- *Corresponding Author:
- M. Ahmed
Institute of Chemistry, University of the Punjab, New Campus, Lahore
E-mail: mahmoodresearchscholar@gmail.com
| Date of Submission | 29 August 2014 |
| Date of Revision | 29 January 2015 |
| Date of Acceptance | 08 September 2015 |
| Indian J Pharm Sci 2015;77(5):515-521 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
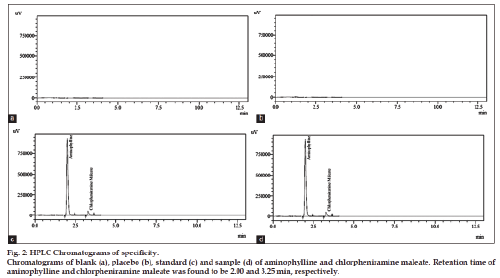
The present work deals with the development and validation of method for simultaneous determination of antihistaminic drugs in pharmaceutical formulations. A precise, specific and accurate reverse phase-high-performance liquid chromatography method for the simultaneous measurement of aminophylline and chlorpheniramine maleate was developed. The separation of drugs was achieved on C-18 (5 μm, 250×4.6 mm) high-performance liquid chromatography column. The runtime for analysis was 10 min. Mobile phase is mixture containing dilute H2SO4:methanol (60:40% v/v) with flow rate adjusted at 1.5 ml/min. The detection of components was performed at a wavelength of 264 nm. Retention times of aminophylline and chlorphinramine maleate were found to be 2.00 and 3.25 min, respectively. Linearity was found in the range of 16-24 μg/ml for chlorpheniramine maleate and 102.4-153.6 μg/ml for aminophylline with a correlation coefficient of 0.9998 and 0.9996, respectively. High peak purity index of 99.99% indicated the complete separation of analytes in the presence of degradation products is justification of method stability. Linearity, accuracy, specificity, precision and robustness studies were performed for method validation.
Keywords
Antihistaminic drugs, degradation products, ICH, RP-HPLC, validations
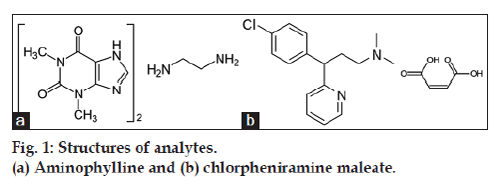
Aminophylline (fig. 1a) is 3,7-dihydro-1,3-dimethyl- 1H-purine-2,6-dione compound with 1,2-ethanediamine (2:1); theophylline ethylenediamine. Theophylline is active component of aminophylline, used as bronchodilator in the treatment of chronic obstructive pulmonary disease and asthma. It is also used in relief of neonatal apnoea, treatment of heart failure and obstructive airways disease. Aminophylline due to greater solubility in water is usually preferred to theophylline, particularly in intravenous formulations [1,2]. Chlorpheniramine maleate (fig. 1b) is 2- [p-chloroa- [ 2-(dimethylamino)ethyl]benzyl]pyridine maleate (1:1). It is a sedating antihistamine which causes a moderate degree of sedation. Its mode of activity is antimuscarinic. The dextrorotatory isomer of chlorpheniramine, dexchlorpheniramine is twice active than chlorpheniramine. Chlorpheniramine maleate and dexchlorpheniramine maleate are used for the treatment of allergic conditions including conjunctivitis, rhinitis, angioedema, urticaria and pruritic skin disorders. Both these are also used in formulations for treatment of coughs and cold [3]. Aminophylline and chlorpheniramine maleate alone or with many other drugs have been estimated in pharmaceutical formulations, Chinese herbals and serum [4-6] by RP-HPLC (linear range for chlorpheniramine maleate: 0.5-50 μg/ml) [7,8], HPLC with fluorescence detector (linear range for chlorpheniramine maleate: 0-40 ng/ml) [9], FT Raman spectroscopy [10], spectrometry (linear range for aminophylline 1-69.5 μg/ml) [11,12], LC-MS/MS(linear range for chlorpheniramine maleate: 0.1-50 ng/ml) [13] and micellar electro kinetic chromatography (MEKC, linear range for chlorpheniramine maleate: 10-250 μg/ml) [14]. The pharmacopoeia (USP, EP, BP, JP) still have not been adopted the combine analysis of aminophylline and chlorphinramine maleate and in best of our knowledge RP-HPLC stability-indicating assay did not revealed for combination of both the drugs. Therefore in present work a stability indicating RP-HPLC assay was attempted and validated as per ICH [15] guidelines for simultaneous estimation of aminophylline and chlorphinramine maleate in pharmaceutical liquid dosage form.
