- *Corresponding Author:
- N. Udupa
Department of Quality Assurance and Dr. T. M. A. Pai Pharmaceutical Research Centre, Manipal College of Pharmaceutical Sciences, MAHE, Manipal-576 104, India.
E-mail: n.udupa@manipal.edu
| Date of Submission | 28-Feb-2011 |
| Date of Revision | 31-Aug-2012 |
| Date of Acceptance | 03-Sep-2012 |
| Date of Web Publication |
Abstract
A reverse phase liquid chromatographic method was developed for the simultaneous estimation of tizanidine and valdecoxib in tablets. This method is based on using a Hypersil BDS C18 column using a mobile phase of 10 mM octane sulphonic acid sodium salt and 0.3% triethylamine (pH adjusted to 3.5±0.1 with orthophosphoric acid) and acetonitrile in the ratio of 70:30 v/v. Rofecoxib was used as an internal standard. The retention time of tizanidine, valdecoxib, and rofecoxib were 3.15, 10.92, and 16.24 min respectively. The method was found to be linear (correlation coefficient r>0.999), precise (Residual standard deviation: 0.62% for tizanidine, 0.86% for valdecoxib), accurate (overall average recovery yields: 98.7% for tizanidine, 99.2% for valdecoxib), and selective.
Introduction
Tizanidine HCl (TIZ) is 5-chloro-4-[2-imidazolin-2-ylamino]-2,1,3-benzothiadiazole. It is used as skeletal muscle relaxant. Valdecoxib (VAL) is used as nonsteroidal antiinflammatory drug. This combination is used for spasm and pain associated with musculoskeletal disorders. Both the drugs are not official in any Pharmacopoeia. Many methods [1-13] have been described in the literature for the determination of TIZ and VAL, individually. However, there is no HPLC method reported for the simultaneous determination of these drugs either as active pharmaceutical ingredient or dosage forms. The present work describes a simple, precise, and accurate reverse phase HPLC method for simultaneous estimation of TIZ and VAL in combined dosage forms.
A gradient high-pressure liquid chromatograph (Shimadzu HPLC Class VP series) with two LC-10AT VP pumps, variable wavelength programmable UV/Vis detector SPD10AVP, SCL-10AVP system controller (Shimadzu) and operating software Shimadzu Class VP version 6.12 SP2 data station was used for the analysis. The drug sample TIZ was obtained as gift sample from the Sun Pharmaceutical Industries, Mumbai. VAL and rofecoxib (ROF) were obtained as gift samples from IPCA Laboratories, Mumbai. Triethylamine AR, orthophosphoric acid AR, octane sulphonic acid sodium salt HPLC grade, and acetonitrile of HPLC grade were supplied by S. D. Fine Chemicals, Mumbai. Water HPLC grade was obtained from a milli-QRO water purification system.
The method was carried out on a Hypersil BDS C18 (150 mm * 4.6 mm i.d., 5 μ) column as a stationary phase. The mobile phase consisted of 10 mM octane sulphonic acid sodium salt and 0.3% triethylamine (pH adjusted to 3.5±0.1 with orthophosphoric acid) as aqueous phase and acetonitrile in the ratio of 70:30 v/v as the mobile phase at the flow rate of 1 ml/min. The mobile phase was filtered through a 0.45 μ membrane filter and degassed before analysis. A Rheodyne 7725 injector with a 20 μl loop was used for the injection of samples. Detection was done at 239 nm and separation was carried out at the room temperature of about 20°.
Standard stock solutions of TIZ, VAL, and ROF (100 μg/ ml) were prepared separately in a mixture of acetonitrile and water (1:1 v/v). From the standard stock solutions, mixed standard solution was prepared containing 0.6 μg/ ml of TIZ, 20 μg/ml of VAL, and 25 μg/ml of ROF as internal standard. Drug
Vorth 20 (Gracewell Pharmaceuticals, Mumbai), ten marketed tablets, each containing 6 mg of TIZ and 20 mg of VAL, were weighed and finely powdered. A quantity of powder equivalent to 6 mg of TIZ and 20 mg of VAL (an average weight) was weighed accurately and transferred to a sintered glass crucible. To this, 10 ml of ROF (2.5 mg/ml prepared in warm acetonitrile) was added and the drugs were extracted with three quantities, each of 20 ml of mixture of acetonitrile and water (1:1 v/ v). The combined extracts were made up to 100 ml with mobile phase, and further dilutions (100 times) were made to get a concentration of 0.6 μg/ml of TIZ, 20 μg/ml of VAL, and 25 μg/ml of ROF (theoretical value). The content was vortexed, filtered through a 0.45 μ membrane filter, and injected in triplicate. The ratio of peak area of drug to that of internal standard was calculated. The mixed standard solution was subjected to proposed HPLC method of analysis for finding out intra- and inter-day variations. Linearity and range of the method was also determined by analysing mixed standard solutions. The calibration curve was plotted using response factor versus concentration of the standard solutions. The recovery studies were carried out by adding known amount of standard drug to the pre-analyzed samples and subjecting them to the proposed HPLC method of analysis.
