- Corresponding Author:
- Fatma Demirkaya−Miloglu Department of Analytical Chemistry, Faculty of Pharmacy, Ataturk University, Erzurum-25240, Turkey. E−mail: fdemirkayamiloglu@gmail.com
| Date of Submission | 06 March 2013 |
| Date of Revision | 27 June 2013 |
| Date of Acceptance | 10 July 2013 |
| Indian J Pharm Sci 2013;75(5):563-568 |
Abstract
A simple, sensitive and rapid spectrofluorimetric method for determination of α-tocopherol in pharmaceutical capsule and human plasma was developed and validated. The native fluorescence of α-tocopherol was measured at 334 nm with excitation at 291 nm, after extraction of α-tocopherol from human plasma hexane:dichloromethane mixture. The calibration curves were linear (R≥0.9993) in the concentration range of 0.25-2.5 μg/ml of α-tocopherol in both standard solutions and plasma samples. The developed method was directly and easily applied for determination of α-tocopherol in the plasma of healthy volunteers and different type of bladder cancer and stomach cancer patients and also pharmaceutical capsule.
Keywords
α−tocopherol, pharmaceutical, human plasma, cancer, spectrofluorimetric method
There are eight main compounds which have vitamin E activity. These eight compounds are available in two forms which are tocotrienols and tocopherols [1]. Tocopherols and tocotrienols are fat−soluble antioxidants. The most common and active form, in terms of chemical and biological activity, of vitamin E compound is α-tocopherol (α-T) in humans. It is a potent peroxyl radical scavenger and a chain−breaking antioxidant that prevents the propagation of free radical damage in biological membranes [2]. According to some research results, scientists claim that vitamin E active compounds help to protect and even to cure human body from cancers of bladder and stomach, and also diseases as atherosclerosis, stroke, heart disease, diabetes mellitus types I and II, Alzheimer and Parkinson caused by free radicals [3,4]. However, there was also no association between dietary intakes or blood concentrations of α−T and cancer in some studies [5]. Mirvish [6] pointed out that vitamin E as an antioxidant is reduced to nitroso compounds which help to inhibit formation of cancer in bladder and stomach cancer, and thus reduces the risk of developing cancer.
Vitamin E is usually taken with food. If this intake is insufficient or if special dietary requirements exist, multivitamins or vitamin preparations can be taken in order to prevent vitamin deficiency. Due to the importance and indication of deficiency, it is important to determine the level of vitamin E both in biological fluids and pharmaceutical preparations. Attempts to determine α−T individually or in its combination with other vitamins in human plasma [7−18] and pharmaceutical preparations [19−21] with different methods, such as UV spectrophotometry [7,8], high−performance liquid chromatography (HPLC) with UV detection [9−11,19−21], diode array detection [12,13], fluorimetric detection [14,15], electrochemical detection [16,17] and mass spectroscopic detection [18] are reported in literature. But we could not find any article on determination of α−T in pharmaceutical preparations and human plasma by spectrofluorimetric method.
Hence, in this manuscript an alternative and novel spectrofluorimetric method, which is applicable for clinical analysis, was proposed for the determination of α−T in pharmaceutical preparations and plasma of healthy volunteers as well as plasma of bladder cancer and stomach cancer patients.
Materials and Methods
HPLC grade (purity≥%96) α−tocopherol (α-T), analytical grade H3BO3, H3PO4, n−hexane and CH3COOH were also bought from Sigma-Aldrich, St. Louis, Mo, USA. GC grade ethanol and dichloromethane (DCM) were obtained from Riedel De Haen, Germany. Evicap® soft gelatin capsule (400 mg α−T, Kocak Farma Pharmaceutical Industry, Istanbul, Turkey) was obtained from local sources in Erzurum, Turkey. Blank plasma was obtained from Yakutiye Hospital, Faculty of Medicine, Ataturk University, Erzurum, Turkey. The fluorescence spectra and measurements were recorded using a Perkin Elmer LS45 fluorescence spectrometer equipped with FL WinLab software, and a 150 W xenon arc lamp. The scan range of emission and excitation are between 290-410 nm and 240−330 nm, respectively. The emission (λem) and excitation (λexc) wavelengths were 334 nm and 291 nm, respectively. Slit width for both monochromators was set at 10 nm. Moreover, sensitivity of each recorder was set at 1.0 nm.