Materials and Methods
Reference standards of aminophylline and chlorpheniramine maleate were obtained from Zhejiang Hongyuan, China. The syrup (32.0 mg of aminophylline and 5.0 mg of chlorpheniramine maleate in 5.0 ml as claimed) was obtained from CCL Pharmaceuticals, Lahore, Pakistan. Methanol (HPLC grade) and sulphuric acid (AR grade) were obtained from Falcon Scientific, Lahore, Pakistan (Merck-origin). Double distilled water was prepared in our own laboratory using Milli-Q system (Millipore, MA, USA). The mobile phase was filtered through 0.45 μm nylon filter (Sartorius-Germany).
The chromatography was performed using Shimadzu HPLC system (Kyoto, Japan) consisting of DGU-4A degasser, LC-20AD pump, a Rheodyne sample injection port with 20 μl loop, SPD20A UV/Vis detector and CTO-20A column oven. At room temperature (25±2º), on Mediterranea™ sea 18 (5μm, 4.6×250 mm) experimental conditions were optimized. Dilute sulphuric acid and methanol (pH 2.8) in ratio of 60:40 (% v/v) was used as mobile phase. Mobile phase flow rate was 1.5 ml/min and detection was performed at 264 nm. Shimadzu LC solution (version 1.227) software program was used for peak areas integration. Dilute sulphuric acid was prepared by taking 29.0 ml of concentrated sulphuric acid in 1.0 dm3 volumentric flask and diluted it up to the mark with distilled water.
Standard solution preparation
For stock standard solution, weighed accurately 128.0 mg of aminophylline and 20.0 mg of chlorpheniramine maleate and transferred in to a volumetric flask, dissolved in distilled water to a final volume of 100 ml. From this 10.0 ml was transferred to 100 ml volumetric flask and volume was made up by mobile phase to prepare working standard equivalent to 128.0 μg/ml of aminophylline and 20.0 μg/ml of chlorpheniramine maleate.
Sample solution preparation
Sample solution was prepared by dissolving 5.0 ml of syrup solution (equivalent to 32 mg of aminophylline and 5 mg of chlorpheniramine maleate) in volumtric flask and dissolved in distilled water to a final volume of 100 ml. From this 10.0 ml was transferred to 25 ml measuring flask and diluted it upto the mark with mobile phase to obtain working concentration of 128 μg/ml for aminophylline and 20 μg/ml for chlorpheniramine maleate.
Wavelength selection
When spectrum of both APIs of same concentration (0.002% in distilled water) was taken from spectrophotometer, aminophylline and chlorpheniramine showed maximum absorbance at wavelength of 269 nm and 264 nm, respectively. The sample and standard solutions were prepared which contain both these APIs of same concentration were shifted on HPLC (Shimadzu LC, 20-A SPD) constructed with spectrophotometer detector (SPD) having spectrum range from 210 nm to 320 nm. The SPD detector showed maximum absorbance at wavelength 264 nm. The wavelength on UV/Vis detector selected was 264 nm.
Linearity
The linearity of proposed method was determined over a concentration range of 102.4-153.6 μg/ml (102.4, 115.2, 128.0, 140.8, 153.6 μg/ml) for aminophylline and 16-24 μg/ml (16, 18, 20, 22, 24 μg/ml) for chlorpheniramine maleate which covers the 80-120% concentration of respective drugs in commercial syrups.
