are prone to increase in mortality in post myocardial infraction patients [2]. In contrast to these Type I agents, amiodarone appears to improve survival in patients after myocardial infarction3 and with congestive heart failure [4]. Amiodarone thus shifted from an agent of last recourse to that of first line therapy [5] and its popularity has further increased by its rapidly growing use in the management of refractory atrial arrhythmias. Amiodarone possesses interesting pharmacokinetic profile with a long elimination half life, large volume of distribution, and erratic and sometimes incomplete bioavailability [6,7]. There is a need for estimation of inter individual variability required to support therapeutic drug monitoring. Though many high performance liquid chromatographic (HPLC) methods of assay [8-16] for use in pharmacokinetic studies are available for determination of amiodarone in biological specimens, as mentioned in Table 1, they are complex, expensive, time consuming and use internal standards that are expensive or not commonly available. , they are complex, expensive, time consuming and use internal standards that are expensive or not commonly available. There is a need for a simple method for the simultaneous estimation of amiodarone and its metabolite desethylamiodarone in human plasma.
| Species | Matrix | Volume (ml) | Validated LLQ (µg/ml) | Reference |
|---|---|---|---|---|
| Human | Serum | 1 | NS | 8 |
| Human | Serum | 1 | 0.008 | 9 |
| Human | Serum | 0.2 | NS | 10 |
| Human | Serum | 1 | 0.1 | 11 |
| Human | Plasma | 0.5 | 0.02 | 12 |
| Human | Plasma | 0.5 | 0.25 | 13 |
| Rat | Plasma | 0.1 | 0.035 | 14 |
| Human | Plasma | 0.2 | 0.3 | 15 |
| Human | Serum | 0.5 | 0.16 | 16 |
Table provides the references of the previously published studies on the development of high-performance liquid chromatographic method for the analysis of amiodarone from blood of animals and human. NS stands for not stated and LLQ is the lowest limit of quantification
Table 1: Previously Published Assay for Amiodarone in Blood Fluids
Here, we describe a HPLC method for simultaneous estimation of amiodarone and its metabolite desethylamiodarone in human plasma. The method can be used in pharmacokinetic studies of amiodarone where multiple, serial blood sample collection is desired in individuals.
Materials and Methods
Amiodarone hydrochloride (Medreich, India), desethylamiodarone (Sanofi-Aventis, Newcastle, UK) and etoricoxib (Glenmark Pharmaceuticals, Mumbai) were obtained as gift samples. Acetonitrile, triethylamine, ammonium acetate, and ortho-phosphoric acid were purchased from Qualigens Fine chemicals (Mumbai, India). Water for analytical purpose was obtained from Milli-Q RO system.
Chromatographic conditions
The HPLC system consisted of a Shimadzu LC-10AT liquid chromatographic pump, SIL-10a auto sampler and SPD-10A UV/Vis UV absorbance detector (Shimadzu, Kyoto, Japan). Data collection, integration and calibration were accomplished using Class VP Chromatography Data System Version 4.2 computer software (Shimadzu, Kyoto, Japan). The chromatographic separations of amiodarone, desethylamiodarone, and internal standard (etoricoxib) were accomplished using a 250 × 4.6 mm I.D Luna 5μ C18 analytical column (Phenomenex, USA). A Guard-Pak pre- column module (Phenomenex, USA) containing an ODS cartridge insert was placed serially just before the analytical column. The mobile phase consisted of acetonitrile: 10 mM ammonium acetate (pH 3.5) in a combination of 60:40 v/v. Before use the mobile phase was degassed by passing it through a 0.22 μm filter.
The mobile phase was pumped at an isocratic flow rate of 1 ml/min at room temperature. The UV detection wavelength was set at 242 nm and 247 in two channels. The wavelengths of 242 and 247 represented the UV maximum of amiodarone and desethylamiodarone, respectively, in acetonitrile: water in 1:1 ratio.
Assay procedure
A stock solution representing 100 μg/ml of amiodarone was prepared in acetonitrile: water in 1:1 ratio. Similarly, a stock solution of desethylamiodarone was also prepared. These solutions were stored at -20° until use. Amiodarone is known to remain stable for at least 3 mo under these conditions. The working standard solutions were prepared prior to use from the stock solution by sequential dilution with a combination of acetonitrile: water in 90:10 ratio to yield final concentrations of 10, 1, 0.1, 0.01, and 0.001 μg/ml of amiodarone. Similarly desethylamiodarone working standards were also prepared. The internal standard stock solution was prepared by dissolving 1 mg of etoricoxib in 100 ml of acetonitrile: water in 1:1 ratio. This solution was stored at 20° until use.