Preparation of calibration standards and quality control samples
The standard stock solution of α−T (2 mg/ml) was prepared by dissolving about 20 mg of α−T in 10 ml ethanol and then stored at 4° in dark bottles until analysis. To obtain working standard solutions (WS) of α−T at 2.5, 2.0, 1.5, 1.0, 0.75, 0.5 and 0.25 μg/ml concentrations, the stock solution was subsequently diluted in the same diluents. Quality control (QC) samples at three concentration levels (0.25, 0.75 and 1.5 μg/ml for plasma and 0.5, 1.0 and 1.5 μg/ml for standard) were also prepared in a similar manner.
Drug sample preparation
The content of capsule (Evicap® soft gelatin capsule) was transferred into 100 ml calibrated flask, dissolved in 50 ml ethanol and volume was made up to mark. This solution was sonicated and filtered through phonomene 0.45 μm size (25−mm filter) and stored in dark glass flasks, in order to protect them from light, and kept in freezer. Required concentrations were prepared by diluting the stock with ethanol.
Plasma sample preparation
Plasma extraction was achieved by modifying extraction method previously developed in our laboratory[22]. According to this process, plasma samples were taken in a test tube and 1.0 ml of ethanol added for protein precipitation and plasma−ethanol solution was mixed for 10 s. Then, 6 ml of hexane:DCM (9:1 v/v) solution was added into the plasma sample and the solution was remixed on vortex for 1 min. This mixture was centrifuged about 7 min at 4000×g. The organic layer was transferred into a clean tube and evaporated to dryness under the stream of nitrogen for 10 min. The residue was dissolved in 12 ml of ethanol for analysis.
Application of the method
Subjects were cancer patients in the Haematology/Oncology and Urology Clinics at the University of Ataturk (experimental group) and healthy volunteers (control group). This study was approved by the Ethics Committee of Health Sciences Institute (2006.2.1/9), Ataturk University, after having received written consents from all the volunteers. The cancer patients were divided into four groups: (a) diffuse stomach cancer patients, (b) intestinal stomach cancer patients, (c) superficial bladder cancer patients, (d) invasive bladder cancer patients. Each group consisted of six subjects and the members of the groups were classified by matching them in terms of their age and sex. The entire group members have not received drug containing α−T for at least 2 weeks before and during the entire study other than study administration. In addition, subjects fasted for 10 h before drawing blood. The experiments were performed between 08:00 and 9:00 a.m. and blood was collected only once for each subjects. Plasma was separated from blood cells by using a clinical centrifuge for 15 min at 25±5º, frozen in 0.3 ml aliquots, and stored at −70±5º until analyzed.
Statistical analysis
For five independent groups each consisting of six subjects comparisons, nonparametric Kruskal−Wallis variance analysis was performed by using SPSS 11.5 software program (SPSS, Chicago, IL, USA). Values were presented as means±standard deviation of the mean. Differences with P<0.05 were considered to be statistically significant.
