- *Corresponding Author:
- Ruckmani Kandasamy
Department of Pharmaceutical Technology, Anna University-BIT Campus, Tiruchirappalli-620 024, India
E-mail: hodpharma@gmail.com
| Date of Submission | 06 June 2016 |
| Date of Revision | 04 April 2017 |
| Date of Acceptance | 05 November 2017 |
| Indian J Pharm Sci 2018;80(1):14-25 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The oral route of drug administration is mostly preferred by paediatric and geriatric patients. Patient non-compliance due to poor taste and swallowing problems are major issues in paediatric and geriatric drug therapy. Lack of age-appropriate formulations, inadequate labelling instructions, non-compatible and non-palatable medicines are the main reasons for medication errors in paediatric patients. This review mainly highlights major issues of paediatric and geriatric drug therapy and provides the design of oral flexible formulations as an alternative formulation design for age appropriate formulation development. Development of solid oral flexible formulations as an alternative to existing marketed formulations such as conventional tablets, orally disintegrating tablets, chewable and dispersible tablets for paediatric and geriatrics patients is described. Details about drug properties, excipient selection and taste masking approaches were described. The need for bioavailability and bioequivalence studies for flexible formulations also described. Development of flexible formulations as an alternative to marketed different formulations of the same drug would reduce the cost of the formulation for patients particularly developing countries where accessibility to various types of formulations is less. Flexible tablet formulations can be conveniently used by the patients as orally swallowable, orally disintegrating, chewable and as dispersible tablets. Sprinkle formulations such as pellets, granules packed in capsules or sachets can be conveniently used by paediatric and geriatric patients. Properly designed and developed flexible formulations for new chemical entities and off-patent drugs are need of the hour to provide better treatment to varying age groups of paediatrics and geriatrics.
Keywords
Flexible formulations, multi-particulates, paediatrics, geriatrics, dysphagia, taste masking
Paediatric and geriatric patients require new modified dosage forms to comply with prescribed dosing regimens due to differences in age variance when compared to another age group. The age classifications for paediatric, adult and geriatric patients are shown in Table 1. Non-compliance of paediatric and geriatric patients is a concern due to issues such as the poor taste of medications and swallowing difficulties (dysphagia) [1]. Most of the available dosage forms across the globe are designed and developed only for adult patients considering the market size of the majority of populations. Off-label use of adult dosage forms by crushing the tablets or capsule contents in water or other liquid can lead to non-compliance and safety issues in paediatrics [2,3]. Extemporaneous drug preparations are commonly practiced in hospital pharmacies across the globe. Safety and effectiveness of extemporaneously prepared medications are a major concern for paediatric and geriatric patients [4]. Main reasons for non-availability of paediatric-specific formulations are non-availability of clinical data on paediatric patients, clinical studies conducted mostly with adult patients, the small number of patients in the paediatric age group, varying age groups of paediatric patients, and business focus on the larger population of adult patients [5]. Geriatric patients mainly suffer due to swallowing difficulties with large size tablets and capsules [6]. Polypharmacy in geriatric patients is leading to consumption of multiple drugs for the treatment of several medical complications in one patient ranging from diabetes, malfunction of liver, kidney, coronary heart disease, arthritis, vision problems, and neurological diseases. Aging, memory loss and comorbidities in patients with poor swallowing ability of large size pills and holding small size pills during handling lead to poor compliance among geriatric patients [7]. Hence, there exists a need and attention of global researchers for appropriate development of patient-centric formulations, which are suitable to be administered for both paediatric and geriatric patients, whilst also being presented in an acceptable dosage form to ensure safety and compliance [8]. The development of paediatric formulations suitable for all the age group of patients from very young children to adolescents is challenging as it involves consideration of drug properties, excipient safety, administration volumes, dosage form size, taste, and acceptability [9]. The oral route of administration is commonly used for dosing medicinal products to paediatric and geriatric patients. The various types of marketed oral dosage forms include solutions, syrups, suspensions, powders, granules, tablets, orally disintegrating tablets (ODT), chewable tablets, dispersible tablets, capsules, oral thin strips, chewing gums, powder for reconstitution, tablets for constituton to suspension, oral drops and troches [10]. Chewable tablets, orodispersible formulations such as ODT, orally disintegrating mini-tablets and orodispersible films (ODF) and multi-particulate formulations can be conveniently consumed by school going children and geriatric patients due to advantages like convenient administration and less swallowing difficulties [11-15]. A flexible tablet formulation administered as chewable tablets, ODT, orally dispersible tablets dispersed in a small quantity of water, and also be orally swallowed. Flexible granules or pellets or powder formulations can be directly swallowed or administered as dispersion by dispersing in a small quantity of water. Development of solid oral flexible tablets (OFT) of cetirizine as a model drug with ion exchange resins (IER) as taste masking and stabilizing agent [16], and development of extended release oral flexible tablets (ER-OFT) of carbamazepine as a model drug with ethyl cellulose as the polymer is described in the literature to improve palatability and compatibility in paediatric and geriatric patients [17]. Rapidly disintegrating levetiracetam tablets for oral suspension (Spritam) using 3D printing technology [18] was recently approved by the United States Food and Drug Administration (USFDA).