Accuracy
Standard addition method and analysis of synthetic mixture of aminophylline and chlorpheniramine maleate was used for accuracy determination in terms of recovery. Previously analyzed sample was fortified with known concentration of aminophylline and chlorpheniramine maleate (50, 100 and 150%) using standard addition method and compared the experimental and true values. Using synthetic mixture method excipients equivalent to 100 ml of syrup i.e. terpine hydrate (200.0 mg), potassium bicarbonate (2.0 mg), ammonium chloride (500.0 mg), menthol (20.0 mg), potassium guaiacol sulphate (100.0 mg), potassium citrate (2.0 mg), aminophylline (128.0 mg) and chlorphinramine maleate (20.0 mg) were transferred in measuring flask and dissolved in water to a final volume of 100 ml and sonicated it for 30 min. Three synthetic mixtures equivalent to 50, 100 and 150% of concentration of aminophylline (128.0 μg/ml) and chlorpheniramine maleate (20.0 μg/ml) were prepared and analyzed by developed method.
Precision
Intraday (repeatability) and interday (intermediate precision) were determined by injecting standard solutions (n=5) of three different concentrations on same day and three consecutive days, respectively.
Robustness
The stability of the developed method was checked by introducing small but deliberate changes in measuring parameters such as flow rate, mobile phase concentration, column temperature, wavelength and pH. Assay of the drug content was performed and noted the chromatographic parameters like tailing factor, retention time and theoretical plates which indicated characteristics robustness of the developed method.
Limit of detection and limit of quantitation
The limit of detection (LOD) is minimum amount of analyte in sample detectable and larger than uncertainty associated with it and the limit of quantitation (LOQ) is amount quantitatively measured with suitable precision and accuracy. LOD and LOQ were determined by standard deviation of the response based on the slope of the calibration by six injections of five working standards each of aminophylline and chlorpheniramine maleate under the optimized chromatographic conditions. LOD=yB+3sB and LOQ=yB+10sB, where yB is intercepts of regression line and sB is standard deviation of intercepts of regression line.
Specificity
Specificity of developed method was determined by chromatographic analysis of placebo only by dissolving the inactive ingredients same as that of sample preparation as well by applying different stress conditions (acid, base, oxidation, thermal and photolytic).
Acid degradation
Stock standard solution of 2.5 ml, and 2.5 ml each of 1.0 M and 5.0 M of HCl were taken into two 25 ml measuring flask and kept at 25º for 20 h and 40º for 1 h, respectively for acidic degradation. Then neutralized the stressed solutions with 1.0 M and 5.0 M NaOH, respectively and made the volume upto mark with mobile phase.
Base degradation
Basic degradation also was performed under two different conditions of temperatures. 2.5 ml of stock standard solution and 2.5 ml each of 1.0 M and 5.0 M of NaOH were taken into two 25 ml volumetric flask and kept at 25º for 20 h and 40º for 1 h, respectively. Then neutralize the stressed solutions with 1.0 M and 5.0 M HCl, respectively and made the volume up to mark with mobile phase.
Oxidative degradation
Environmental condition of 40º and 75% RH for 16 h were set in stability chamber to perform oxidative degradation. Stock standard solution (2.5 ml) was transferred in 25.0 ml of flask followed by addition of 2.5 ml of 10% H2O2; the solution was made upto mark with mobile phase after completion of oxidative degradation.
Thermal degradation
Thermal degradation was performed at 60o for 4 h. Stock standard solution (2.5 ml) was transferred in 25.0 ml of flask and kept into oven (Gallenkamp, UK) for dry heat thermolysis, after completion of thermal stress solution was made upto mark with mobile phase.
Photolytic degradation
Stock standard solution (2.5 ml) was transferred in 25.0 ml of flask and kept in direct sunlight for 8 h for photolytic degradation studies. The solution was completed upto mark with mobile phase.