Results and Discussion
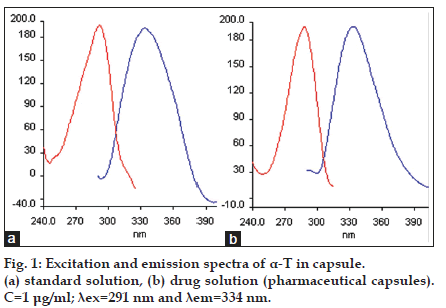
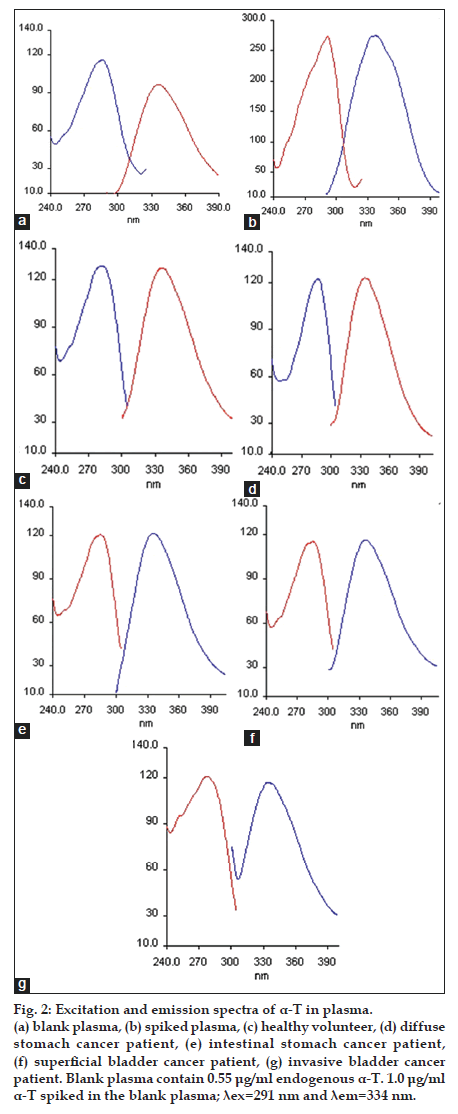
Different experimental factors such as pH, type and volume of the diluting solvent and ionic strength affected the formation and stability of the fluorescence of the compound studied. Variation of pH from 2.0 to 12.0 was investigated with Britton−Robinson buffer solution and it was determined that relative fluorescence intensity did not change with pH. Therefore, buffer solution was not used in this study. Ethanol was selected as the best solvent for the dilution. In addition to this, different volumes of ethanol were tested to observe the effect of the volume of solvent on relative fluorescence intensity. According to the obtained data, fluorescence intensity was unaffected with increasing the volume of the solvent. In order to reduce endogenous tocopherol concentration, the extracted plasma residues were reconstituted in 12 ml ethanol, while the standard solutions of α−T were prepared in 1 ml ethanol. The fluorescence spectra (excitation and emission) obtained for α−T standard solutions and also the solutions extracted from plasma reveal that the maximum lexc and lem showed a band peak at 291 nm and 334 nm, respectively. The fluorescence spectra obtained from 1.0 μg/ml of a−T standard solution, blank plasma containing endogenous a−T and plasma spiked with 1.0 μg/ml a−T were given in figs. 1 and 2.
Figure 2:Excitation and emission spectra of α-T in plasma. (a) blank plasma, (b) spiked plasma, (c) healthy volunteer, (d) diffuse stomach cancer patient, (e) intestinal stomach cancer patient, (f) superficial bladder cancer patient, (g) invasive bladder cancer patient. Blank plasma contain 0.55 μg/ml endogenous α-T. 1.0 μg/ml α-T spiked in the blank plasma; λex=291 nm and λem=334 nm.