| Classification | Age | Currently marketed Dosage Form |
|---|---|---|
| Preterm newborn infants | 23 w to 25 w gestation | Liquids |
| Term newborn infants | 0-27 d | Liquids |
| Infants and toddlers | 1 to 23 mo | Liquids |
| Pre-school children | 2-5 y | Liquids, multiparticle, dispersible tablets, chewable tablets and ODT |
| School going children | 6-11 y | Liquids, multiparticle, chewable tablets, ODT, dispersible tablets, swallowable tablets, and capsules |
| Adolescents | 12 to 16-18 y | |
| Adults | 18-60 y | |
| Geriatrics | 60-65 y | |
| 65-70 y | ||
| 75-80 y | ||
| >85 y |
Table 1: Preferred Dosage Forms According to Age of Patients
Global Regulations and Guidances for Paediatric Drug Development
Global regulatory agencies such as Central Drugs Standard Control Organization (CDSCO), European Medicines Evaluation Agency (EMEA) and USFDA had passed regulatory acts to assess safety and effectiveness of new drugs and off-patent drugs in paediatric populations [19]. In India, Schedule Y of Drugs and Cosmetics Act 1940 and Rules 1945 details guidelines for conducting clinical studies in paediatric patients and are ensured by the CDSCO and Drug Control General of India (DCGI). In 2000 the International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use issued the ICH E11 guideline regarding the clinical investigation of medicinal products in the paediatric population [20]. In 2002, Best Pharmaceuticals for Children Act (BPCA) was passed by US Congress to conduct voluntary clinical studies on certain drugs in paediatric populations. As per BPCA, USFDA may issue a written request (WR) to a sponsor for voluntary paediatric studies that may lead to health benefits in that population and sponsors who submit studies fulfilling a WR are eligible to receive paediatric exclusivity. Paediatric exclusivity does not require positive paediatric studies. In 2003, Paediatric Research Equity Act (PREA) was passed by US Congress to evaluate any new drug, new indication, new dosage form, new dosing regimen or new route of administration in paediatric patients. In 2012, FDA Safety and Innovation Act (FDASIA) includes a provision that requires sponsors planning to submit an application for a drug subject to PREA to submit an initial paediatric study plan (iPSP) early in the development process. As per FDASIA, USFDA issued draft guidance in March 2016 on content and process for submitting an iPSP [21]. In Europe, paediatric regulation came into force from January 2007 for the development of paediatric-specific drug products and generation of clinical data in approved medicines. This EU regulation applies to both new products, new formulations, new indications protected under the protection of a Supplementary Protection Certificate (SPC) and off-patent drug products to conduct clinical studies in children under the age of 18 in accordance with a Paediatric Investigation Plan (PIP). Upon approval of PIP, a new product can be rewarded with a 6-mo extension to SPC and for off-patent drug products, 10-y market exclusivity can be obtained under Paediatric Use Marketing Authorization (PUMA) [22,23]. In 2006, European Medicines Agency (EMA) published reflection paper on formulation choice for a paediatric population which details the range of formulations, dosage forms of choice related to age, excipient factors, dosing devices and taste evaluations to be considered in developing paediatric formulations [24]. In 2013, EMA guideline on pharmaceutical development of medicines for paediatric use came into effect, which details various aspects of formulation development, such as drug properties, excipient selection, dosing frequency, modified release formulation, patient acceptability, container closure systems, dose-measuring device, administration device, and packaging of medicines for paediatrics [25]. In 2007, European Paediatric Formulation Initiative (EPFI) was created to promote and facilitate the development of better and safe paediatric drug products by linking research and information dissemination [26]. In 2007, WHO launched “make medicines child size” initiative to improve awareness for improved availability and access to child-specific medicines. WHO published a guidance document on “points to consider” in development of paediatric medicines, which details development aspects such as desirable features of paediatric medicines, flexible dosage forms, formulation design, excipient safety, taste masking, dosage forms relevant to oral, rectal, parenteral, dermal, inhalation route of administration, packaging, and labelling [27].
Geriatric Concerns and Regulations
Oral administration is the most preferred route of drug administration as it is easy and more convenient for geriatric (elderly persons aged 65 y and over) patients. The geriatric patient can be classified into three major categories based on their age, 60-70, 70-80 and >85 y. Many of the geriatric patients suffer from issues such as swallowing difficulty (dysphagia) and taste disturbances (dysgeusia). Taste sensation is often affected in certain disease conditions and in patients undergoing chemotherapy. In the case of mentally ill patients hiding of tablets in cheeks (cheeking) to avoid swallowing tablets were reported. Polypharmacy due to comorbidities in geriatric patients aged >65 y and 75 y is common due to a range of diseases such as renal or hepatic impairment, impaired cardiac function, hypertension and Alzheimer's disease. It is recommended to include an adequate number of geriatric patients of age ranging from 65 to >85 y in clinical studies to understand the safety and efficacy of drugs compared to younger patients. In 1997, FDA established the Geriatric Use subsection, as a part of the precautions section, in the labeling for human prescription drugs to include a complete information about the use of a drug in the elderly (persons aged 65 y and over). Global regulatory agencies require drug firms to include geriatric use details in drug product label [28,29]. Priority implementation of geriatric labeling is required for certain classes of drugs such as psychotropic drugs, cardiac drugs, hypoglycaemic agents, non-steroidal antiinflammatory drugs (NSAID), anticoagulants and quinolones [30].
Marketed Oral Formulations
Based on the currently available literature and review of approved products in the globe, various types of formulations such as solutions, suspensions, ODTs, and chewable tablets are prescribed for paediatric and geriatric patients in addition to conventional orally swallowable tablets and capsules [31]. As each of the formulations are unique in design and require careful selection of excipients and formulation process. The quality attributes of the formulations were widely different for each of the formulation. For example, taste masking and stability are challenging quality attributes for a solution, syrups, drops and suspension formulations. Hardness and chewability are key attributes for chewable tablet formulations. Dispersibility and stability of the dispersion are quality attributes for dispersible tablet formulation. Rapid in vitro and in vivo disintegration, hardness, friability, and stability are critical for ODTs. As these formulations are developed based on safety and efficacy profile generated on adult patients, compatibility of excipients used in these formulations to paediatric patients are a concern [32-35].
Advantages and disadvantages of marketed formulations
Solid oral tablet formulations in the market were mostly prescribed for adult and geriatric patients. Solution and suspension formulations were better choices for paediatric patients of <2 y of age. Marketed formulations such as dispersible tablets, ODT, and chewable tablets were the choice for paediatric patients of age >2 y to avoid swallowing issues [24]. However, non-compliance of these preferred dosage forms were mainly due to the poor taste of medications. Most of the bitter taste drugs such as antibacterial drugs and central nervous system (CNS) acting drugs were marketed with incomplete taste masking [36]. Lack of appropriate paediatric drug development resulted in administration of various types of colors, flavours and preservatives in solutions, suspension, chewable tablets and dispersible tablets to paediatric patients. Many of the excipients used in the marketed dosage forms are reported to cause adverse reactions in paediatric patients [37]. Measurement of dose with a liquid formulation is the challenge for accurate delivery of the desired dose. Inappropriate liquid dose measurement leads to toxicity and less efficacy during treatment. Bitter taste, grittiness (texture), high dose volume, larger size of capsule or tablet, higher cost, non-availability of suitable dosage forms leads to poor patient compliance among paediatric and geriatric patients [38]. Due to the varying age group of paediatric and geriatric patients, selection of appropriate and suitable formulation according to age of patient is a critical requirement for many of over the counter medications (OTC). Drug products sold in many of the developing countries lack appropriate information such as precautions, dosage administration and warning instructions in drug product labels [39].
