- *Corresponding Author:
- N. R. Chatterjee
Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Pimpri, Pune-411 018
E-mail: nitharc@yahoo.com
| Date of Submission | 31 October 2005 |
| Date of Revision | 19 June 2006 |
| Date of Acceptance | 13 February 2007 |
| Indian J. Pharm. Sci., 2007, 69 (1): 135-137 |
Abstract
Stability studies of some novel dual action fluoroquinolonyl-3'-penicillin amides (1a-e) in aqueous DMF (1:1) solution has been carried out in presence of Cu (ic) or Hg (ic) ions and their degradation rate was monitored by UV/Vis spectrophotometric method. The results thus obtained indicate that ciprofloxacin moiety offered greater stability to the β-lactam moiety of the hybrid (1d or 1e) than norfloxacin (1a or 1b) in the presence of heavy metal ion degradation; whereas the degradation pattern of β-lactam moiety in the hybrids under investigation (1a-e) was found to be in agreement with the previously reported heavy metal ion degradation profile of the penicillins.
Degradation studies on fl-lactam antibiotics have been studied at length by various methods; which helped significantly in development of their dosage form as well as in evaluation of their physico-chemical properties [1]. Dual action hybrids of fluoroquinolones and fl-lactam antibiotics have recently come into prominence e.g., cefotoxime-fleroxacin (Ro-23-9424) due to their novel mechanism of action; wherein the fl-lactam moiety acts as a carrier for the quinolone antibiotic. The major thrust in the construction of dual action hybrids has been linking the penicillins or cephalosporins at 3′ or 4′ position to the different point (s) of any fluoroquinolone through ester, carbamate or amide linkages [2].
We have synthesized some novel fluoroquinolonyl-3′- penicillanic and 4′-cephalosporanic acid amides, wherein some fluoroquinolonyl penicillin amides (la-1e) have shown higher Gram-negative activity than their corresponding cephalosporanic acid derivatives [3]. Since no report on stability studies of this class of hybrid antibiotics was found in literature; therefore it appeared worthwhile to study the stability of some of these fluoroquinolonyl-3′-penicillin amides in presence of heavy metal ions (Cu++ or Hg++) under controlled pH condition by following UV/Vis spectrophotometric method as established earlier [4-5]. The purpose of this study is therefore to investigate the stability of the fl-lactam moiety in this group of hybrid antibiotics in presence of heavy metal ions like copper or mercury.
Since the quinolone moiety showed specific absorption at 320 and 270 nm, it appeared more logical to monitor the 320 and 270 nm, it appeared more logical to monitor the rate of change of absorption at 260 nm (specific for fl- lactams) for the determining the stability of hybrid molecules5. However, the rate of change of absorption at 280 nm due to formation of penicilloic acid derivatives also revealed the similar stability of penicillin moiety as obtained in case of the reported penicillin degradation studies. Therefore, we intended to follow the degradation pattern of each of these compounds (la-e) in presence of Cu++ or Hg++ ions by measuring the absorbance of its aqueous DMF (1:1) solution at 260 nm and 280 nm, respectively as a function of time.
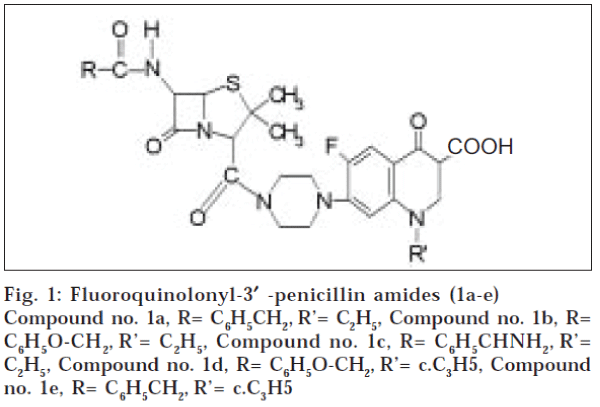
The title compounds (la-e) under stability study were prepared following our reported method (fig.1) and their purity was checked by TLC, reported mp and mixed melting point with an authentic sample3. The experimental solution (50 ml, 1.5 M) of each of the said compounds (1a-1e) under study was then prepared by dissolving the required amount of the compound in DMF (25 ml) and subsequently diluted with water to make the volume up to 50 ml under stirring to obtain a clear stock solution. Cupric chloride or mercuric chloride stock solution (100 ml, 0.01 M) was prepared using AR grade salt. UV spectral measurements were performed with a Bausch and Lomb spectronic 21 DB spectrophotometer using matched 1 cm2 silica cells.
