- Corresponding Author:
- P. Pattanayak
Jeypore College of Pharmacy, Jeypore - 764 002
E-mail: priyabrata2005@gmail.com
| Date of Submission | 25 March 2010 |
| Date of Revision | 15 September 2010 |
| Date of Acceptance | 10 January 2011 |
| Indian J Pharm Sci, 2011, 73 (1): 65-70 |
Abstract
Quality assurance of herbal products may be ensured by proper quality control of the herbal ingredients and by means of good manufacturing practice. We have developed a simple scheme for the standardization and authentication of Sulaharan Yoga a poly herbal formulation. Sulaharan Yoga was prepared as per Ayurvedic Formulary of India. Inhouse and marketed preparation has been standardized on the basis of organoleptic characters, physical characteristics and physico-chemical properties. The set parameters were found to be sufficient to standardize the Sulaharan Yoga and can be used as reference standards for the quality control/ quality assurance study.
Keywords
Organoleptic characters, physicochemical parameters, standardization, traditional medicine
Sulaharan Yoga (SY-1), an Ayurvedic polyherbal formulation, consists of Strychnos nux-vomica and other seven ingredients in Vati (Tablet) form. It is one of the most widely used Ayurvedic product indicated for pain/colic, malabsorption syndrome, diarrhea, abdominal lump and digestive impairment. The World Health Organization (WHO) has appreciated the importance of medicinal plants for public health care in developing nations and has evolved guidelines to support the member states in their efforts to formulate national policies on traditional medicine and to study their potential usefulness including evaluation, safety and efficacy [1,2].
The need of quality control for Ayurvedic drug is due to the fact that the preparation of drug according to the ancient method has been reduced due to the commercialization of Ayurvedic pharmacy during past era [3]. The present paper reports on the standardization of Sulaharan Yoga based on organoleptic characters, physical characteristics; physico-chemical properties and HPTLC fingerprint study.
All the chemicals used in the experiment were of analytical grade. Gallic acid, gingerol, piperine, brucine, strychine, umbelliferone and scopoletin were purchased from Sigma Aldrich, USA. All the solvents used in the experiment were procured from Merck Specialties Pvt. Ltd, Mumbai, India.
Sulaharan Yoga consists of 1. Terminalia chebula (T, Combretaceae, dried fruit), 2. Zingiber officinale (Z, Zingiberaceae, dried rhizome), 3. Piper nigrum (PN, Piperaceae, dried fruit), 4. Piper longum (PL, Piperaceae, dried fruit), 5. Strychnos nux-vomica (S, Fabaceae, dried seed), 6. Ferula foetida (F, oleo-gumresin), 7. sulphur and 8. rock salt (Saindhava lavana). All these ingredients were procured from the local market of Jeypore, Koraput, Odisha, India.
In-house formulation of Sulaharan Yoga was prepared as per Ayurvedic Formulary of India. The ingredients number 1 to 4 were washed, dried and powdered and passed through 80# sieve. The ingredients number 5, 6, 7 and 8 were cleaned and powdered individually and passed through 80# sieve. All the ingredients were mixed thoroughly in specified ratio (1 parts each) to obtain a homogeneous blend. The blended mass was expelled through tablet punch machine fitted with suitable die. The rolled vatis (Tablets) were dried in a tray-dryer at a temperature not exceeding 60º. It was packed in a tightly closed glass containers for further use. One marketed sample of Sulaharna Yoga (Sarmayu, SY-2) were chosen for evaluation. Sarmayu and the in-house preparation were standardized based on their oganoleptic characters, physical characteristics and physicochemical properties.
The organoleptic characters of the samples were carried out based on the method described by Siddique et al. [4] Microscopic analysis of in-house formulation and the marketed formulation were carried out by classical pharmacognostical methods [5]. The authenticity of the individual ingredients was confirmed by comparison of the power characteristics with those given in the literature.