Challenges in Development of Age-Appropriate Formulations
Age-appropriate formulations are required for paediatric patients due to varying age group in paediatrics [40]. Currently, liquids, suspensions, tablets, chewable tablets, ODTs are used in paediatric patients according to the age of the patients. Liquids and suspension products are mostly suitable to paediatric patients of <2 y of age. Solid dose forms such as chewable tablets, ODT are suitable for paediatric patients of >2 y of age and non-disintegrating type solids such as chewing gums, tablets and capsules are suitable for children of >6 y of age. Development of age-appropriate formulations suitable for paediatric patients involves evaluation of various aspects such as drug properties, dose, pharmacokinetic and pharmacodynamic properties, taste masking, the safety of excipients, size of the dosage form, dose volume, dosing frequency, administration methods, palatability and stability of the formulation [41]. The preferred dosage forms according to the age of paediatric and geriatric patients are listed in Table 1. The size of the dosage form is related to the dose of the drug indicated for paediatric patients. As dose increases, the size of dosage form increases. The increase in the size of the dosage form leads to poor patient compliance in paediatric and geriatric patients due to swallowing difficulties. Beads or sprinkles size of greater than 2.0 mm is not recommended for oral administration by dispersing in water or sprinkling over foods and administration by nasogastric tubes [42]. Rapid disintegration of the formulation is a desired quality attribute for dispersible and ODT, which are preferred in paediatric patients of age <2 y and in geriatric patients. Dose volume of <5 ml and <10 ml is preferred in patients with 0-3 y of age and 4-12 y of age, respectively. Excipient selection for paediatric formulation must be based on the precedence of use in paediatric patients and toxicity data in animals, adults and in paediatric patients. Comorbidities in geriatric patients require flexible ways of drug administration of multiple drugs to avoid swallowing difficulties. Polypharmacy in geriatric patients may cause taste disturbances (dysgeusia) particularly with anticholinergics, antibiotics and anticancer drugs. Taste masking of bitter tasting drugs is a critical quality requirement for patient compliance in paediatric and geriatric patients. Combinations of acceptable taste with less oral grittiness, small size, rapid disintegration, accurate dose measurement, minimal dosing frequency, easy swallowability, and chewability are critical quality requirements for development of age-appropriate formulations suitable for paediatric and geriatric patients.
Design of Flexible Formulations for Paediatrics and Geriatrics
Flexible formulations, which can be used in flexible ways according to the specific age group of paediatric and geriatric patients are ideal requirement to improve the patient compliance. A flexible formulation can be tablets, granules, and multi-particulates such as pellets. Dose, dosage form size, drug release pattern, taste masking and rapid dispersion in water, non-grittiness in mouth, palatability, stability and robustness of formulation are critical design features to be considered for development of flexible formulation. OFT can be used as chewable tablets, ODT or dispersed in a small quantity of water and administered. Pellets, powders or granules can be filled into sachets or capsules and can be dispersed in water or soft foods such as yogurts or apple juice prior to administration. A flexible formulation can be designed to provide immediate release, delayed release and extended release drug pattern based on the therapeutic need. The design of flexible formulations with modified release characteristics such as extended release multi-particulates and disintegrating type extended release flexible tablets can be used to reduce dosing frequency. Dose reduction by nanotechnology, micronization or drug solubilisation methods such as solid dispersions can be used to design small size flexible formulation [43].
Drug properties
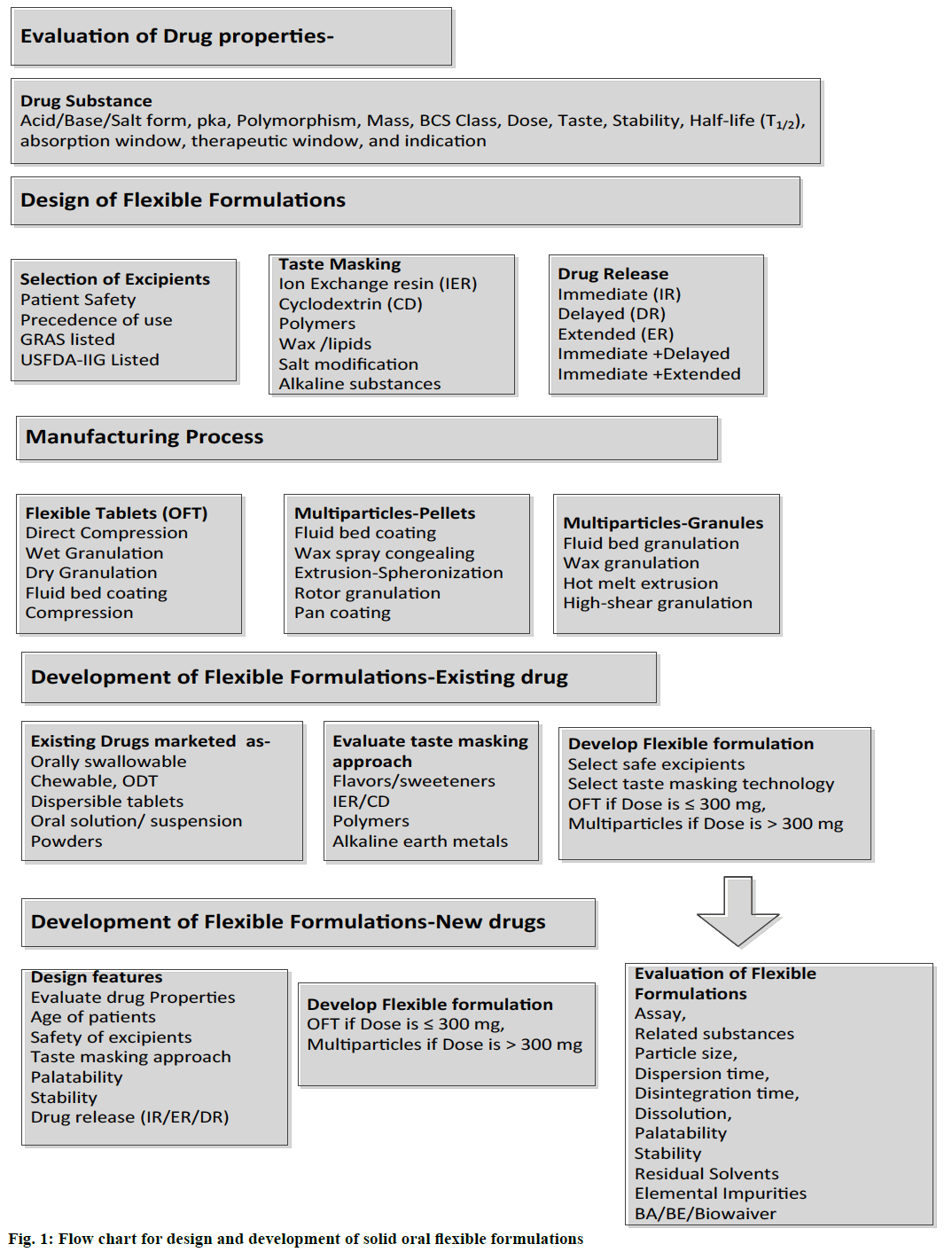
Drug substances are available as different types such as acidic, basic, ampholytic, or zwitterionic and salt form. Drug properties such as molecular weight, dissociation constant (pKa), polymorphic form, particle size, solubility, permeability (logP), stability and taste are important factors to be considered during development of flexible formulations. Based on solubility and permeability characteristics, drugs are classified into four groups as per Biopharmaceutical Classification System (BCS) [44]. The solubility of low soluble drugs can be increased by micronization, nano milling, inclusion complexes and solid dispersion methods. Solubility enhancement of aripiprazole using hydroxypropyl betacyclodextrin (HPß-CD) is reported [45]. Nanocrystals of aprepitant is marketed as capsules