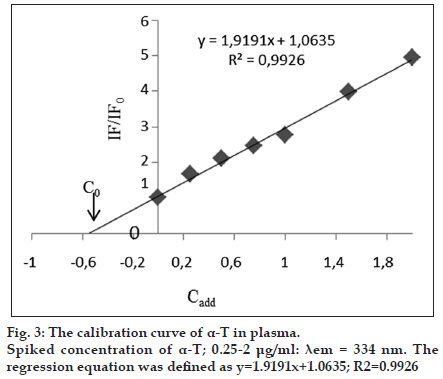
Endogenous α-T was present in the pooled blank human plasma. In order to determine endogenous level of concentration of α-T (C0), standard addition method was developed in human plasma. The followed equation defines the calibration curve as IF* =AC+B, which is also represented as IF*=[(IF0*/ C0)×Cadd]+IF0*. Where, C0 is endogenous concentration of α-T to be estimated in blank human plasma, IF0* is normalized fluorescence intensity of the blank human plasma, and Cadd is added α-T concentrations and IF* is normalized fluorescence intensity and is calculated by the ratio of (IF/IF0), where IF is after the standard addition and IF0 is the fluorescence intensity before the standard addition. In order to construct a calibration curve with normalized fluorescence intensity (IF*) of the added analyte concentration (Cadd), different concentrations (0.25-2.0 μg/ml) was added into the blank human plasma (fig. 3). By the negative intercept of the curve with the abscissa axis, C0 value is calculated to be 0.55 μg/ml [23].
The proposed method was validated with parameters such as specificity, linearity, sensitivity, precision, accuracy, recovery and stability parameters in accordance with International Conference on Harmonization (ICH) Q2B guidance [24].
Method was determined to be linear and is in accordance with the values of regression equation obtained by plotting calibration curves between fluorescence intensity and concentrations added. The least squares method was used to calculate the regression equation. The equations for calibration curve and statistical parameters were given in Table 1.
| Parameters | Standard solution | Human plasma |
|---|---|---|
| Linearity (µg/ml) | 0.25−2.5 | 0.25−2.0 |
| Regression equationa | bI=149.52C+28.38 | bI=140.17C+25.15 |
| Standard deviation of slope (Sa) | 1.808 | 3.049 |
| Standard deviation of intercept (Sb) | 2.659 | 1.96 |
| Correlation coefficient | 0.9996 | 0.9993 |
| Standard deviation of correlation coefficient | 0.0043 | 0.0056 |
aAverage of six replicate determinations, bI=The fluorescence intensity, C=a-T concentration
Table 1: Results of regression analysis of α−t
LOD and LOQ were determined with ratio of signal to noise which were lower than 3 and 8, respectively. These values were obtained by going down to lower values than calibration values for standard solution and by diluting blank human plasma containing endogenous α−T for human plasma. For both human plasma and standard, LOD and LOQ were 0.09 and 0.25 μg/ml, respectively.
The precision of the analytical method was determined by repeatability (intraday) and intermediate precision (interday). Repeatability was evaluated by analyzing spiked blank human plasma samples six times at QC samples (0.5, 1 and 2 μg/ml for standard solution and 0.25, 0.75 and 1.5 μg/ml for human plasma) and during the same day. The intermediate precision was evaluated by assaying the same plasma samples in six times once daily for 6 days. The precision of the method was expressed as the percent relative standard deviation (%RSD=(standard deviation/mean)×100) and the accuracy of method was expressed as the relative error (relative error (RE)=(found-known)/known×100). The precision of method was adequate, because the RSD% values for standard solutions and human plasma samples were less than 6.76 and 7.94%, respectively. Accuracy of method (RE) was less than 6.31 (for standard solution) and 5.48 (for plasma samples). The obtained results were shown in Table 2.