Results and Discussion
In RP-HPLC separation of analytes containing nitrogen atom exposed to silanol group in silica based columns results in peak tailing. Also separation depends on the hydrophobicity of stationary phase so choice of column for reverse phase is important [16,17]. In this study, a simple and specific method for simultaneous determination of aminophylline and chlorpheniramine maleate and their degraded products is described. Various experiments were performed by changing the concentration of different mobile phases, pH of mobile phase and stationary phase selection to optimize the chromatographic conditions. Method optimization was started with four different mobile phases such as dilute sulphuric acid:methanol, dilute sulphuric acid:acetonitrile, methanol:0.5 M potassium dihydrogen phosphate buffer, methanol: 0.05 M ammonium acetate buffer with ratios (60:40, 60:40, 70:30, 30:70), respectively. These mobile phases were run on five different stationary phases (Purespher® RP- 18 endcapped, Hypersil ODS, Spheri-5 monofunctional C18, mediterranea™ sea 18, Venusil XBP C18) at different pH (2.8, 3.0, 3.5). The chromatographic parameters such as tailing factor, capacity factor, resolution and separation efficiency are effected by change of mobile phase and pH of mobile phase as well as stationary phases. Best separation efficiency was achieved using the dilute sulphuric acid:methanol (60:40) at pH 2.8 on Mediterranea™ sea 18 column. Also the tailing factor, capacity factor, resolution and theoretical plates were in compliance with standard requirements (ICH guidelines).
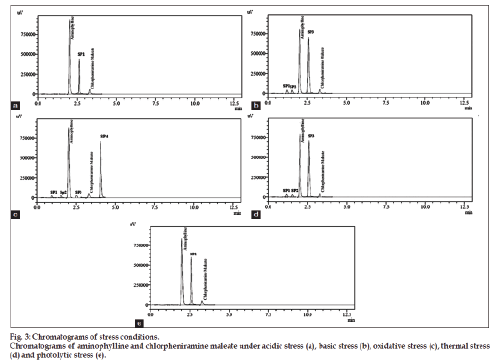
The optimized method was also validated as per validation parameters comprised of accuracy, linearity, precision, specificity, robustness, limit of detection and quantitation. Five different concentrations in range of 102.4-153.6 μg/ml (102.4, 115.2, 128.0, 140.8, 153.6 μg/ml) for aminophylline and 16-24 μg/ ml (16, 18, 20, 22, 24 μg/ml) for chlorpheniramine maleate were plotted for linearity studies. The linear regression equation was found to be y=29654x+18298 (R2=0.9996) for aminophylline and y=19748x+55219 (R2=0.9998) for chlorpheniramine maleate. Limit of detection and limit of quantitation of proposed method was determined by using the linear regression equation. LOD was found to be 1.6 and 0.25 μg/ml for aminophylline and chlorpheniramine maleate, respectively. While LOQ for aminophylline and chlorpheniramine maleate were found to be 3.3 and 0.62 μg/ml, respectively. Standard addition (known amount added to sample solution) and synthetic mixture (known amount added to placebo) techniques were used for accuracy determination at three levels of concentration (50, 100 and 150%). The results (n=5) are reported (Table 1) in terms of standard deviation and relative standard deviation. Recovery results indicated the accuracy of method and its suitability for its intended use. The results of intraday and interday precision are given in Table 2 by injecting standard solutions (n=5) of three different concentrations (80, 100 and 120% of analyte) on same day and three consecutive days. Percent RSD of peak area measurement represented the precision. The specificity of proposed method is justified by the chromatograms (fig. 2) of blank, placebo, standard and sample solutions under same chromatographic conditions. The placebos did not interfere in determination of aminophylline and chlorpheniramine maleate in commercial syrup. Specificity of the developed method was also evaluated by applying different stress conditions (oxidation, acid, base, thermal and photolytic) to aminophylline and chlorpheniramine maleate syrup. From the results of forced degradation studies showed that these components remained intact under stressed conditions. The specificity studies showed that the