and powder for oral suspension for chemotherapy-induced nausea and vomiting (CINV) and post-operative nausea and vomiting [46]. Selection of appropriate base, salt or a polymorphic form with minimum bitter taste is critical for paediatric and geriatric formulation design. Evaluation of molecular size, the dissociation constant (pKa), solubility is critical for selection of taste masking technologies. Molecular size and ionization of drug are critical for preparing inclusion complexes using cyclodextrins (CD). Ionization at different pH (pKa), acidity, basicity, and dose are critical properties to be considered for preparation of ion-exchange drug complexes for successful taste masking and drug release modifications. Weakly acidic drugs can be complexed with strongly basic anionic exchange resins such as cholestyramine resin. Weakly basic drugs can be complexed with weakly acidic polacrilex resin and strongly acidic polistirex cation exchange resins. Drug solubility, stability at different pH and hygroscopicity are critical for selection of polymer type and suitable formulation design. The design of flexible formulations such as flexible tablets, multi-particulates can be selected based on dose size, solubility, taste, stability, and pharmacokinetic properties. Flowchart for design of oral flexible formulation is presented as Figure 1.
Excipient selection
Excipients are inactive ingredients used to formulate drug substances into a suitable medicinal product. Excipient selection in the development of flexible formulations is an important quality requirement to develop a formulation appropriate for paediatric patients [47]. The selection of excipients and level of use for formulation development must be based on dose, frequency of administration, patient age, safety, biopharmaceutical and physiochemical characteristics of excipients. Level of selected excipients must be based on the safety factors, content of elemental impurities, residual solvents, acceptable daily intake (ADI) as per FDA, EU, WHO, Generally Recognized as Safe (GRAS) status and as per inactive ingredients database (IID) of FDA. A minimum number of excipients with minimal level is preferred for compliance of paediatric patients. Colors such as tartrazine (E102), quinoline yellow (E104), sunset yellow FCF (E110), Carmoisine (E122), Ponceau 4R (E124) and Allura Red (E129) that were commonly used in food for children were associated with an increased risk for hyperactivity [48]. The 2007 Guideline on Excipients in the Dossier for Application for Marketing Authorisation of a Medicinal Product indicates that azo dyes and other synthetic colouring agents should not be used in new paediatric drug products. The EPFI in collaboration with the US paediatric Formulation Initiative (USPFI) and Global Research in Paediatrics (GRP) had developed a Safety and Toxicity of Excipients for Paediatrics (STEP) database [49,50], which provides published information on the safety and toxicity of commonly used excipients. Some of the excipients such as sorbitol are known to affect the bioavailability (BA) of poorly permeable drugs when used at higher levels [51]. FDA safety announcement in 2011 reported increased risk of adverse events such as serious heart, kidney, or breathing problems in premature babies with use of Kaletra (lopinavir/ritonavir) oral solution containing alcohol and propylene glycol [52].
Taste masking technologies
The selection of taste masking approach must be based on the organoleptic properties of the drug, dose, stability and its physicochemical properties such as solubility, pka, particle size [53]. Based on the level of bitterness and dose of drugs, various taste masking approaches such as, modification of drug solubility using pH modifiers, alternate salt or prodrug forms, and complexation with IER, CD and matrixing or coating with polymers and lipid materials can be used. Most commonly used technologies are inclusion complexes with CD, drug complexation with IER, matrixing of drug and polymer using aqueous or solvent granulation techniques and fluid bed coating of drug using pH independent/pHdependent polymers and microencapsulation of drugs in polymers, and by hot-melt technology such as hotmelt coating, hot-melt granulation, hot-melt extrusion and hot-melt spray congealing using waxes and fatty acids [24]. Taste masking can also achieved by reducing the solubility of drug in salivary pH conditions by keeping the drug in unionized state by using materials such as alkali earth metals or salts. Drug complexation with IER is a preferable technology as it provides a combination of taste masking, rapid disintegration and option of modified release of drugs and in many cases, it improves the stability of drugs. Taste masking with water permeable pH dependent or pH independent polymers is the cost effective technology as it combines conventional methods of manufacturing such as drug granulation with polymers and coating of drugs with polymers [54].