To determine the total ash value 2 g of powdered material of each formulation were placed separately in a suitable tared crucible [6] of silica previously ignited and weighed. The powdered drugs were spread into an even layer and weighed accurately. The materials were incinerated by gradually increasing the heat, not exceeding 450° until free from carbon, cooled in a desiccator, weighed and percentage ash was calculated by taking in account the difference of empty weight of crucible and that of crucible with total ash. The ash obtained as above was boiled for 5 min with 25 ml of dilute hydrochloric acid; the insoluble matter was collected on an ash less filter paper, washed with hot water and ignited to constant weight. The percentage of acid-insoluble ash with reference to the air-dried drug was calculated.
Five grams each of the two formulations were macerated with 100 ml of alcohol [7] in a closed flask for 24 h, shaking frequently during 6 h and allowed to stand for 18 h. It was then filtered rapidly; taking precautions against loss of solvent. Twenty-five milliliters of the each filtrate was evaporated to dryness in a tared flat-bottomed shallow dish at 105° to constant weight and weighed. The percentage of alcohol-soluble extractive was calculated with reference to the air-dried drug and is represented as % value. Five grams each of the two formulations were macerated with 100 ml of chloroform water and the percentage of water-soluble extractive was calculated as above. About 10 g of dug samples of each formulation was accurately weighed in a dried and tared flat weighing bottle and dried at 105º for 5 h. Percentage loss on drying was calculated with reference to initial weight.
pH of different formulations in 10% w/v of water soluble portions was determined using standard glass electrode at 240 according to the prescribed standard method in Indian Pharmacopoeia. Thickness of tablets was measured using sliding calipers. Hardness indicates the tensile strength of a tablet. It was measured using a Monsanto hardness tester. Twenty tablets were taken in a Roche Friabilator rotating at a speed of 100 rpm for 10 min and reweighed after the treatment. Friability was expressed as % loss in weight. Twenty tablets were weighed individually and collectively. Average weight per tablet was calculated from the collective weight. Then the weights of individual tablets were compared with the average weight to determine weight variation.
Disintegration test was performed in a basket rack assembly supporting six glass tubes of 3 inches long, open at the top and held against a 10-mesh screen at the bottom end of the basket rack assembly. One tablet was placed in each tube and the assembly was moved up and down at a frequency of 28 to 30 cycles per min at a temperature of 38º. For fluorescence test, 1 mg of powdered drugs of each formulation were exposed to ultraviolet light at wavelength of 366 nm and daylight [5] while wet, after being treated with different reagents.
Bulk density [7,8] was determined using the formula Db= M/Vb,,where M is the mass of the particles and Vb is the total volume of the packing. Vb was determined by placing 100 g of the powdered formulation in a Jolting volumeter, the initial volume was noted and the sample was then tapped until no further reduction in volume was noted. The initial volume gave the bulk density value and the reduced volume after tapping gave the tapped density. For determining angle of repose, the fixed funnel and the free standing cone method was employed. Powder or granules was carefully poured through the funnel until the apex of the conical pile just touched the tip of the funnel placed 2.5 cm above the surface. Tan a= H/R or a= arc tan H/R, where a is the angle of repose, R being the radius of the conical pile.
Hausner ratio is related to interparticle friction and as such can be used to predict the powder flow properties. The formula for measuring the Hausner ratio used was Df/Do, where Df= tapped density and Do= bulk density. Another indirect method of measuring the powder flow from bulk density is Carr’s index and it was determined using the formula, % compressibility= (Df-Do/Do)×100, where DF= tapped density and Do= bulk density.