| Matrix | Added | Intraday | Interday | ||||
|---|---|---|---|---|---|---|---|
| (µg/ml) | Found | Precision (%RSD) | Accuracy (RE) | Found | Precision (%RSD) | Accuracy (RE) | |
| Standard solution | 0.5 | 0.53±0.014 | 2.71 | 6.31 | 0.52±0.018 | 3.37 | 4.41 |
| 1 | 1.01±0.017 | 1.71 | 0.81 | 1.02±0.034 | 3.29 | 2.2 | |
| 2 | 1.98±0.015 | 0.74 | −1.06 | 2.01±0.136 | 6.76 | 0.28 | |
| Human plasma | 0 (basal) | 0.55±0.006 | - | - | - | - | - |
| 0.25 | 0.24±0.019 | 7.94 | −5.48 | 0.24±0.016 | 6.69 | −3.97 | |
| 0.75 | 0.78±0.030 | 3.86 | 4.56 | 0.74±0.043 | 5.81 | −1.17 | |
| 1.5 | 1.49±0.009 | 0.63 | −0.91 | 1.44±0.041 | 2.84 | −3.85 | |
Vaues are expressed as mean±SD. SD=Standard deviation, RSD=relative standard deviation, RE=relative error
Table 2: Accuracy and precision of proposed method
Recoveries (%R) were carried out by spiking known quantities (0.5, 1 and 2 μg/ml for standard solution and 0.25, 0.75 and 1.5 μg/ml for human plasma) of standard in blank human plasma or pharmaceutical capsule and calculated as %R=(Ct-Ce/Ca)×100, where Ct is the total concentration of α-T in the added samples, Ce is the concentration α-T to be estimated in blank human plasma or pharmaceutical capsule and Ca is the concentration of standard α-T added. The recovery values of α−T from human plasma and pharmaceutical capsule for intraday and interday analysis were higher than 89.3 and 100.5%, respectively. The obtained results were presented in Table 3.
| Matrix | Added (µg/ml) | Intraday (%) | Interday (%) | ||
|---|---|---|---|---|---|
| aRecovery | RSD | Recovery | RSD | ||
| Human plasma | 0.25 | 91.9 | 6.08 | 89.25 | 5.06 |
| (0.55 µg/ml | 0.75 | 97.1 | 3.21 | 92.04 | 5.09 |
| endogenous α−T) | 1.5 | 91.6 | 1.68 | 90.75 | 2.69 |
| Evicap® gelatin | |||||
| soft capsule | 0.5 | 108.3 | 2.26 | − | |
| (0.5 µg/ml) | 1 | 103.1 | 2.98 | − | |
| 2 | 100.5 | 3.46 | − | ||
RSD=Relative standard deviation, aAverage of six replicate determinations
Table 3: Recovery values of α−t in human plasma and pharmaceutical capsule
The developed and validated spectrofluorimetric method was applied to determine the α-T in human plasma and pharmaceutical capsule. The emission fluorescence at 334 nm (excitation at 291 nm) of the pharmaceutical capsule solution and the human plasma sample against blank which is prepared by extraction process was measured. The amounts of α−T in human plasma samples were calculated from the calibration curve of human plasma samples and the obtained value was multiplied with the dilution factor of 12 (12 ml of ethanol as reconstitution solvent/1 ml real sample solution) to find the real amount of α−T. Furthermore, the amount of α−T in pharmaceutical capsule was calculated directly from the standard calibration curve.
The fluorescence spectra of the solution of pharmaceutical capsule and the sample of human plasma (healthy volunteer and different type stomach and bladder cancer patients) were shown in figs. 1 and 2. It was indicated that the α−T contents measured by the proposed methods in pharmaceutical were in good agreement with the values supplied by the manufacturers (Table 4). The α−T levels obtained from plasma of each group were determined and given in Table 5. In addition to this, the data obtained from group a (control), b and c (stomach cancer patients) and group a (control), d and e (bladder cancer patients) were compared. Kruskal–Wallis variance analysis was applied for independent groups each consisting of six subjects. According to data obtained from this analysis, there was no significant difference between human plasma endogenous a−T concentrations in groups a, b and c and in groups a, d and e (P=0.121 and P=0.884, respectively). The results obtained were verified with Mann−Whitney Test (Table 5).