In a 2 ml micro-centrifuge tube, 500 μl of plasma was added along with 500 μl of internal standard solution. The plasma proteins were precipitated by the addition of 500 μl of acetonitrile, and then the tubes were vortex mixed for 30 sec and centrifuged at 11 000×g for 8 min. The supernatant was transferred to a clean, similarly labeled tube and was subsequently recentrifuged for 2 min. The resultant solution was injected in to the HPLC system.
Assay parameters
The extraction efficiency was determined by comparing the peak area of known amounts of amiodarone and desethylamiodarone (unextracted) in mobile phase directly injected to peak areas of samples containing the same amounts of amiodarone in plasma after extraction.
Quantification was based on calibration curves constructed using peak area ratios of drug to internal standard vs. nominal concentration. Intra-day reproducibility was tested by using four different concentrations (0.010, 0.100, 1, and 10 μg/ml). The procedure was repeated on three separate days to allow determination of inter-day precision and accuracy. Intra-day accuracy was estimated based on the mean percentage error, and the inter-day accuracy was calculated as the mean of the intra-day accuracy determinations. The precision, expressed as a percentage, was evaluated by calculating the intra- and inter-day relative standard deviations.
The standard drug solutions in varying concentrations ranging from 0.01 μg/ml to 10.0 μg/ml were examined by the assay procedure. The response factor was calculated. The calibration curve was plotted using response factor Vs concentration of the standard solutions. The calibration curve showed a linear response over the range of concentrations used in the assay procedure. The calibration curve passes through the origin, which justifies the use of single point calibration.
Patient samples
Patient plasma samples were obtained from patients who were on long-term oral amiodarone therapy. The protocol was approved by the institutional ethical committee. Two blood samples were collected i.e., 6-10 h and 24 h postdose of amiodarone. The samples were placed on ice immediately after collection. Subsequently, separated plasma samples were kept frozen at -70° until analysis. Blank plasma samples were collected from volunteers for method validation and were stored at -70°.
Results and Discussion
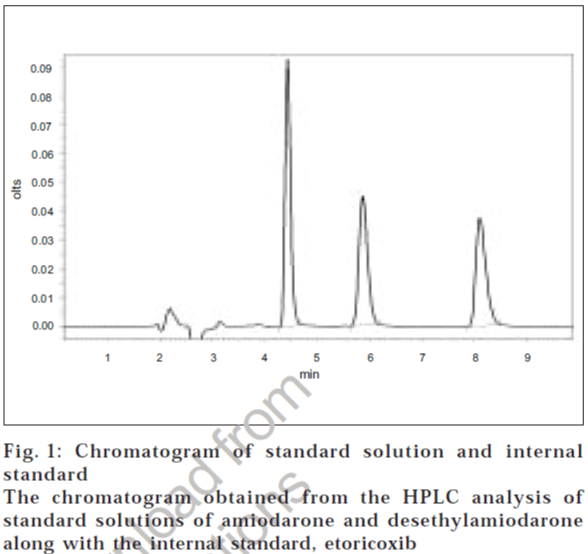
Peaks corresponding to internal standard, desethylamiodarone and amiodarone eluted free of interfering substances, at 4.4, 5.6 and 7.6 min, respectively (fig. 1). The analytical run time was 10 min for each plasma sample. The mean extraction efficiencies of amiodarone from 500 μl of plasma at concentrations of 0.01 μg/ml to 10 μg/ml were 90-92% and of desethylamiodarone were 90-93%. Good linear relationship were obtained between peak area ratios and the corresponding plasma concentration over a range of 0.01-10 μg/ml of amiodarone.
The inter-day and intra-day coefficients of variation were less than 14% for amiodarone and less than 19% for desethylamiodarone (Table 2). Over the range of concentrations from 0.01-10 μg/ml, the intra-day accuracies ranged from 90 to 99.7% for amiodarone and 91-98% for desethylamiodarone, and average inter-day accuracy ranged from 93.2 to 98.3% for amiodarone and 91 to 98.1% for desethylamiodarone. Based on this data, the validated lower limit of quantification of the method was 0.01 μg/ml based on 500 μl of human plasma. In patients taking a dose of 50-400 mg/day of amiodarone, plasma samples were analyzed using the described HPLC method (fig. 2).