principle peaks were well resolved (peak purity 99.99%) and free from any interference from the degradation product. The stress conditions were applied and degraded products of both the drugs are compared. Under acidic degradation aminophylline was degraded upto 0.7% and chlorpheniramine maleate upto 2.31%. Under basic degradation aminophylline was degraded upto 7.4% and chlorpheniramine maleate upto 10.49%. Under oxidative stress aminophylline was degraded upto 5.86% and chlorpheniramine maleate upto 4.10%. Under thermal degradation aminophylline was degraded upto 7.39% and chlorpheniramine maleate upto 10.39%. Under photolytic stress aminophylline was degraded up to 5.44% and chlorpheniramine maleate upto 3.59%. From the stress studies it is concluded that substantial degradation of aminophylline and chlorpheniramine maleate was occurred basic, oxidative and thermal stress conditions while the slight degradation of both the drugs observed under acidic and photolytic stress conditions (chlorpheniramine maleate only). The results of stress conditions are presented in Table 3. The degradation products (impurities) in addition to percent degradation under acid, base, oxidation, thermal and photolytic stresses have unique retention times (RT) to acidic stress (one impurity, RT: 2.54 min impurity peak SP1), basic stress (three impurities, RT: 1.25 min, 1.53 min, at 2.52 min major impurity peak SP3), oxidative stress (four impurities, RT: 1.15 min, 1.51 min, 2.56 min, at 4.23 min major impurity peak SP4), thermal stress (three impurities, RT: 1.41 min, 1.53 min, at 2.52 min major impurity peak SP3) and photolytic stress (one impurity, RT: 2.50 min impurity peak SP1). Chromatograms under basic and thermal stress are presented in fig. 3. Degradation studies justified the method specificity for its intended application. Slight changes in chromatographic parameters were done for robustness of proposed method and the results (assay, retention time, theoretical plates, resolution and tailing factor) are affected negligibly. This showed the robustness of proposed method for its intended use. The results of robustness data are presented in Table 4. The proposed method was applied for analysis of both the drugs in commercial syrup, high recovery and low % RSD were obtained, and results are reported in Table 5.
| Drugs | Spiked concentration (µg/ml) | Standard addition (%) | Synthetic mixture (%) | ||
|---|---|---|---|---|---|
| Concentration found (µg/ml) ± SD; RSD | Recovery | Concentration found (µg/ml) ± SD; RSD | Recovery | ||
| Aminophylline | 64 | 63.55 ± 0.67; 0.30 | 99.3 | 64.05 ± 0.13; 0.31 | 100.1 |
| 128 | 128.51 ± 0.43; 0.96 | 100.4 | 128.27 ± 0.53; 0.61 | 100.2 | |
| 192 | 192.38 ± 0.25; 0.46 | 100.2 | 192.11 ± 0.09; 0.33 | 100.1 | |
| Chlorpheniramine maleate | 10 | 10.02 ± 0.11; 0.08 | 100.2 | 10.11 ± 0.42; 0.15 | 101.1 |
| 20 | 19.96 ± 0.28; 0.26 | 99.8 | 20.15 ± 0.49; 0.11 | 100.8 | |
| 30 | 30.27 ± 0.38; 0.29 | 100.9 | 30.31 ± 0.45; 0.29 | 101.0 | |
Table 1: Accuracy Studies
| Drugs | Concentration (µg/ml) | Concentration found (µg/ml) ± SD; RSD | |||
|---|---|---|---|---|---|
| Intraday precision | Interday precision | ||||
| Day 1 | Day 2 | Day 3 | |||
| Aminophylline | 102.4 | 102.5 ± 0.43; 0.22 | 102.54 ± 0.34; 0.78 | 103.63 ± 0.90; 1.82 | 103.71 ± 0.72; 0.32 |
| 128 | 128.53 ± 0.83; 0.41 | 128.64 ± 0.45; 0.92 | 129.74 ± 0.87; 1.03 | 129.48 ± 0.48; 0.76 | |
| 153.6 | 153.4 ± 0.23; 0.60 | 153.89 ± 0.37; 1.22 | 154.44 ± 0.48; 0.91 | 154.32 ± 0.19; 0.42 | |
| Chlorpheniramine maleate | 16 | 16.12 ± 0.33; 0.68 | 15.96 ± 0.13; 0.94 | 16.08 ± 0.32; 0.27 | 16.24 ± 0.24; 1.14 |
| 20 | 20.3 ± 0.34; 1.45 | 19.91 ± 0.12; 0.52 | 20.18 ± 0.31; 0.53 | 20.22 ± 0.62; 0.12 | |
| 24 | 23.92 ± 0.93; 0.17 | 24.15 ± 0.84; 0.34 | 24.35 ± 0.76; 0.33 | 24.41 ± 0.28; 0.49 | |
Table 2: Intra-Day And Inter-Day Precision