Sohi et al. reviewed various taste masking approaches and technologies used in oral formulations such as taste masking by sweeteners and flavours, polymeric coating, inclusion complexes using CD, drugresin complexation using IER, lipophilic vehicles, hydrophilic vehicles, salt formation, prodrug approach, freeze drying solid dispersion and by polymeric systems [55]. Taste masking of lamotrigine (LMT) by salt formation with cyclamic acid, a commonly used sweetener that has increased the aqueous solubility and dissolution rate of LMT is reported [56]. US Patent 5,024,997 describes taste masking of ibuprofen using hydroxypropyl-beta-cyclodextrin in a palatable solution formulation [57]. Taste masked formulations containing IER complexes of ambroxol [58], ciprofloxacin [59,60], levofloxacin [61], metoclopramide [62], metformin [63], ondansetron [64] and risperidone [65] was evaluated. US Patent 5,980,882 describes sustained release drugresin complexes of dextromethorphan coated with ethyl cellulose and stabilized by EDTA as a chelating agent [66]. US patent 4,999,189 describes coating of drug-resin complex coated with wax by hot-melt coating followed by coating with ethyl cellulose and dibutyl sebacate dissolved in alcohol/water mixture suing fluid bed coating system [67]. US Patents 6,984,403 and 7,887,844 describes the preparation of extended release azithromycin multiparticle using glyceryl dibehenate and poloxamer with holt-melt extrusion and hot-spinning disk method [68,69]. US Patent 5,405,617 describes taste masking of pharmaceutical actives such as acetaminophen, ibuprofen and loperamide using aliphatic fatty acids such as stearyl stearate and glyceryl mono, di or tri behenate by spray congealing of hot-melt of fatty acid and drug mixture to a particle size of <100 μm [70]. US Patent 4,865,851 describes taste masking of cefuroxime axetil using stearic acid by preparing lipid matrix by spray congealing technique [71]. US Patent 4,808,411 describes taste masking of clarithromycin using carbomer by preparing a solid dispersion [72]. US Patent 5,286,489 describes the porous chewable matrix of active ingredients with methacrylic acid copolymer by dissolving in non-aqueous solvents followed by vacuum drying [73]. US Patent 4,851,226 describes chewable tablet formulation of coated granules containing drug coated with cellulose acetate or cellulose acetate butyrate and polyvinyl pyrrolidone polymers dissolved in a non-aqueous solvent system using fluid bed coater [74]. US Patent 6,565,877 describes taste masking of bitter drugs such as cefuroxime axetil, and clarithromycin using a combination of the methacrylic acid copolymer and a phthalate polymer by preparing solvent-based solid dispersion followed by vacuum drying [75]. US Patent 8,168,228 describes taste masking of clarithromycin by preparing micropellets of a core containing drug followed by coating with one layer of cellulosic polymer and an outer layer of enteric polymer, wherein the coated particles are less than 500 μm [76]. WIPO/PCT publication WO2006030297A1 describes taste masking of clarithromycin using alginic acid and hydrocolloids followed by coating with hypromellose phthalate for preparing into powder for oral suspension, wherein the coated particles are <500 μm [77]. US Patent 5,633,006 describes taste masking of azithromycin using alkaline earth oxides for chewable tablets and liquid suspension preparation [78].
Solid Oral Flexible Formulations
Various types of solid oral flexible formulations can be developed for use in paediatric and geriatric applications. Each of the formulation types can possess common characteristic requirements such as taste masking, rapid disintegration, less grittiness, ease of swallowing. The design and development of a suitable type of formulation technology can be based on drug substance physicochemical properties, pharmacokinetics, dose size, frequency of use, stability of the drug substance, and drug release pattern.
Multiparticulates
Multi-particles can be manufactured by technologies such as granulation, extrusion-spheronization, spray congealing, and fluid bed coating methods. Multiparticulates of size <1.5 mm can be used as flexible formulations in paediatrics and geriatrics. Multiparticle can be spherical round pellets or small irregular particles and extrudates. Multiparticle technology provides the option of low dose to high dose drug loading, modified drug release for most of the soluble and insoluble drugs, taste masking and improves the stability of drugs, and fixed dose combination of 2 to 3 drugs. In addition, multiparticulates provide reproducible gastrointestinal transit times compared to monolithic dosage forms and so there is a lower risk for dose dumping. Multiparticles can be dispersed in water or suitable diluent and administered orally or via nasogastric tube to hospitalized patients who are unable to swallow.
OFT
Tablets are the most commonly used unit dose solid oral formulation in many of the patients across the globe. Flexible tablet formulation can be designed to possess all the quality attributes of dispersible, chewable and ODT. Critical quality attributes of flexible tablet formulations are rapid disintegration within 30 s, acceptable mouth-feel without grittiness, taste-masked drug particles, stability and convenient drug administration to the various age group of paediatric and geriatric patients. Flexible tablet formulation can be designed to be of round, caplet or oval shape and size can be smaller with a maximum weight of 500 mg as recommended by guidelines [79]. Drug substances with a dose less than 300 mg can be formulated into OFT considering maximum tablet size of ~500 mg. Ionisable drug substances with bitter taste characteristics and with a dose of ≤100-150 mg can be complexed with suitable ion-exchange resins and formulated into OFTs.
Flexible tablets can be designed to provide immediate release, delayed release and extended drug release properties. Immediate release type flexible tablet formulation can be manufactured by conventional methods of granulation such as wet, dry or roller compaction using diluents, disintegrating agents, flavours, sweeteners, and lubricants. Extended-release oral flexible tablet formulations can be obtained by coating of drug particles or drug cores with pH-dependent, pH independent polymers, lipid materials and by drug complexation using IER resins followed by polymeric or lipidic coatings. The US Patents 8,778,390 and 8,956,649 describes orally effective extended release IER complexes of methylphenidate [80,81]. The US Patents 8,790,700 and 8,883,217 describes modified release formulations containing IER complexes [82,83]. The US patents 8,999,386 and 9,295,642 describe extended release chewable tablet formulation containing methylphenidate IER complexes [84,85]. US patents 9,017,731 and 9,265,737 describes composition of dextro and levo amphetamine IER complexes [86,87]. The coated drug particles can be further processed into a flexible tablet formulation with suitable tableting excipients such as diluents, disintegrating agents, flavours, sweeteners, and lubricants [88]. Advantages of oral flexible formulations are presented in Table 2.
