- *Corresponding Author:
- S. B. Wankhede
Department of Pharmaceutical Chemistry, Pad. Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Sant Tukaram Nagar, Pimpri, Pune-401 018, India
E-mail: sagar2277@rediffmail.com
| Date of Submission | 18 August 2008 |
| Date of Revision | 16 February 2009 |
| Date of Acceptance | 16 June 2009 |
| Indian J Pharm Sci, 2011, 73 (3): 300-302 |
Abstract
Three simple, accurate and economical methods have been developed for the estimation of norfloxacin and ornidazole in tablet dosage form. First method is based on the simultaneous equations, wavelengths selected for analysis were 273.0 nm (λmax of norfloxacin) and 318.5 nm (λmax of ornidazole), respectively, in 0.1N NaOH. Second method is Q-analysis method, based on absorbance ratio at two selected wavelengths 297.0 nm (iso-absorptive point) and 318.5 nm (λmax of ornidazole). Third method is first order derivative spectroscopy using 297.5 nm (zero cross for norfloxacin) and 264.0 nm (zero cross for ornidazole). The linearity was obtained in the concentration range of 4-20 μg/ml and 5-25 μg/ml for norfloxacin and ornidazole, respectively. The results of the analysis have been validated statistically and by recovery studies.
Keywords
Norfloxacin, ornidazole, simultaneous equation, Q-analysis, derivative spectroscopy
Norfloxacin (NF), chemically 1-ethyl-6-fluoro-1,4- dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline carboxylic acid [1], is a synthetic broad spectrum fluoroquinolone antibacterial agent used in the treatment of urinary and genital tract infection [2,3]. Ornidazole (OZ), chemically 1-chloro-3-(2-methyl-5-nitro-imidazol- 1-yl) propan-2-ol, is an antimicrobial agent used in treatment of susceptible protozoal infections and anaerobic bacterial infection [4,5]. NF is official in USP [1], BP [6] and IP [7] whereas OZ is not official in any pharmacopoeia. Both the drugs are marketed as combined dose tablet formulation in the ratio of NF:OZ 400:500 mg. Literature survey revealed that a number of methods have been reported for estimation of NF [8-11] and OZ [12-17] individually or in combination with other drugs. However, there is no analytical method reported for the simultaneous estimation of norfloxacin and ornidazole in a combined dosage formulation. Present work describes three simple, accurate, reproducible, rapid and economical methods for simultaneous estimation of NF and OZ in tablet formulation.
A double-beam Shimadzu UV/Vis spectrophotometer, 1700 Pharmaspec, with spectral bandwidth of 2 nm, wavelength accuracy of ±0.5 nm and a pair of 1-cm matched quartz cells, was used to measure absorbance of the resulting solution. Standard gift sample of norfloxacin was provided by Emcure Pharmaceuticals Ltd., Pune and ornidazole by Aristo Pharmaceuticals Pvt. Ltd., Mumbai. Combined dose NF and OZ tablets (Norrit-Ord, 400 mg norfloxacin and 500 mg ornidazole; Ind-Swift Ltd., Chandigarh), were purchased from the local pharmacy. Sodium hydroxide, 0.1N, was prepared from analytical reagent grade sodium hydroxide in double distilled water and used as a solvent. Standard stock solutions of NF (100 μg/ml) and OZ (100 μg/ml) were prepared and used for the analysis.
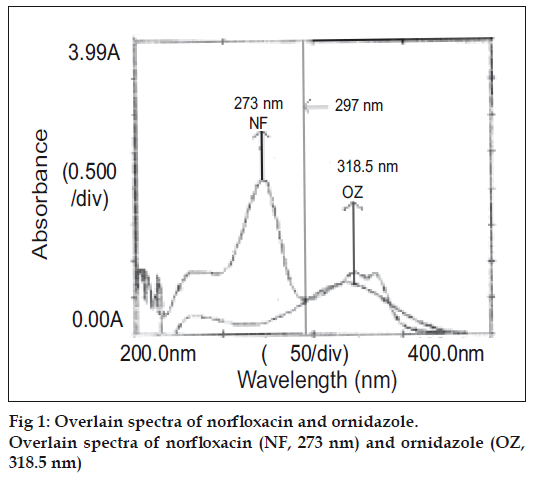
For the selection of analytical wavelength for the simultaneous equation method (Method-A), solutions of NF and OZ (20 μg/ml, each), were prepared separately by appropriate dilution of standard stock solution and scanned in the spectrum mode from 200 nm to 400 nm. From the overlain spectra of both drugs (fig. 1), wavelengths 273.0 nm (λmax of NF) and 318.5 nm (λmax of OZ) were selected for the simultaneous equations. The calibration curves for NF and OZ were prepared in the concentration range of 4-20 μg/ml and 5-25 μg/ml at both the wavelengths respectively. The absorptivity values were determined for both the drugs at both the wavelengths and following Eqns were used, A1 = 110.9CNF+15.3COZ (1) and A2 = 43.7CNF+28.4COZ (2), where A1 and A2 are absorbances of the sample at 273 nm and 318.5 nm, respectively, 110.9 and 43.7 are absorptivities of NF at 273.0 and 318.5 nm, respectively, 15.3 and 28.7 are the absorptivities of OZ at 273.0 nm and 318.5 nm, respectively. CNF is the concentration of NF and COZ is the concentration of the OZ. The mixture concentration was determined by using the Eqns. 1 and 2.