| Drug | Added conc(μg/ml) | Intraday (n=5) | Interday (n=5) | ||||
|---|---|---|---|---|---|---|---|
| Observed conc (μg/ml) | CV (%) | Accuracy (%) | Observed conc(μg/ml) | CV (%) | Accuracy (%) | ||
| Amiodarone | 0.01 | 0.009 ± 0.0011 | 12.2 | 90 | 0.0095 ± 0.001 | 12.63 | 95 |
| 0.1 | 0.098 ± 0.013 | 13.2 | 98 | 0.0932 ± 0.011 | 11.83 | 93.2 | |
| 1 | 0.96 ± 0.043 | 4.47 | 96 | 0.981 ± 0.043 | 4.3 | 98.1 | |
| 10 | 9.97 ± 0.13 | 1.43 | 99.7 | 9.83 ± 0.57 | 5.7 | 98.3 | |
| Desethylamiodarone | 0.01 | 0.0091 ± 0.001 | 10.98 | 91 | 0.0091 ± 0.001 | 12.08 | 91 |
| 0.1 | 0.098 ± 0.012 | 12.24 | 98 | 0.096 ± 0.012 | 12.5 | 96 | |
| 1 | 0.96 ± 0.18 | 18.75 | 96 | 0.952 ± 0.14 | 11.5 | 95.2 | |
| 10 | 9.78 ± 0.15 | 1.53 | 97.8 | 9.81 ± 0.42 | 4.2 | 98.1 | |
The intra-day and inter-day precision and accuracy data for the simultaneous analysis of amiodarone and desethylamiodarone from human plasma samples. *CV is coefficient of variation
Table 2: Intraday and Interday Precision and Accuracy Data
The HPLC method described here is accurate and precise, and is capable of determining concentrations of amiodarone and desethylamiodarone in small volumes of human plasma. The extraction procedure was simple and the procedure used a commercially available inexpensive internal standard (etoricoxib). The method performed well with respect to reproducibility and accuracy over therange of concentrations studied (Table 3). This assay method was rapid, simple, and accurate. The chromatographic run time was less than 10 min. The lower limits of quantification and the small plasma volume necessary for the assay were ideal for studying amiodarone pharmacokinetics. The assay was found to be suitable for pharmacokinetic studies in humans treated with oral dose of amiodarone, and was validated for measurement of concentration as low as 10 ng/ml of amiodarone based on 500 μl of human plasma.
| Drug | Nominal spike concentration (µg/ml) |
Observed concentration (µg/ml) |
CV (%) | Recovery (%) |
|---|---|---|---|---|
| Amiodarone | 0.01 | 0.0092± 0.0012 | 13.04 | 92 |
| 0.1 | 0.460 ± 0.0012 | 2.17 | 92 | |
| 1.0 | 0.091± 0.005 | 5.49 | 91 | |
| 10 | 0.90± 0.08 | 8.89 | 90 | |
| Desethylamiodarone | 0.01 | 0.0090± 0.0010 | 11.11 | 90 |
| 0.1 | 0.450 ± 0.0033 | 7.33 | 90 | |
| 1.0 | 0.093 ± 0.005 | 5.38 | 93 | |
| 10.0 | 0.92± 0.08 | 8.70 | 92 |
Table describes the percentage recovery data of the drug amiodarone and the metabolite desethylamiodarone from human plasma samples at various concentrations of the drug and the metabolite. *CV is coefficient of variation
Table 3: Accuracy – Recovery Studies
References
- Jafari-Fesharaki, M. and Scheinman, M.M., Pacing Clin. Electrophys. 1998, 21, 108.
- Lazzara, R., Amer. J. Cardiol., 1996, 78, 28.
- Burkart, F., Pfisher, M., Kiowski, W. and Follath, F., J. Amer. Coll. Cardiol., 1990,16, 1711.
- Cairns, J.A, Coonnolly, S.J. and Roberts, R., Lancet, 1997, 349, 675.
- Singh, B. N., Clin. Cardiol., 1997, 20, 608.
- Pollak, P. T., Bouillon, T. and Shafer, S. L., Clin. Pharmacol. Ther., 2000, 67, 642.
- Meng, X., Mojaverian, P., Doedee, M., Lin, E., Weinryb, I., Chiang, S. T. and Kowey, P.R., Amer. J. Cardiol., 2001, 87, 432.
- Ress, K., Liebich, H. M., Kramer, B., Ickrath, O., Risler, T. and Seipel,L., J. Chromatogr., 1987, 417, 465.
- Bliss, M., Mayersohn, M. and Nolan, P., J. Chromatogr., 1986, 381, 179.
- Kannan, R., Miller, S., Perez, V. and Singh, B.N., J. Chromatogr., 1987, 385, 225.
- Brien, J.F., Jimmo, S. and Armstrong, P.W., Can. J. Physiol. Pharmacol., 1983, 61, 245.
- Hutchings, A., Spragg, B.P. and Routledge, P. A., J. Chromatogr., 1986, 382, 389.
- Duranti, L., Caracciolo, M. and Oriani, G., J. Chromatogr., 1983,277, 401.
- Jun, A.S. and Brocks, D.R., J. Pharm. Pharm. Sci., 2001, 4 (3), 263.
- Juenke, J.M., Brown, P.I., McMillin, G.A. and Urry, F.M., J. Anal. Toxicol., 2004, 28, 63.
- Jandreski, M.A. and Vanderslice, W.E., Clin. Chem., 1993, 39,496.