Sodium content [9,10] was estimated by flame photometry. NaCl content of the in-house and marketed formulation was determined by titrating a solution prepared by dissolving 2-3 g in 25 ml of purified water against standard silver nitrate solution (0.1N) using potassium chromate as indicator. Each ml of 0.1 N AgNo3 solutions is equivalent to 0.005845 g of NaCl. For determing sulfur, 0.5-1 g powdered sample was mixed with 10 ml of carbon tetrachloride saturated with bromine in a 250 ml beaker, the mixture was kept cold and left over night in a fume chamber. The mixture was then digested on a water bath with 10-15 ml of concentrated nitric acid. It was further digested with 10 ml concentrated hydrochloric acid till a syrupy mass formed. The mass was extracted with 100 ml hydrochloric acid, boiled and filtered through Whatman No. 40 filter paper. The residue was washed with hot water, filtered through Whatman No. 41 paper in to a 600 ml beaker and the filtrate was acidified with hydrochloric acid, 20 ml of 10 per cent barium chloride solution was added stirred and digested on a burner. The BaSO4 precipitate was then filtered through a Whatman No. 42 filter paper, washed with water, ignited at 8500 in a muffle furnace in a pre weighed platinum crucible, cooled and weighed. Each g of the precipitate is equivalent to 0.13734 g of sulphur.
HPTLC study [11,12] of methanol extracts of the individual ingredients, in-house formulation and marketed formulation were carried out along with different marker compounds corresponding to the active ingredients to ensure the presence of active ingredients in all the formulations. For HPTLC, 2 g of each sample were extracted with 25 ml of methanol on a boiling water bath for 25 min consecutively three times using fresh portion of 25 ml methanol, filtered and concentrated. The chromatograph was performed by spotting standards and extracted samples on pre coated silica gel aluminum plate 60F-254 (10×10 cm with 250 μm thickness) using Camag Linomat IV sample applicator and 100 μl Hamilton syringe. The samples, in the form of bands of length 5 mm, were spotted 15 mm from the bottom, 10 mm apart, at a constant application rate of 15 nl/s using nitrogen aspirator. Subsequent to the development, TLC plates were dried in a current of air with the help of an air-dryer. Densitometric scanning was performed on Camag TLC scanner III in the absorbance/reflectance mode.
The protocol employed to develop pharmacoepial standards are mostly based on triple P (pharmacognostical, physicochemical and phytochemical screening). As part of standardization procedure, the finished in-house product Sulaharan Yoga (SY-1) was tested for relevant physical and chemical parameters along with the marketed formulation (SY-2). All the samples were grayish yellow in color, smooth with fine tablets, having characteristic of asafoetida odour, possessing pungent taste (Table 1).
| Parameter (s) | In-house formulation (SY-1) | Marketed sample (SY-2) |
|---|---|---|
| Color | Grayish yellow | Grayish yellow |
| Odor | Asafoetida odor | Asafoetida odor |
| Taste | Pungent | Pungent |
| Diameter (mm) | 12±0.02 | 11.8±0.35 |
| Thickness (mm) | 3.2±0.05 | 2.9±0.020 |
| Hardness (kg/cm2) | 4.7±0.02 | 4±0.01 |
| Friability (%) | 1.6±0.12 | 1.2±0.32 |
| Weight variation (%) | 5.82±2.89 | 5±1.45 |
| Disintegration time (min) | 11 | 7 |
| Loss on drying (%) | <6 | <4 |
| Total ash (%) | <16 | <16 |
| Acid insoluble ash (%) | <2 | <2 |
| Alcohol soluble extractive | <16 | <14 |
| Water soluble extractive | <39 | <35 |
| pH | 6-8 | 6-8 |
| Sodium content (%) | 30 | 39 |
| Chloride content (%) | 11 | 12 |
| Sulphur content (%) | 56 | 61 |
Table 1: Physicochemical Analysis Of Sulaharana Yoga Samples
Microscopic examinations were carried out to see the presence of T. chebula, P. nigrum, Z. officinale, S. nux-vomica and P. longum in both the samples of Sulaharana yoga. Spherical pitted stone cells, criss cross fibers; elongated pitted sclereids and fibers with peg like out growth indicated the presence of T. chebula. Endocarp cells and stone cells intercepted with parenchyma cells indicated the presence of P. nigrum. Perisperm cells containing compacted masses of starch grains indicated the presence of P. longum. Vessels and vessels with parenchyma cells indicated the presence of Z. officinale. fragments of trichome rods and outer layer of endocarp indicated the presence of S. nux-vomica.
