- Corresponding Author:
- A. Shirwaikar
Department of Pharmaceutics, Manipal College of Pharmaceutical Sciences, Manipal - 576 104, India. E-mail: arunshirwaikar@yahoo.co.in
| Date of Submission | 15 March 2007 |
| Date of Revision | 10 September 2007 |
| Date of Acceptance | 14 February 2008 |
| Indian J Pharm Sci, 2008, 70 (1): 128-131 |
Abstract
A novel, simple, sensitive and rapid spectrophotometric method has been developed for simultaneous estimation of esomeprazole and domperidone. The method involved solving simultaneous equations based on measurement of absorbance at two wavelengths, 301 nm and 284 nm, 'λ max of esomeprazole and domperidone respectively. Beer's law was obeyed in the concentration range of 5-20 µg/ml and 8-30 µg/ml for esomeprazole and domperidone respectively. The method was found to be precise, accurate, and specific. The proposed method was successfully applied to estimation of esomeprazole and domperidone in combined solid dosage form.
Keywords
Esomeprazole, domeperidone, λ max, spectrophotometric method
Esomeprazole magnesium trihydrate [1] (ESO) is chemically bis(5-methoxy-2-[(S)-[(4-methoxy- 3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]- 1-H-benzimidazole-1-yl) magnesium trihydrate, a compound that inhibits gastric acid secretion. Esomeprazole is cost effective in the treatment of gastric oesophageal reß ux diseases. Esomeprazole is the S-isomer of omeprazole, the first single optical isomer proton pump inhibitor, generally provides better acid control than current racemic proton pump inhibitors and has a favorable pharmacokinetic proÞ le relative to omeprazole [2]. Domperidone [3] (DOM) chemically, [5-chloro-1-[1,3-(2,3-dihydro-2-oxo-1Hbenzmidazole- 1yl)propyl)-4-piperdinyl-1,3-dihydro- 2H-benzimidazole-2-one] is a dopamine antagonist. A detailed survey of literature revealed the estimation of omeprazole by gas chromatographic method [4], UV spectrophotometric method [5,6], TLC [7] and several HPLC [8-20] methods. Estimation of DOM included spectrophotometric methods [21,22], HPLC [23-26] and HPTLC [27] in dosage forms. Combination of these two is used for the treatment of gastric esophagus reß ux disease. However, no references have been found for simultaneous determination of ESO and DOM in pharmaceutical formulations. A successful attempt has been made to estimate two drugs simultaneously by spectrophotometric analysis.
A Shimadzu UV/Vis spectrophotometer, model 1601 (Japan) was employed with spectral bandwidth of 0.1 nm and wavelength accuracy of ± 0.5 nm with automatic wavelength correction with a pair of 3 mm quartz cells. ESO, DOM (obtained as gift samples from Themis Laboratories Pvt. Ltd., Thane), methanol (Merck India Ltd., Mumbai) and distilled water were used in the present study.
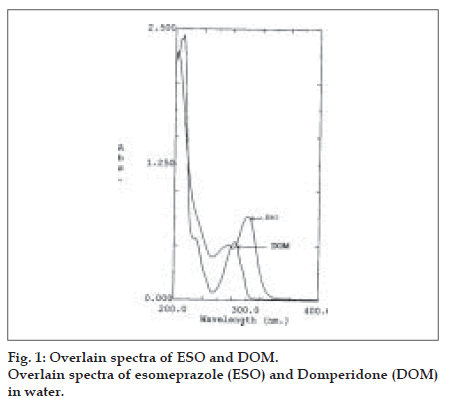
Stock solutions (500 µg/ml) of ESO and DOM were prepared by dissolving separately 50 mg in 50 ml of methanol in 100 ml volumetric flasks, and the volume was made up to 100 ml with distilled water. The maximum absorbances of ESO and DOM were obtained at 301 nm (λ1) and 284 nm (λ2), respectively. ESO and DOM showed linearity with absorbance in the range of 5-20 µg/ml and 8-30 µg/ml at their respective maxima, which were validated by least square method. Coefficients of correlation were found to be 0.9972 for ESO and 0.9986 for DOM. For simultaneous estimation of ESO and DOM, a series of standard solutions in concentration range of 5 to 20 µg/ml, were prepared by diluting appropriate volumes of the standard stock solutions. The scanning solutions of ESO and DOM were carried out in the range of 200 to 400 nm against water as blank for obtaining the overlain spectra that are used in the analysis (fig. 1). Absorbance and absorptivities of series of standard solutions were recorded at selected wavelengths λ1 and λ2.