In the absorption ratio method (Method-B), from the overlain spectra of both drugs (fig.1), wavelengths 297.0 nm (iso-absorptive point) and 318.5 nm (λmax of OZ) were selected for the analysis. The calibration curves for NF and OZ were plotted in the concentration range of 4-20 μg/ml and 5-25 μg/ml at both the wavelengths respectively. The absorptivity values were determined for both the drugs at both the wavelengths. From the following set of Eqns the concentration of each component in the sample can be calculated, Cx = Qm–Qy/Qx–Qy×A1/a (1) and Cy = Qm–Qx/Qy–Qx×A1/a (2), where Cx is the concentration of NF, Cy is the concentration of OZ, A1 is the absobance of sample at iso-absorptive wavelength 297.0 nm, a is the mean absorptivity of NF and OZ at iso-absorptive wavelength 297.0 nm, Qm is the ratio of absorbance of sample solution at 318.5 nm and at 297.0 nm, Qx is the ratio of absorptivities of NF at 318.5 nm and at 297.0 nm and Qy is the ratio of absorptivities of OZ at 318.5 nm and at 297.0 nm.
In first order derivative spectroscopy (Method-C) solutions of NF and OZ (20 μg/ml, each), were prepared separately by appropriate dilution of standard stock solution and scanned in the spectrum mode from 200 nm to 400 nm. The absorption spectra thus obtained were derivatized from first to fourth order. First order derivative spectrum was selected for analysis of both drugs. The zero crossing wavelengths 297.5 nm (zero cross for NF) and 264.0 nm (zero cross for OZ) were selected for the analysis. The calibration curves for NF and OZ were plotted in the concentration range of 4-20 μg/ml and 5-25 μg/ml at both the wavelengths, respectively. The concentration of the individual drug present in the mixture was determined against the calibration curve in quantitation mode.
For the estimation of drugs in the commercial formulations, twenty tablets were weighed and average weight was calculated. The tablets were crushed to obtain fine powder. Tablet powder equivalent to 80 mg of NF was transferred to 100.0 ml volumetric flask containing 40 ml of 0.1N NaOH and ultrasonicated for 10 min and diluted to the mark with 0.1N NaOH. The solution was then filtered through a Whatmann filter paper No. 41. From the filtrate 5.0 ml was transferred to a 50.0 ml volumetric flask and diluted to the mark with 0.1N NaOH to obtain 8 μg/ml of NF and 10 μg/ml of OZ. The concentration of both NF and OZ was determined by measuring the absorbance of the sample at 273.0 nm and 318.5 nm (Method-A) and at 297.0 nm and 318.5 nm (method B) in the spectrum mode and values were substituted in the respective formulae to obtain concentrations. For Method-C concentration of both NF and OZ was determined by measuring the absorbance of the sample at 297.5 nm and 264.0 nm in first order spectrum mode. The results of the tablet analysis were calculated against the calibration curve in quantitation mode.
Recovery studies were carried out by standard addition method at three different levels 80%, 100% and 120%. The % recovery of NF and OZ in the sample mixture was determined. The results of tablet analysis and recovery studies obtained by proposed method were validated by statistical evaluation and are recorded in Tables 1 and 2.
| Method | Component | Label Claim (mg/tab) | Amount Found | Estimated Label Claim* | SD | CV |
|---|---|---|---|---|---|---|
| A | NF | 400 | 398.89 | 99.73 | 0.5932 | 0.5948 |
| OZ | 500 | 496.60 | 99.32 | 0.8720 | 0.8780 | |
| B | NF | 400 | 396.51 | 99.13 | 0.6855 | 0.6915 |
| OZ | 500 | 498.89 | 99.78 | 0.7358 | 0.7374 | |
| C | NF | 400 | 400.10 | 100.03 | 0.7842 | 0.7840 |
| OZ | 500 | 498.62 | 99.68 | 0.9286 | 0.9316 |
Table 1: Analysis of tablet formulation.
| Level of Recovery (%) |
Amt. of Pure | Method-A | Method-B | Method-C | ||||
|---|---|---|---|---|---|---|---|---|
| Drug Added (mg) | % Recovery | % Recovery | % Recovery | |||||
| NF | OZ | NF | OZ | NF | OZ | NF | OZ | |
| 80 | 64 | 80 | 99.41 | 99.98 | 98.32 | 100.80 | 99.49 | 99.49 |
| 100 | 80 | 100 | 99.65 | 99.94 | 98.49 | 100.91 | 99.22 | 99.55 |
| 120 | 96 | 120 | 99.80 | 99.65 | 98.57 | 100.76 | 100.31 | 99.57 |
| Mean % Recovery | 99.62 | 99.86 | 98.46 | 100.82 | 99.81 | 99.53 | ||
| SD | 0.2147 | 0.2910 | 0.1578 | 0.2231 | 0.6711 | 0.7216 | ||
| CV | 0.2155 | 0.2914 | 0.1603 | 0.2213 | 0.6724 | 0.7250 | ||
| SE | 0.0877 | 0.1188 | 0.0644 | 0.0911 | 0.2740 | 0.2946 | ||
Table 2: Results of recovery studies.