| Commercial preparation |
n | aFound ±SD (mg) |
Recovery (%) |
RSD % |
aConfidence interval |
|---|---|---|---|---|---|
| Evicap® gelatin soft capsule (135.23 mg/capsule) |
|||||
| 12 | 136.5±5.17 | 101.7 | 3.79 | 96.8−109.0 | |
RSD=Relative standard deviation, SD=Standard deviation of six replicate determinations, aAverage of six replicate determinations
Table 4: Determination of α−t in pharmaceutical capsule
| Groups | n | Mean α−T concentration (µg/ml) | Confidence interval | χ2 values |
|---|---|---|---|---|
| 1 | 6 | 9.018c | 6.248a−11.57c | 4.227 (P=0.121) |
| 2 | 6 | 6.501c | 4.337a−8.321c | |
| 3 | 6 | 7.113c | 4.508a−9.696c | |
| 1 | 6 | 9.018c | 6.248a−11.57c | 0.246 (P=0.884) |
| 4 | 6 | 8.750c | 5.552a−13.26c | |
| 5 | 6 | 9.018c | 3.77a−13.55c |
aAverage of six replicate determinations, cObtained concentration by multiplying with the dilution factor of concentration calculated from the calibration curve, Group 1=Healthy volunteers, Group 2= Diffuse stomach cancer patients, Group 3=Intestinal stomach cancer patients, Group 4=Superficial bladder cancer patients, Group 5=Invasive bladder cancer patients, H0=No statistically significant difference exists between groups (P>0.05)
Table 5: Endogenous α−t concentrations in plasma samples and their statistical comparison
The performance of the spectrofluorimetric method was compared with HPLC and spectrophotometric methods, it seems that spectrophotometric method is rapid but accuracy and sensitivity are poorer with respect to HPLC. The HPLC method is sensitive and accurate enough but it requires pretreatments causing time consuming (mobile phase preparation, column equilibration before analysis) and also this technique is expensive in terms of column, mobile phase and instrumentation cost. As mentioned above, using HPLC and spectrophotometry has several disadvantages whereas spectrofluorimetric method offer combination of advantages in both methods. Spectrofluorimetric method is as sensitive as HPLC methods and as rapid as spectrophotometric method which can be applied on human plasma at low concentrations.
There are two study about determination of α-T by HPLC with fluorescence dedection [14,15] in literature. When comparing these two methods with our proposed method, very similar results were obtained in terms of accuracy, recovery and precision. However, proposed method (0.25 μg/ml) was around two−fold more sensitive than the method of Siluk et al. (0.5 μg/ml) [15]. Furthermore, our proposed method is much more simple, cheap and rapid than the mentioned HPLC methods. Because of this advantages, its usage is more applicable in routine analysis of α-T in plasma samples and pharmaceutical formulations.
In this study, new spectrofluorimetric method was developed to provide a very sensitive and quantitative assay for determination of a−T in pharmaceutical capsule and human plasma. This is the first study about determination of a−T in human plasma by spectrofluorimetry in accordance with our research, which point out the novelty of this study. The proposed method was applied to plasma of healthy volunteers and patients with bladder and stomach cancer. An important aspect of the implementing a new assay in routine quality control analysis is that it should be thoroughly evaluated before introduction for routine use.
References
- Sen S, Khanna S, Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci 2006;78:2088−98.
- Brigelius−Flohe R, Traber MG. Vitamin E: Function and metabolism. FASEB J 1999;13:1145−55.
- Mancini M, Parfitt VJ, Rubba P. Antioxidants in the Mediterranean diet. Can J Cardiol 1995;11:105−9.
- Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: A review. J BiochemMolToxicol 2003;17:24−38.
- Knekt P, Aromaa A, Maatela J, Aaran RK, Nikkari T, Hakama M. Vitamin E and cancer prevention. Am J ClinNutr 1991;53:283−6.
- Mirvish SS. Effects of vitamins C and E on N-nitroso compound formation, carcinogenesis, and cancer. Cancer 1986;59:1842−50.
- Kayden HJ, Chow CK, Bjornson LK. Spectrophotometric method for determination of tocopherol in red blood cells. J Lipid Res 1973;14:533−40.