| Nature of stress | Storage condition | Time (h) | Amount remaining (mean ± SD) | Extent of degradation | ||
|---|---|---|---|---|---|---|
| Aminophylline | Chlorpheniramine maleate | Aminophylline | Chlorpheniramine maleate | |||
| 1 M HCl | 25° | 20 | 99.30 ± 1.21 | 97.69 ± 1.43 | None | None |
| 5 M HCl | 40° | 1 | 99.44 ± 1.17 | 97.87 ± 1.21 | None | None |
| 1 M NaOH | 25° | 20 | 92.60 ± 1.31 | 89.51 ± 1.86 | Substantial | Substantial |
| 5 M NaOH | 40° | 1 | 92.53 ± 1.42 | 89.64 ± 1.79 | Substantial | Substantial |
| 10% H2O2 | 40°, 75% RH | 16 | 94.14 ± 1.09 | 95.90 ± 1.27 | Slight | Slight |
| Thermal | 60° | 4 | 92.61 ± 1.98 | 89.61 ± 1.54 | Substantial | Substantial |
| Photolytic | Sunlight | 8 | 94.56 ± 1.85 | 96.41 ± 1.12 | Slight | Slight |
Table 3: Stress Testing Results Of Aminophylline And Chlorpheniramine Maleate
| Chromatographic conditions | Aminophylline | Chlorpheniramine maleate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Assay (%) | tR (min) | n | TF | Assay (%) | tR (min) | n | TF | Rs | |
| Flow rate: 1.6 | 101.1 | 1.98 | 21,540 | 1.45 | 101.7 | 3.15 | 27,065 | 1.53 | 8.47 |
| Flow rate: 1.4 | 100.4 | 2.06 | 21,351 | 1.49 | 100.3 | 3.30 | 27,233 | 1.58 | 8.41 |
| Mobile phase (65:35) | 100.5 | 2.0 | 21,040 | 1.53 | 99.7 | 3.25 | 27,865 | 1.59 | 8.47 |
| Mobile phase (65:35) | 99.8 | 2.0 | 21,259 | 1.58 | 99.3 | 3.25 | 27,033 | 1.53 | 8.41 |
| Column temperature (20°) | 99.87 | 2.03 | 21,390 | 1.47 | 101.0 | 3.28 | 27,444 | 1.56 | 8.39 |
| Column temperature (30°) | 100.2 | 2.02 | 21,287 | 1.46 | 100.7 | 3.21 | 27,344 | 1.58 | 8.58 |
| Wavelength (262 nm) | 100.2 | 2.03 | 21,289 | 1.49 | 100.8 | 3.25 | 27,109 | 1.54 | 8.39 |
| Wavelength (266 nm) | 100.4 | 2.01 | 21,401 | 1.46 | 100.1 | 3.25 | 27,009 | 1.55 | 8.58 |
| pH: 2.7 | 99.9 | 2.01 | 21,540 | 1.46 | 100.9 | 3.27 | 27,165 | 1.54 | 8.39 |
| pH: 2.9 | 99.8 | 2.0 | 21,387 | 1.43 | 100.2 | 3.28 | 27,098 | 1.55 | 8.58 |
Table 4: Robustness Study Of Aminophylline And Chlorpheniramine Maleate
| Product | Ingredients | Label claim (mg per 5 ml) | Found (mg) | Recovery | |
|---|---|---|---|---|---|
| (mean ± SD); | %RSD | ||||
| Syrup | Aminophylline | 128 | 128.03 | 100.02 ± 0.07; | 0.65 |
| Chlorpheniramine maleate | 20 | 20.1 | 100.5 ± 0.09; | 0.94 | |
Table 5: Assay result of aminophylline and Chlorpheniramine maleate in commercial Syrup
A reverse phase HPLC method was developed for the simultaneous determination of aminophylline and chlorpheniramine maleate in pharmaceutical formulations. The developed method has proved to be simple, accurate and reproducible. The verification of the developed HPLC method was done by validation parameters. The results of validation indicated good precision, accuracy, linearity and reliability. The developed reverse phase HPLC method has many advantages in terms of simplicity of mobile phase, isocratic mode of elution, short run time, good resolution, less expensive chemicals and simple method of sample and standard solutions preparation. The method has efficiently separated the peaks of aminophylline and chlorpheniramine maleate from the degradation products during stress conditions. The method accurately determined the amounts of both API in the presence of impurities and excipients. The aminophylline and chlorpheniramine maleate drug combination studied, still not reported in any official pharmacopeia. The study convinced to conclude that the developed HPLC method can be successfully used as routine analysis for the determination of the aminophylline and chlorpheniramine maleate in pharmaceutical formulations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Mader TJ, Smithline HA, Durkin L, Scriver G. A randomized controlled trial of intravenous aminophylline for atropine-resistant out-of-hospital asystolic cardiac arrest. Acad Emerg Med 2003;10:192-7.