Variations were observed in most of the physicochemical parameters studied. The diameter of SY-1 was found to be higher than that of SY-2. Similarly thickness, hardness, friability, weight variation, disintegration time (Table 1) were also relatively higher in the case of SY-1. When subjected to assay, the sulphur, sodium and chloride contents of SY-1 sample were found to be 11, 30 and 56%, respectively, whereas, those of SY-2 sample were 12, 39 and 61%. The sodium percentage for SY-2 was much higher than other (39%). While the chloride percentage was also higher in SY-2 sample (61%).
Quality tests (Table 1) for different Sulaharan Yoga were performed for moisture content, water soluble extractive, methanol soluble extractive, ash content, and acid insoluble ash, and were found to be within standard ranges [13]. Loss on drying at 105º, alcohol soluble extractive, water soluble extractive values of SY-1were found to be much higher when compared to SY-2. However total ash, acid insoluble ash and pH were similar to that for both the samples. In fluorescence analysis the power samples were exposed to day light and ultraviolet light at wavelength of 366 nm after being treated with different reagents as reported in Table 2. Fluorescence analysis results indicated no fluorescent material in any of formulation.
| Materials | In house preparation | Market formulation | ||
|---|---|---|---|---|
| Day light | UV 366nm | Day light | UV 366nm | |
| Powder as such | Grayish yellow | Blackish gray | Grayish yellow | Blackish gray |
| P+ Conc. H2SO4 | Pale purple | Pale purple | Pale purple | Pale purple |
| P+ In NaOH (1N) in H2O | Pale parrot green | Black | Pale parrot green | Black |
| P + In HCl (1N) | Parrot green | Brown | Parrot green | Brown |
| P + In NaOH (1N) in MeOH | Gray | Black | Gray | Black |
Table 2: Powder Fluorescence Test Of Different Sulaharan Yoga Formulations
Physical characteristics of the in-house formulation and marketed formulation are shown in Table 3. Results of the marketed formulation and in-house formulation were found to be comparable. Flowability of the formulation was found to be good in both marketed and in-house formulation, which was further confirmed by high values of Hausner ratio and Carr’s index.
| Formulations | Tap density* | Bulk density | Angle of repose | Hausner ratio | Carr's Index |
|---|---|---|---|---|---|
| In-house | 0.54±0.23 | 0.4±0.11 | 32±0.41 | 1.22±0.02 | 12.3±0.14 |
| Marketed | 0.44±0.16 | 0.32±0.24 | 40±0.28 | 1.4±0.12 | 13±0.31 |
*Measurement is the average of three readings
Table 3: Physical Characteristic Of Different Sulaharan Yoga Formulations
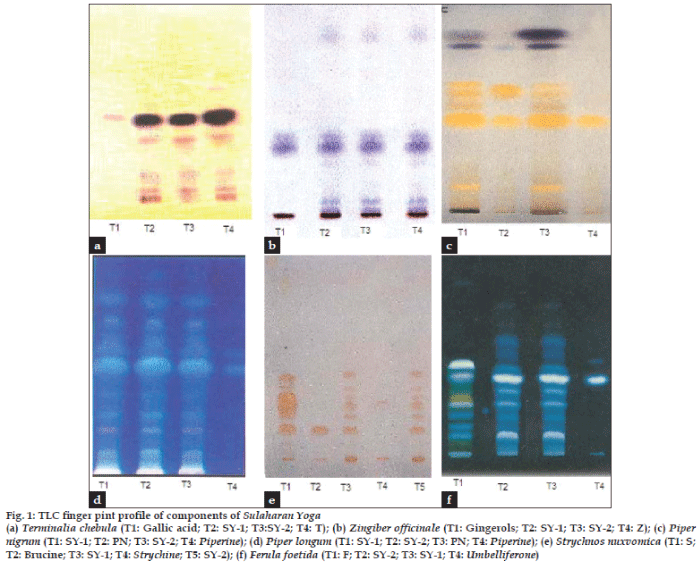
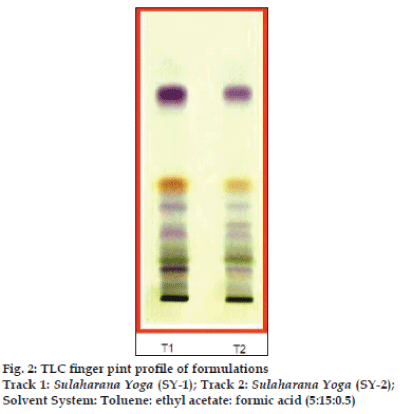
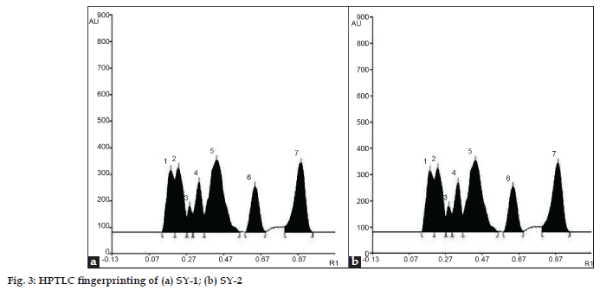