- Unalacak M, Atmaca H, Gurel A, Armutcu F, Demircan N, Aktunç E. Levels of serum malondialdehyde, α-tocopherol and β-Carotene in hyperglycemic patients with impaired glucose metabolism.Firat Medical Journal 2005;10:113-6.
- Gonzalez−Corbella MJ, Lloberas−Blanch N, Castellote−Bargallo AI, Lopez−Sabater M, Rivero−Urgell M. Determination of α−tocopherol in plasma and erythrocytes by high−performance liquid chromatography. J Chromatogr B 1994;660:395−400.
- Nirungsan K, Thongnopnua P. Simple and rapid high-performance liquid chromatographic method for endogenous α-tocopherol determination in human plasma. Biomed Chromatogr 2006;20:774−81.
- Julianto T, Yuen KH, Noor AM. Simple high−performance liquid chromatographic method for determination of α−tocopherol in human plasma. J Chromatogr B 1999;732:227−31.
- Zaman Z, Fielden P, Frost PG. Simultaneous determination of vitamins A and E and carotenoids in plasma by reversed−phase HPLC in elderly and younger subjects. ClinChem 1993;39:2229−34.
- Karppi J, Nurmi T, Olmedilla−Alonso B, Granado−Lorencio F, Nyyssönen K. Simultaneous measurement of retinol, α−tocopherol and six carotenoids in human plasma by using an isocratic reversed−phase HPLC method. J Chromatogr B 2008;867:226−32.
- Teissier E, Walters−Laporte E, Duhem C, Luc G, Fruchart JC, Duriez P. Rapid quantification of alpha−tocopherol in plasma and low− and high−density lipoproteins. ClinChem 1996;42:430−5.
- Siluk D, Oliveira RV, Rosas ME, Ling S, Bos A, Ferrucci L, et al. A validated liquid chromatography method for the simultaneous determination of vitamins A and E in human plasma. J Pharm Biomed Anal 2007;44:1001−7.
- Corrado S, Edoardo M, Marco Vincenti MN, Flavio G. Determination of plasma tocopherols by high−performance liquid chromatography with coulometric detection. J Chromatogr B 1993;620:268−72.
- Takeda H, Shibuya T, Yanagawa K, Kanoh H, Takasaki M. Simultaneous determination of α−tocopherol and α−tocopherolquinone by high−performance liquid chromatography and coulometric detection in the redox mode. J Chromatogr A 1996;722:287−94.
- Mottier P, Gremaud E, Guy PA, Turesky RJ. Comparison of gas chromatography: Mass spectrometry and liquid chromatography– tandem mass spectrometry methods to quantify α−tocopherol and α−tocopherolquinone levels in human plasma. Anal Biochem 2002;301:128−35.
- Scalia S, Ruberto G, Bonina F. Determination of vitamin A, vitamin E, and their esters in tablet preparations using supercritical fuid extraction and HPLC. J Pharm Sci 1995;84:433−6.
- Moreno P, Salvado V. Determination of eight water−and fat−soluble vitamins in multi−vitamin pharmaceutical formulations by high−performance liquid chromatography. J Chromatogr A 2000;870:207−15.
- Wang LH, Huand SH. Determination of vitamins A, D, E, and K in human and bovine serum, and β−carotene and vitamin A palmitate in cosmetic and pharmaceutical products, by isocratic HPLC. Chromatographia 2002;55:289−96.
- Demirkaya F, Kadioglu Y. Simple GC−FID method development and validation for determination of α−tocopherol (vitamin E) in human plasma. J BiochemBiophys Methods 2007;70:363−8.
- Rima J, Abourida M, Xu T, Cho IK, Kyriacos S. New spectrophotometric method for the quantitative determination of melamine using Mannich reaction. J Food Compos Anal 2009;22:689−93.
- ICH. Q2B, Harmonized tripartite guideline, validation of analytical procedure: Methodology, IFPMA. In: Proceedings of the International Conference on Harmonization, Geneva, 1996.