The absorptivity values for ESO and DOM at 284 nm were 243 ± 1.73 and 266 ± 1.80, respectively. At 301 nm the absorptivity of ESO and DOM were 383 ± 1.87 and 47 ± 1.73, respectively. The optical characteristics and regression values for the calibration curve are presented in Table 1. The method employed simultaneous equations using Cramer′s rule and matrices (C1 = λ2e2 × Aλ1-λ1e2 × Aλ2/λ1e1 × λ2e2-λ1e2 × λ2 e1 and C2 = λ1e1 × Aλ2-λ2e1 × Aλ1/λ1e1 × λ2e2-λ1e2 × λ2 e1). A set of two simultaneous equations was framed using the mean of absorptivity values, given as Aλ1 = 243C1 + 266C2 and Aλ2 = 383C1 + 47C2, where, C1 and C2 are the concentrations of ESO and DOM respectively in simple solution (µg/ml). Aλ1 and Aλ2 are the absorbances of the sample solutions measured at 284 and 301 nm, respectively.
| Parameters | ESO | DOM |
|---|---|---|
| Beer’s law range | 5-20 μg/ml | 8-30 μg/ml |
| Molar absorptivity (0.001 | 1.864 ´104 | 1.133 ´104 |
| absorbance unit/mole.cm/dm3) | ||
| Sandell’s sensitivity (μg/cm2/0.001 | 0.0412 | 0.0376 |
| absorbance unit) | ||
| Regression coefficient (r) | 0.9972 | 0.9986 |
| Slope | 0.2038 | 0.1285 |
| Intercept | 0.1729 | 0.1505 |
Table 1: Regression and optical characteristics of esomeprazole and domperidone
Twenty capsules were weighed accurately. The average weight was determined and then ground to a fine powder. A quantity equivalent to 30 mg of DOM and 20 mg of ESO were transferred to a 100 ml volumetric ß ask. The contents were ultrasonicated for 10 min with 50 ml of methanol, made to volume with distilled water. Then the solution was Þ ltered through a Whatman filter paper (No. 40). The filtrate was further diluted with distilled water. The absorbance of the resulting solution was measured at 284 and 301 nm. The result of the analysis of the capsule formulation is presented in Table 2.
| BrandA | BrandB | ||
|---|---|---|---|
| % ESO | % DOM | % ESO | % DOM |
| 99.54 ± 0.89 | 100.21 ± 0.63 | 99.92 ± 1.05 | 99.65 ± 0.77 |
Brand A: Ignis 20 (Alembic Ltd, Vadodara) and Brand B: Izra - D (Unichem India Ltd, Mumbai) containing 20 mg of Esomeprazole magnesium trihydrate equivalent to 20 mg of esomeprazole and 30 mg of domperidone
Table 2: Analysis of commercial formulation
To study accuracy, reproducibility and precision of the proposed methods, recovery studies were carried out at three different levels by addition of standard drug solution to preanalysed sample. Results of recovery studies were found to be satisfactory and the results are presented in Table 3.
| Drug in standard mixture solution (μg/ml) | % Recovery ±SD | Coefficient of variance | |||
|---|---|---|---|---|---|
| ESO | DOM | ESO | DOM | ESO | DOM |
| 2 | 2 | 99.36 ±0.144 | 99.30 ±0.261 | 0.332 | 0.511 |
| 4 | 4 | 99.32 ±0.271 | 99.11 ±0.447 | 0.424 | 0.358 |
| 6 | 6 | 99.64 ±0.355 | 99.52 ±0.591 | 0.297 | 0.493 |
SD stands for standard deviation, the results are mean of three readings (n = 3)
Table 3: Recovery studies
The proposed method for simultaneous estimation of ESO and DOM in combined sample solutions was found to be simple, accurate and reproducible. Beer′s law was obeyed in the concentration range of 5-20 µg/ml and 8-30 µg/ml for esomeprazole and domperidone respectively. Co-efficient of variation was found to be 0.9972 and 0.9986 for ESO and DOM, respectively. The percentage recoveries were found to be in the range of 99.32 to 99.64% and 99.11 to 99.42% for ESO and DOM, respectively. Once the equations are determined, analysis requires only the measuring of the absorbance of the sample solution at two wavelengths selected, followed by a few simple calculations. It is a new and novel method and can be employed for routine analysis in quality control laboratories.
Acknowledgements
The authors thank Themis Laboratories Pvt. Ltd., Thane, for supplying gift samples of Esomeprazole and Domperidone to carry out this work.
References
- Andersson T, Hassan-Alin M, Hasselgren G, Rohss K, Weidolf L. Pharmacokinetic studies with Esomeprazole, the (S)-Isomer of omeprazole. Clinical Pharmacokinetics 200l;40:4ll-26.
- Scott LJ, Dunn CJ, Mallarkey G, Sharpe M. Esomeprazole- A review of its use in the management of acid-related disorders. Drugs 2002;62:l503-38.
- British Pharmacopoeia, Vol.l, London: Her Majesty’s Stationary Office; l993. p. 5l9.
- Petersen KU, Schmutzler W. Proton pump inhibitors release of active substance from various preparation. Detsche Apotheker Zeitung l999;l39:68-9.