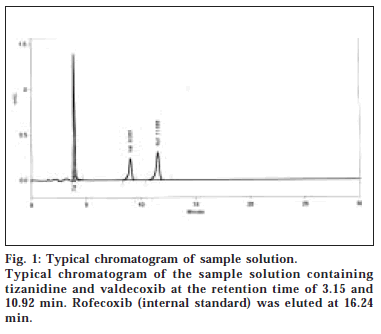
The present study was carried out to develop a simple and rapid HPLC method for the simultaneous estimation of TIZ and VAL, using most widely used Hypersil BDS column. The typical chromatogram of TIZ, VAL with the internal standard ROF in the formulation is presented in fig. 1. The retention time of TIZ, VAL, and ROF were found to be 3.15, 10.92, and 16.24 min, respectively. The assay concentration of 0.6 μg/ml TIZ and 20 μg/ml of VAL was selected according to the labelled claim. The peaks were well resolved and the capacity factor (k’) between TIZ and VAL was found to be 5.54. The drug peaks were symmetrical in shape and asymmetry factor for all the peaks were found to be less than 1.20. There was good repeatability of the proposed method as the precision of the method was less than 2% for all the three drugs. The coefficient of variance was found to be 0.62% for TIZ and 0.86% for VAL; that shows the method is highly precise.
Linearity experiment was performed thrice for all the three components, and response was found to be linear in the concentration range of 0.42-0.78 μg/ml for TIZ, 14.0-26.0 μg/ml for VAL. Regression lines obtained at 95% confidence interval using least square method. Correlation coefficient ‘r’ values (n=3) for all three drugs were ≥0.999. Accuracy of method was determined by recovery studies (n=3). The concentration of standard spiked to the sample was 0.07-0.13 μg/ml for TIZ, 1.753.25 μg/ml for DCL, and 11.375-21.125 μg/ml for PAR. Recovery data from the study are reported in Table 1. The mean % recovery was found to be 99.1% for TIZ, 99.3% for DCL, and 98.6% for PAR. The content of the drugs in the commercial dosage form was found to be 98.7% for TIZ and 99.2% for VAL by this method. The estimated amount was within the acceptable limits of the labelled claim of the formulation.
| Drug | Amount added (µg/ml) | Amount recovered (µg/ml) | Recovery (%) | Average recovery (%) |
|---|---|---|---|---|
| Tizanidine | 0.480 | 0.471 | 98.13 | |
| 0.596 | 0.584 | 97.99 | 98.62 | |
| 0.724 | 0.715 | 99.75 | ||
| Valdecoxib | 15.900 | 15.878 | 99.86 | |
| 19.928 | 19.860 | 99.65 | 99.73 | |
| 24.184 | 24.105 | 99.68 | ||
| Recovery experiment data for TIZ and VAL showing the amounts of drug added and recovered from sample solution at each level (n=3), percentage recovery and the average percentage recovery. TIZ stands for tizanidine and VAL stands for valdecoxib | ||||
Table 1: Recovery Studies
The developed RP-HPLC method provides a convenient and efficient method for the separation and estimation of TIZ and VAL in combined dosage form. There was no interference from the excipients used in the tablet formulation and hence the method is suitable for analysis of tablets. The results of validation showed that the proposed method is simple, linear, precise, accurate, and selective and is employed in routine assay of TIZ and VAL in tablets.
References
- Reddy, K.S.S.R.K., Babu, M.J., Dubey, P.K., Shekar, B.C., Reddy, O.G. and Vyas, K., J. Pharm. Biomed. Anal., 2002, 29, 355
- Reynolds, J.E.F. Eds in, Martindale, the Extra Pharmacopoeia, 29thEdn,, Pharmaceutical Press, London, 1989, 31
- Radhakrishna, T., Rao, S.D. and Reddy, G.O., J. Pharm. Biomed.Anal., 2001, 26, 617
- Aruna, A., Anusuya, P., Balamarriappan, C., Lakshmanan, M.K.,Nanjappan, K and Kumarpavan, A.R., Indian Drugs, 2001, 38, 523
- Woolf, E., Fu I and Matuszeweski, B., J. Chromtatogr. B.Biomed Sci. Appl., 1999, 730, 221
- Chevez-Eng, C.M., Constanzer, M.L., and Matuszeweski, B., J.Chromtatogr. B. Biomed Sci. Appl., 2000, 748, 38
- Shingare, M.S., Naidu, K.R. and Kale, U.N., Indian J. Pharm.Sci., 2003, 65, 315
- Tuncel, M. and Dogrulol, D., Anal. Lett., 1992, 25, 1087
- Bouklouge, A.A., EI Jammal, A., Vire, J-C and Patriache, G.J., Anal.Chim. Acta., 1992, 257, 41
- Bhoir, I.C., Raman, B., Sundaresan, M. and Bhagawat, A.M., Anal.Lett., 1998, 31, 1533
- Ramakrishna, N. V., Vishwottam, K. N., Wishu, S. and Koteshwara, M., J Chromatogr B AnalytTechnol Biomed Life Sci., 2004, 802(2), 271.
- ZhangJ Y, Fast D M and Breau A P, J Chromatogr B AnalytTechnol Biomed Life Sci., 2003, 785, 123
- Subramanian G., Pandey S. and Udupa N., Indian J. Pharm. Sci., 2004, 5, 699.
- International Conference on Harmonization, Guidance for Industry In; Q2B Validation on Analytical Procedures: Methodology, 1996, 2.