| Condition | Advantages |
|---|---|
| Dysphagia | Easily swallowed by mixing with water |
| Dysgeusia | Improved taste of drugs by protective coatings/complexes/flavours/sweeteners |
| Frequency of dose | Modified release Multiparticulates by coating with polymers leads to reduced dosing frequency |
| Polypharmacy | FDC of 2 to 3 drugs can be achieved by mixing different drug-coated Multiparticulates |
| Volume of dose | Can be dispersed in 5 to 10 ml of water |
| Dose administration | Dose dispersion can be administered using syringe or measuring cups to paediatrics |
| Flexibility method of administration | Orally swallowed/dispersed in water/chewable tablets/orally disintegrating tablets |
| Flexible tablets ≤500 mg | Flexibility in dose administration |
| Multiparticulates |
Table 2: Advantages of Oral Flexible Formulations
Bioavailability (BA) and Bioequivalence (BE)
As per regulatory requirements, the sponsors must use the most accurate, sensitive, and reproducible method to demonstrate BA or BE of a product. Several in vivo and in vitro methods can be used to measure BA and to establish BE. These include, in general order of preference, pharmacokinetic studies, in vitro tests predictive of human in vivo BA, pharmacodynamic studies, studies with clinical benefit endpoints, and other in vitro studies are followed [89,90]. In general single-dose PK studies are most commonly used for BE comparison of the test product and a reference product. BA/BE evaluation by pharmacokinetic study includes measurement of peak concentration (Cmax) and time to peak concentration (Tmax) for the rate of absorption and area under the curve (AUC0-t and AUC0-Inf) for the extent of absorption into systemic circulation. A typical PK study is conducted as a crossover study under fasting and fed conditions as the crossover design reduces variability caused by patient-specific factors, thereby increasing the ability to discern differences due to formulation [91]. Solid oral flexible formulations of a new drug can be evaluated for BA and BE studies in adult patients including geriatric patients and also in relevant age groups of paediatric patients. Based on BCS class and drug release type, flexible formulations developed using existing drugs can be evaluated by comparing BE using PK and in vitro studies with marketed formulation such as conventional orally swallowable or chewable or orally disintegrating or dispersible tablets. BA studies in paediatrics are a developing science and as recommended by regulatory agencies, clinical evaluations in paediatric age groups are warranted for new drugs once the safety and efficacy are established for an indication in adult patients [92]. Biowaiver for BA/ BE studies can be obtained for immediate drug products made using BCS class I drugs (highly permeable and highly soluble) and class III (highly soluble and low permeable) type drug candidates [93].
Palatability Evaluation
Solid oral flexible formulations can be evaluated for palatability studies using healthy adult volunteers. Taste evaluations by evaluating initial taste, after taste, mouth feel, flavour, oral disintegration, chewability and overall acceptability. Taste evaluation can also be done by artificial methods using the electronic tasting system such as e-tongue to compare the taste of various formulations [94]. Based on the taste of drug substance, type of flavoring, sweetener, and taste masking methods, polymers can be selected to develop taste masked palatable formulation. Taste of flexible formulations can also be evaluated in paediatric populations particularly in school going children of >2 y of age with consent of children and caretakers [95].
Oral solid flexible formulations platform can provide age appropriate formulation choice to all age groups of paediatrics and geriatric patients as they provide flexibility in drug administration. Design flexible formulations such as multiparticle, granules or flexible tablets can be a cost effective pharmaceutical alternative to existing formulations in the market as they are palatable and compatible to paediatric s and geriatric populations. Patient compliance can be further improved by the development of suitable long-acting flexible formulations to reduce dosing frequency among paediatric and geriatric patients. Flexible formulations such as sprinkles and granules can be a platform for long-acting drugs and fixed-dose combinations of two or more drugs to improve patient compliance among children with cancer, HIV/AIDS and in elderly patients with comorbidities.
Acknowledgements
The authors duly acknowledge the Department of Science and Technology, New Delhi, Government of India for supporting the establishment the National Facility for Drug Development for Academia, Pharmaceutical and Allied Industries at Bharathidasan Institute of Technology, Anna University, Tiruchirappalli, and providing constant support.
Conflict of interest
The authors report no declarations of interest.
Financial support and sponsorship
Nil.
References
- Ivansovska V, Carin MA, Rademaker CM, Dijk L, Aukje K, Teeuwisse M. Pediatric Drug Formulations: A review of Challenges and Progress. Pediatrics 2014;134:361-72.
- Nunn AJ. Making medicines that children can take. Arch Dis Child 2003;88(5):369-71.
- Mir AN, Geer MI. Off-label use of medicines in children. Int J Pharm Sci Res 2016;7:1820-28.
- Yewale VN, Dharmapalan D. Promoting appropriate use of Drugs in Children. Int J Pediatr 2012;2012:906570.
- Preis M, Breitkreutz J. Pediatric Drug Development and Dosage Form Design. AAPS PharmSciTech 2017;18:239-40.
- Stegemann S, Ecker F, Maio M, Kraahs P, Wohlfart R, Breitkreutz J, et al. Geriatric drug therapy: Neglecting the inevitable majority. Ageing Res Rev 2010;9:384-98.
- Shah RR. Drug development and use in the elderly: search for the right dose and dosing regimen. Br J ClinPharmacol 2004;58:452-69.
- Hanning SM, Lopez FL, Wong IC, Ernest TB, Tuleu C, Orlu GM. Patient-centric formulations for paediatrics and geriatrics: Similarities and differences. Int J Pharm 2016;512:355-9.
- Gupta A, Khan MA. Challenges of pediatric formulations: a FDA science perspective. Int J Pharm 2013;457(1):346-8.
- Strickley RG, Iwata Q, Wu S, Dahl TC. Pediatric drugs--a review of commercially available oral formulations. J Pharm Sci 2008;97(5):1731-74.
- Lopez FL, Ernest TB, Tuleu C, Gul MO. Formulation approaches to pediatric oral drug delivery: benefits and limitations of current platforms. Expert Opin Drug Deliv 2015;12(11):1727-40
- Stoltenberg I, Breitkreutz J. Orally disintegrating mini-tablets (ODMTs)--a novel solid oral dosage form for paediatric use. Eur J Pharm Biopharm 2011;78(3):462-9.
- Slavkova M, Breitkreutz J. Orodispersible drug formulations for children and elderly. Eur J Pharm Sci 2015;75:2-9.
- Visser JC, Woerdenbag HJ, Hanff LM, Frijlink HW. Personalized Medicine in Pediatrics: The Clinical Potential of Orodispersible Films. AAPS PharmSciTech 2017;18:267-72.
- Klingmann V. Acceptability of Mini-Tablets in Young Children: Results from Three Prospective Cross-over Studies. AAPS PharmSciTech 2017;18(2):263-66.