- Castro D, Moreno MA, Torrado S, Lastres JL. Comparison of derivative spectrophotometric and liquid chromatographic methods for the determination of omeprazole in aqueous solution during stability studies. J Pharm Biomed Anal l999;2l:29l-8.
- Ozaltin N, Kocer A. Determination of omeprazole in pharmaceuticals by derivative spectroscopy. J Pharm Biomed Anal l997;l6:337-42.
- Dogrukol AK, Tunalier Z, Tuncel M. TLC densitometric determination of omeprazole in pharmaceutical preparations. Pharmazie l998;53:272-3.
- Sluggett GW, Stong JD, Adams JH, Zhao Z. Omeprazole determination using HPLC with coulometric detection. J Pharm Biomed Anal 200l;25:357-6l.
- Mathew M, Gupta VD, Bailery RE. Stability of omeprazole solutions at various pH values as determined by high performance liquid chromatography. Drug Develop Ind Pharm l998;2l:965-7l.
- l0. Ding L, Yang J, Yan HL, Zhang ZX, An DK. Determination of omeprazole and its pharmacokinetic in human plasma by an improved HPLC method. Chinese J Pharm Anal l999;l7:458-6l.
- ll. Shim SH, Bok SJ, Kwon KI. Determination of omeprazole in rat plasma by HPLC with column switching. Arch Pharm Res l994;l7:458-6l.
- l2. Zhi XJ, Hunang J, Zhang JH, Wang HT, Zhang LL. Determination of omeprazole and its metabolites in plasma by RP-HPLC. Chinese J Pharm l999;30:l66-8.
- l3. Motevalian M, Saeedi G, Keyhanfar F, Tayebi L, Mahmoudian M. Simultaneous determination of omeprazole and its metabolites in human plasma by HPLC using solid phase extraction. Pharm Pharmacol Commun l999;5:265-8.
- l4. Yeung PK, Little R, Jiang YQ, Buckley SJ, Veldhuyzen SJ, Zanten VN. Simple high performance liquid chromatography assay for simultaneous determination of omeprazole and metronidazole in human plasma and gastric fluid. J Pharm Biomed Anal l998;l7:l393-8.
- l5. Kobayashi K, Chiba KO, Sohn DR, Kato Y, Ishizaki T. Simultaneous determination of omeprazole and its metabolites in plasma and urine by reversed phase high performance liquid chromatography with an alkaline resistant polymer coated Cl8 column. J Chromatogr B l992;ll7:299-305.
- l6. Amantea MA, Narang PK. Improved procedure for quantization of omeprazole and metabolites using reversed phase high performance liquid chromatography. J Chromatogr A l988;426:2l6-22.
- l7. Hassan-Alin M, Andersson T, Bredberg E, Rohss K. Pharmacokinetics of esomeprazole after oral and intravenous administration of single and repeated doses to healthy subjects. Eur J Clin Pharmacol 2000;56- l:665-70.
- l8. Johnson DA, Roach AC, Carlsson AS, Karlsson AA, Behr DE. Stability of esomeprazole capsule contents after in vitro suspension in common soft foods and beverages. Pharmacotherapy 2003;23:73l-4.
- l9. Li XQ, Anderson TB, Ahlstrom M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities Drug Metab Dispos 2004;32:82l-7.
- Shetty R, Subramanian G, Ranjith Kumar A, Pandey S, Udupa N. Estimation of esomeprazole in human plasma by reverse phase high performance liquid chromatography. Indian Drugs 2005;42:l58-6l.
- 2l. Al Khamis KL, Hagga ME, Al-Khamis HA. Spectrophotometric determination of domperidone using absorbance difference method. Anal Lett l990;23:45l-60.
- Ramamohan Y, Avadhanulu AB. Extractive spectrophotometric determination of domperidone in its pharmaceutical dosage forms. Indian Drugs l998;35:754-6.
- Yamamoto K, Hagino M, Kotaki H, Iga I. Quantitative determination of domperidone in rat plasma by high performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Appl l998;720:252-5.
- Argekar AP, Shah SJ. Simultaneous determination of cinnarizine and domperidone maleate from tablet dosage form by reverse phase ion pair high performance liquid chromatography. J Pharm Biomed Anal l999;l9:8l3-7.
- Zarapkar SS, Kanyawar NS. Simultaneous estimation of domperidone and omeprazole in pharmaceutical dosage by reverse phase high performance liquid chromatography. Indian Drugs 2002;39:2l7-2l.
- Kobylinska M, Kobylinska K. High-performance liquid chromatographic analysis for the determination of domperidone in human plasma. J Chromatogr B Biomed Sci Appl 2000;744:207-l2.
- Zarapkar SS, Salunkhe BB. Determination of domperidone by high performance thinlayer chromatography in pharmaceutical preparations. Indian Drugs l990;27:537-40.