- Jasmeet S. Aminophylline in bradyasystolic cardiac arrest. Emerg Med J 2007;24:582-3.
- Carlsson A, Lindqvist M. Central and peripheral monoaminergic membrane-pump blockade by some addictive analgesics and antihistamines. J Pharm Pharmacol 1969;21:460-4.
- Yamaguchi M, Monji H, Yamashita K, Aoki I, Yashiki T. Sensitive high-performance liquid chromatographic determination of chlorpheniramine in human serum using column switching. J Chromatogr B Biomed Appl 1994;661:168-72.
- Redasani VK, Gorle AP, Badhan RA, Jain PS, Surana SJ. Simultaneous determination of chlorpheniramine maleate, phenylephrine hydrochloride, paracetamol and caffeine in pharmaceutical preparation by RP-HPLC. Chem Ind Chem Eng Q 2013;19:57-65.
- Tong S, Yang Y, Ding L, Long-Feng XU, Ximing XU, Jiang-Nan YU. HPLC simultaneous determination of theophylline and chlorpheniramine maleate in Shunqi huatan tablets and granules. Chin J Pharm Anal 2012;32:583-6.
- Marín A, García E, García A, Barbas C. Validation of a HPLC quantification of acetaminophen, phenylephrine and chlorpheniramine in pharmaceutical formulations: Capsules and sachets. J Pharm Biomed Anal 2002;29:701-14.
- Sanchaniya PM, Mehta FA, Uchadadiya NB. Development and validation of an RP-HPLC method for estimation of chlorpheniramine maleate, ibuprofen, and phenylephrine hydrochloride in combined pharmaceutical dosage form. Chromatogr Res Int 2013;2013:6.
- Miyamoto Y. Highly sensitive determination of chlorpheniramine as fluorescence derivative by high-performance liquid chromatography. J Chromatogr 1987;420:63-72.
- Mazurek S, Szostak R. Quantitative determination of diclofenac sodium and aminophylline in injection solutions by FT-Raman spectroscopy. J Pharm Biomed Anal 2006;40:1235-42.
- Li Q, Zhang H. A novel spectrophotometric method for the determination of aminophylline in pharmaceutical samples in the presence of methanol. Spectrochim Acta A Mol Biomol Spectrosc 2008;70:284-9.
- El-Shabouri SR, Hussein SA, Emara SE. Colorimetric determination of theophylline and aminophylline with diazotized p-nitroaniline. Talanta 1989;36:1288-90.
- Lou HG, Yuan H, Ruan ZR, Jiang B. Simultaneous determination of paracetamol, pseudoephedrine, dextrophan and chlorpheniramine in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2010;878:682-8.
- Suntornsuk L, Pipitharome O, Wilairat P. Simultaneous determination of paracetamol and chlorpheniramine maleate by micellar electrokinetic chromatography. J Pharm Biomed Anal 2003;33:441-9.
- ICH (Q2B): Note for Guidance on Validation of Analytical Procedures: Methodology. Geneva: International Conference on Harmonization, IFPMA; 1996.
- Razzaq SN, Ashfaq M, Khan IU, Mariam I. Stability-indicating HPLC method for the simultaneous determination of ofloxacin and ketorolac tromethamine in pharmaceutical formulations. Anal Methods 2012;4:2121-6.
- Razzaq SN, Khan IU, Mariam I, Razzaq SS. Stability indicating HPLC method for the simultaneous determination of moxifloxacin and prednisolone in pharmaceutical formulations. Chem Cent J 2012;6:94.