- Chandrasekaran P, Kandasamy R. Development of Oral Flexible Tablet (OFT) Formulation for Pediatric and Geriatric Patients: a Novel Age-Appropriate Formulation Platform. AAPS PharmSciTech 2017;18:1972-86.
- Chandrasekaran P, Kandasamy R. Development of Extended-Release Oral Flexible Tablet (ER-OFT) Formulation for Pediatric and Geriatric Compliance: an Age-Appropriate Formulation. AAPS PharmSciTech2017;18:2394-409.
- Preis M, Öblom H. 3D-Printed Drugs for Children-Are We Ready Yet? AAPS PharmSciTech 2017;18(2):303-08.
- Venkatesh MP. Regulation for pediatric drug development in India. J Clin Studies2014;6:14-7.
- https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E11/Step4/E11_Guideline.pdf.
- https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm360507.pdf.
- Breitkreutz J. European perspectives on pediatric formulations. ClinTher 2008;30:2146-54.
- Walsh J. Reflection on the Pharmaceutical Formulation Challenges Associated with a Paediatric Investigation Plan for an Off-Patent Drug. AAPS PharmSciTech 2017;18:250-56.
- http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003782.pdf.
- http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/07/WC500147002.pdf.
- Salunke S, Liu F, Batchelor H, Walsh J, Turner R, Ju TR, et al. European Paediatric Formulation Initiative (EuPFI)- Formulating Ideas for Better Medicines for Children. AAPS PharmSciTech 2017;18(2):257-62.
- http://www.who.int/medicines/areas/quality_safety/quality_assurance/Rev3-PaediatricMedicinesDevelopment_QAS08-257Rev3_17082011.pdf.
- http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002875.pdf.
- https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM189544.pdf.
- https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM075062.pdf.
- Lajoinie A, Henin E, Nguyen KA, Malik S, Mimouni Y, Sapori JM, et al. Oral drug dosage forms administered to hospitalized children: Analysis of 117,665 oral administrations in a French paediatric hospital over a 1-year period. Int J Pharm 2016;500:336-44
- Kumar A, Rawlings RD, Beaman DC. The mystery ingredients: sweeteners, flavorings, dyes, and preservatives in analgesic/antipyretic, antihistamine/decongestant, cough and cold, antidiarrheal, and liquid theophylline preparations. Pediatrics 1993;91(5):927-33.
- Kumar A, Weatherly MR, Beaman DC. Sweeteners, flavorings, and dyes in antibiotic preparations. Pediatrics 1991;87(3):352-60.
- “Inactive” Ingredients in Pharmaceutical products. Committee of Drugs. American Academy of pediatrics. Pediatrics 1985;76;635-43.
- “Inactive” Ingredients in Pharmaceutical Products: Update (Subject Review). Committee of Drugs. American Academy of pediatrics. Pediatrics 1997;99:268-78.
- Davies HE, Tuleu C. Medicines for children: a matter of taste. J Pediatr 2008;153(5):599-604.
- Pawar S, Kumar A. Issues in the formulation of drugs for oral use in children: role of excipients. Paediatric Drugs 2002;4(6):371-9.
- Venables R, Batchelor H, Hodson J, Stirling H, Marriott J. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int J Pharm 2015;480:55-62.
- Roberts R, Rodriquez W, Murphy D, Crescenzi T. Paediatric Drug Labelling. Improving the Safety and Efficacy of Pediatric Therapies. JAMA Pediatr 2003;290:905-11.
- https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073143.pdf.
- Zajicek A, Fossler MJ, Barrett JS, Worthington JH, Ternik R, Charkoftaki G, et al. A report from the pediatric formulations task force: perspectives on the state of child-friendly oral dosage forms. AAPS J 2013;15(4):1072-81.
- https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm240243.pdf.
- Olver I, Shelukar S, Thompson KC. Nanomedicines in the treatment of emesis during chemotherapy: focus on Aprepitant. Int J Nanomedicine 2007;2(1):13-18.
- Lennernas H, Abrahamsson B. The use of Biopharmaceutic classification of drugs in drug discovery and development: current status and future extension. J Pharm Pharmacol 2005;57:273-85.
- Mihajlovic T, Kachrimanis K, Graovac A, Djuric Z, Ibric S. Improvement of aripiprazole solubility by complexation with (2-hydroxy)propyl-β-cyclodextrin using spray drying technique. AAPS PharmSciTech 2012;13(2):623-31.
- Wu Y, Loper A, Landis E, Hettrick L, Novak L, Lynn K, et al. The role of biopharmaceutics in the development of a clinical nanoparticle formulation of MK-0869: a Beagle dog model predicts improved bioavailability and diminished food effect on absorption in human. Int J Pharm 2004;285(1-2):135-46.
- Zajicek A, Fossler MJ, Barrett JS, Worthington JH, Ternik R, Charkoftaki G, et al. A report from the pediatric formulations task force: perspectives on the state of child-friendly oral dosage forms.AAPS J 2013;15(4):1072-81.
- Van Riet-Nales DA, Kozarewicz P, Aylward B, de Vries R, Egberts TC, Rademaker CM, et al. Paediatric Drug Development and Formulation Design-a European Perspective. AAPS PharmSciTech 2017;18(2):241-49.
- Salunke S, Giacoia G, Tuleu C. The STEP (safety and toxicity of excipients for paediatrics) database. Part 1-A need assessment study. Int J Pharm 2012;435(2):101-11.
- Salunke S, Brandys B, Giacoia G, Tuleu C. The STEP (Safety and Toxicity of Excipients for Paediatrics) database: part 2 - the pilot version. Int J Pharm 2013;457(1):310-22.
- Chen ML, Straughn AB, Sadrieh N, Meyer M, Faustino PJ, Ciavarella AB, et al. A Modern View of Excipient Effects on Bioequivalence: Case Study of Sorbitol. Pharm Res 2007;24(1):73-80.
- http://www.fda.gov/Drugs/DrugSafety/ucm246002.htm.
- Walsh J, Cram A, Woertz K, Breitkreutz J, Winzenburg G, Turner R, et al. European Formulation Initiative. Playing hide and seek with poorly tasting paediatric medicines: do not forget the excipients. Adv Drug Deliv Rev 2014;73:14-33.
- Mizumoto T, Tamura T, Kawai H, Kajiyama A, Itai S. Formulation design of an Oral Fast-disintegrating Dosage form Containing Taste- masked Particles of Famotidine. Chem Pharm Bull (Tokyo) 2008;56(7):946-50.