The methods discussed in the present work provide a convenient and accurate way for simultaneous analysis of NF and OZ. Percent label claim for NF and OZ in tablet, by all the methods, was found in the range of 98.15% to 101.03%. Standard deviation and coefficient of variance for six determinations of tablet sample, by both the methods, was found to be less than ±2.0 indicating the precision of both the methods. Accuracy of proposed methods was ascertained by recovery studies and the results are expressed as % recovery. Percent recovery for NF and OZ, by all three methods, was found in the range of 98.27% to 101.07%, values of standard deviation and coefficient of variation were in the range of ±0.1578 to ±0.7216 and 0.1603 to 0.7250, respectively indicating the accuracy of proposed methods. Based on the results obtained, it is found that the proposed methods are accurate, precise, reproducible and economical and can be employed for routine quality control of norfloxacin and ornidazole in combined dose tablet formulation.
Acknowledgements
The authors wish to thank Dr. Avinash D. Deshpande, Director of Pharmacy, Pad. Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Pimpri, Pune for providing necessary facilities. The authors also thank Emcure Pharmaceuticals Ltd., Pune and Aristo Pharmaceuticals Pvt. Ltd., Mumbai for providing gift samples of drugs NF and OZ.
References
- The United State Pharmacopoeia, 26th Revision, Rockville, MD: US Pharmacopoeial Convention Inc; 2003.
- Petri WA Jr. Antimicrobial agents: Sulfonamides, trimethoprim-sulfamethoxazole, quinolones and agents for urinary tract infections. In: Hardman JG, Limbird LE, Gilman AG. Goodman and Gillman’s the pharmacological basis of therapeutics. 10th ed. New York, McGraw- Hill; 2001.p. 1180.
- Tripathi KD. Essentials of medical pharmacolgy. 5th ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd.; 2004. p. 650.
- Sweetman SC, editors. Martindale, The complete drug reference, 32nd ed. London: Pharmaceutical Press; 2002.
- Budavari S. editors. In; The Merck Index, 12th ed. Whitehouse Station, NJ: Merck and Co. Inc; 1996. p. 1150.
- British Pharmacopoeia, London: Her Majesty’s Stationary Office; 2007. p. 522-3.
- Indian Pharmacopoeia, Vol. I, Government of India, Delhi: The Controller of Publications; 1996. p. 522-3.
- More HN, Mahadik KR, Kadam SS. Simultaneous estimation of Norfloxacin and Tinidazole using UV visible spectrophotometer. Indian Drugs 1994;36:144-6.
- Shrinivas Reddy GK, Jain DK, Trivedi P. Derivative spectrophotometric and graphical absorbance ratio method for simultaneous estimation of norfloxacin and tinidazole in two component tablet formulation. Indian J Pharm Sci 1999;61:16-9.
- Mahareshwari RK, Chaturvedi SC, Jain NK. Novel spectrophotometric estimation of some poorly water soluble drugs using hydrotropic solubilizing agents. Indian J Pharm Sci 2006;68:195-8.
- Argekar AP, Kapadia SP, Raj SV. Simultaneous determination of norfoxacin and tinidazole in tablets by reverse phase-high performance liquid chromatography. Anal Lett 1996;29:1539-49.
- Kasture VS, Bhagat AP, Puro NC. Spectrophotometric method for simultaneous estimation of ofloxacin and ornidazole in Tablet Dosage Form. Indian Drugs 2004;41:51-3.
- Patel PU, Suhagia BN, Patel CN, Patel MM, Patel GC, Patel GM. Simultaneous spectrophotometric estimation of gatifloxacin and ornidazole in mixture. Indian J Pharm Sci 2005;67:356-7.
- Paramane S, Kothapalli L, Thomas A, Deshpande AD. Simultaneous spectrophotometric estimation of gatifloxacin and ornidazole in tablet dosage form. Indian J Pharm Sci 2006;68:819-21.
- Wate SP, Nimje H, Ramtake M. Simultaneous spectrophotometric estimation of gatifloxacin and ornidazole in tablets. Indian J Pharm Edu Res 2007;41:236-9.
- Kamble NS, Venkatachalam A. High performance liquid chromatographic determination of ornidazole and ofloxacin in solid dosage forms. Indian Drugs 2005;42:723-30.
- Nagavallai D, Sankar AS, Karunambigni AK, Raja MS. Reverse phase-HPLC method for simultaneous estimation of gatifloxacin and ornidazole in tablets. Indian J Pharm Sci 2007;69:333-5.