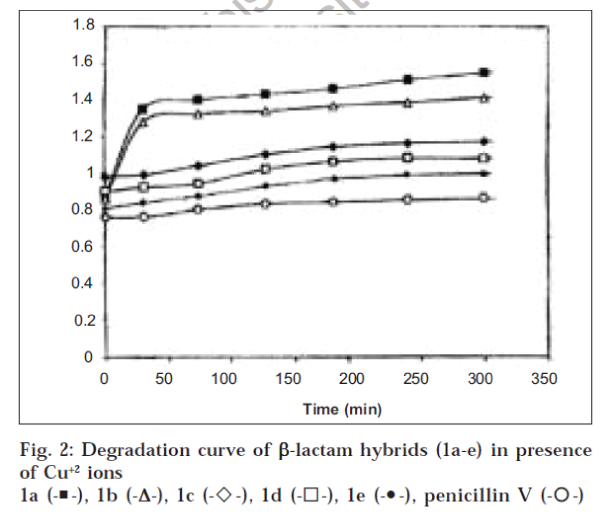
To a gently stirred stock solution (25 ml) of the compound under investigation study at 24±1°, 2 ml of the cupric chloride stock solution was added and kept under stirring. After a regular interval of time, a sample of the solution under study was withdrawn and its absorbance was measured at 260 and 280 nm against a reagent blank in aqueous DMF (1:1) solution; followed by its addition to the main experimental solution. The average of two such readings was taken and each experiment was continued for 6 h. A set of identical experiments were carried out with each of the compounds (1a-e) under investigation replacing cupric chloride solution with mercuric chloride stock solution and the spectrophotometric degradation curves for each compound in aqueous DMF (1:1) solution with Cu++ and Hg++ ions were obtained by plotting the extinction value at 260 and 280 nm against the time interval. Since the degradation curves for the compounds under investigation showed better sensitivity at 260 nm than 280 nm, possibly due to interference of specific absorption of quinolone moiety; the degradation curves of title compounds at 260 nm in presence of Cu++ ions have been depicted in fig. 2. The pattern of degradation curves of these compounds in case of Hg++ ions was observed to be similar to fig. 2 and therefore have not been reported separately.
It has been observed from the above study (fig. 1) that the hybrid molecules incorporating penicillin V or G and ciprofloxacin (1d or 1e) offer greater stability than the hybrids of penicillin G or penicillin V with norfloxacin (la and lb). This could possibly be well explained on the basis of their complexation with metal ions due to their respective ionic nature in aqueous-DMF (1:1) solution, which plays significant role in the penicillin degradation [7]. The identical experiments carried out in presence of Hg++ ions for determining the stability of these compounds (1a-e) also substantiated the said results. The results reported herein also indicate that fl-lactam hybrids bearing ciprofloxacin moiety (1b and 1e) showed greater resistance to heavy metal ion degradation than those of the hybrids containing norfloxacin unit (1a and 1d) in presence of heavy metal ions; possibly due to better complex formation [8]. The conjugates bearing penicillin V moiety (1b and 1d) offer more stability in comparison to those of penicillin G linked hybrids (1a and 1e); whereas ampicillin moiety in the hybrid (1c) offered better resistance to that of penicillin V containing hybrid (1b). These observations are in conformity with the earlier established results of heavy metal ion and acid degradation profile of fl-lactam antibiotics1. Furthermore, comparison of the degradation profile of the fl-lactam hybrids (1a-e) in presence of Cu (ic) ions with penicillin V (fig. 2) reveals that the hybrid formation of fl-lactams through amide linkage do not attribute any resistance to the fl-lactam stability in case of heavy metal ion degradation.
Acknowledgements
Our sincere thanks are due to Drs. A. D. Deshpande and S. R. Naik for their support and encouragement during the work
References
- Chatterjee, N.R, Baheti, K.G. and Deshpande, A.D., Curr. Sci., 2004, 87, 1684
- Chatterjee, N.R. Bhanot, S.K. and Singh, M., Curr. Pharm. Design, 2001, 7, 311.
- Chatterjee, N.R., Bhanot, S.K. and Naik, S.R., Arzneim. Forsch., 1994, 44, 663.
- Chatterjee, N.R., Degani, M.S. and Singh, C.B., Indian J. Pharm. Sci. 1998, 50, 128.
- Raja, M.S. and Pai, P.N.S., Indian J. Pharm. Sci., 2002, 64, 400.
- Rao, S., Chowdary, K.P.R. and Rao, S., Indian J. Pharm. Sci., 2003, 65, 307.
- Krischbaum J., In: Florey K. Eds., Analytical Profile of Drugs Substances, Academic Press New York, 1986, 15, 436.
- Cressman, W.A., Sugita, E. and Nibergall, T., J. Pharm. Sci., 1969, 58, 1471.