- Sohi H, Sultana Y, Khar RK. Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug DevInd Pharm 2004;30(5):429-48.
- Rahman Z, Siddiqui A, Khan MA. Orally disintegrating tablet of novel salt of antiepileptic drug: formulation strategy and evaluation. Eur J Pharm Biopharm 2013;85:1300-9.
- Motola S, Agisim GR, Mogavero A. Palatable ibuprofen solutions. US Patent No: 5,024,997; 1991.
- Venkatesh DP, Geetha Rao CG. Formulation of taste masked oro-dispersible tablets of ambroxol hydrochloride. Asian J of Pharmaceutics 2008;2(4):261-64.
- Pisal S, Zainnuddin R, Nalawade P, Mahadik K, Kadam S. Drug release properties of polyethylene-glycol-treated ciprofloxacin-Indion 234 complexes. AAPS PharmSciTech 2004;5(4):e64.
- Pisal S, Zainnuddin R, Nalawade P, Mahadik K, Kadam S. Molecular properties of ciprofloxacin-Indion 234 complexes. AAPS PharmSciTech 2004;5(4):e62.
- Bilandi A, Mishra AK. Design and Evaluation of Taste masked Ion Exchange Resin Complex of Levofloxacin Hemihydrate: A Flouroquinolone Antibiotic. Int J Pharm Sci 2015;5(1):512-19.
- Dahima R, Sharma R. Comparative study of ion-exchange resin Indion 204 and Indion 214 for the taste masking of metoclopramide hydrochloride and formulation of rapid-disintegrating tablets. Asian J Pharm 2010;4(2):110-15.
- Bhoyar PK, Amgaonkar Y. Taste Masking and Molecular Properties of Metformin Hydrochloride-Indion 234 Complexes. J Young Pharm 2011;3(2):112-8.
- Bhoyar PK, Biyani DM, Umekar M. Formulation and Characterization of Patient-Friendly Dosage Form of Ondansetron Hydrochloride. J Young Pharm 2010;2(3):240-6.
- Shukla D, Chakraborty S, Singh S, Mishra B. Fabrication and evaluation of taste masked resinate of risperidone and its orally disintegrating tablets. Chem Pharm Bull (Tokyo) 2009;57(4):337-45.
- Eichman ML. Drug-resin complexes stabilized by chelating agents. US Patent No: 5,980,882; 1999.
- Kogan PW, Rudnic EM, Sequeira JA, Chaudry IA. Sustained release oral suspensions. US Patent No: 4,999,189; 1991.
- Hagen TA, Lo JB, Thombre AG, Herbig SM, Appel LE, Crew MD, et al. Azithromycin dosage forms with reduced side effects. US Patent No: 6,984,403; 2006.
- Appel LE, Ray RJ, Lyon DK, West JB, McCray SB, Crew MD, et al. Multiparticulate crystalline drug compositions having controlled release profiles. US Patent No: 7,887,844; 2011.
- Gowan, Jr WG, Bruce RD. Aliphatic or fatty acid esters as a solvent less carrier for pharmaceuticals. US Patent No: 5,405,617; 1995.
- James MB, Elliott LG. Pharmaceutical composition comprising cefuroxime axetil. US Patent No: 4,865,851; 1989.
- Lu MY, Borodkin S. Antibiotic-polymer compositions. US Patent No: 4,808,411; 1989.
- Tsau JH, Damani NC. Taste masking compositions. US Patent No: 5286489; 1994.
- Julian TN, Radebaugh GW. Chewable medicament tablet containing means for taste masking. US Patent No: 4,851,226; 1989.
- Mukherji G, Goel S, Arora VK. Taste masked compositions. US Patent No: 6,565,877; 2003.
- Nandi I, Guo M, Gassert CM, Schwarz FX, Kosilek I. Antibiotic clarithromycin micropellet compositions. US Patent No: 8,168,228; 2012.
- Mathur RS, Mehta K, Prabagaran C. Taste masked granules comprising clarithromycin, hydrocolloids and a coating. PCT Publication WO2006030297A1; 2006.
- Catania JS, Johnson AD. Taste-masking composition of bitter pharmaceutical agents. US Patent No: 5,633,006; 1997.
- https://www.fda.gov/downloads/Drugs/.../Guidances/ucm070578.pdf.
- Mehta K, Tu YH, Perumal A. Orally effective methylphenidate extended release powder and aqueous suspension product. US Patent No: 8,778,390; 2015.
- Mehta K, Tu YH, Perumal A. Orally effective methylphenidate extended release powder and aqueous suspension product. US Patent No: 8,956,649; 2015.
- Mehta K, Tu YH. Modified release formulations containing drug-ion exchange resin complexes. US Patent No: 8,790,700; 2014.
- Mehta K, Tu YH. Modified release formulations containing drug-ion exchange resin complexes. US Patent No: 8,883,217; 2014.
- Tu YH, Perumal A, Kathala K. Methylphenidate extended release chewable tablet. US Patent No: 8,999,386; 2015.
- Tu YH, Perumal A, Kathala K. Methylphenidate extended release chewable tablet. US Patent No: 9,295,642; 2016.
- Tengler M, McMahen R. Composition comprising a mixture of dextro- and levo-amphetamines complexed with ion-exchange resin particles to form drug resin particles. US Patent No: 9,017,731; 2015.
- Tengler M, McMahen R. Pharmaceutical composition comprising amphetamines complexed with ion-exchange resin particles. US Patent No: 9,265,737; 2016.
- Licht D, Shmuel G, Zholkousky M, Yam B, Kaplan R, Holon, etal. Sustained Release Formulation of Carbamazepine. US Patent No: 6,1614,66; 2000.
- https://www.fda.gov/downloads/Drugs/.../Guidances/ucm070124.pdf.
- https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208745Orig1s000AdminCorres.pdf.
- https://www.fda.gov/ohrms/dockets/ac/03/briefing/3995B1_07_GFI-BioAvail-BioEquiv.pdf.
- http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003066.pdf.
- https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070246.pdf.
- Ishizaka T, Miyanaga Y, Mukai J, Asaka K, NakaiY,Tsuji E, etal. Bitterness evaluation of medicines for pediatric use by a taste sensor. Chem Pharm Bull (Tokyo) 2004;52(8):943-8.
- http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/09/WC500132555.pdf.