TLC profile of the maker compounds corresponding to the individual ingredients of Sulaharana yoga was presented in fig. 1a-e, to authenticate the presence of these ingredients in all the samples of Sulaharana yoga. A band (Rf 0.44) corresponding to gallic acid (fig.1a) is visible in both T. chebula (T) and Sulaharana yoga formulations, indicate the presence of T. chebula in the fomulations. Band (Rf 0.35) corresponding to gingerols (blue spot) is visible in both the ingredient and formulations, indicate the presence of Z. officinale (Z) (fig.1b). Similarly bands with Rf values 0.50 corresponding to piperine indicates the presence of both P. nigrum (PN) (fig.1c) and P. longum (PL) (fig.1d) in the formulations. Bands (Rf 0.15 and 0.33) corresponding to brucine and strychine is visible in both the ingredient and formulations indicate the presence of S. nux-vomica (S) (fig.1e). A band (Rf 0.40) corresponding to umbelliferone (fig.1f) is visible in both the ingredient and formulations, indicate the presence of F. foetida (F) in the formulations. The TLC profile of both the formulations indicates the uniformity in the preparation of in-house and marketed formulation (fig. 2). HPTLC fingerprint profile of the Sulaharan Yoga formulations are depicted in figs. 3a and 3b indicates the presence of all the ingredients in proportional quantity in the formulations without any impurity. This confirms the brand-to-brand consistency of the finished products.
Fig. 1: TLC finger pint profile of components of Sulaharan Yoga
(a) Terminalia chebula (T1: Gallic acid; T2: SY-1; T3:SY-2; T4: T); (b) Zingiber officinale (T1: Gingerols; T2: SY-1; T3: SY-2; T4: Z); (c) Piper
nigrum (T1: SY-1; T2: PN; T3: SY-2; T4: Piperine); (d) Piper longum (T1: SY-1; T2: SY-2; T3: PN; T4: Piperine); (e) Strychnos nuxvomica (T1: S;
T2: Brucine; T3: SY-1; T4: Strychine; T5: SY-2); (f) Ferula foetida (T1: F; T2: SY-2; T3: SY-1; T4: Umbelliferone)
In conclusion, the pharmacognostic study, physicochemical constant, preliminary phytochemical studies and TLC, HPTLC fingerprint profiles have been useful for deciding the identity, purity and strength of the polyherbal formulation and also for fixing standards for this Ayurvedic formulation. Thus the present study will certainly help to build a monograph of the Ayurvedic formulation in the Indian Formulary.
Acknowledgements
Authors gratefully acknowledge Mr. S. R. Dash, H.O.D., Dept of Botany, Vikram Dev College Jeypore, Koraput, Odisa for plant material authentification.
References
- Govind D. BhaishajyaRatnavali, New Delhi: MotilalBanarasidas Publishers; 2002. p. 461.
- Asokar LV, Kakkar KK, Chakra OJ. Glossary of Indian medicinal plants with active pinciples. New Delhi: Publication and Information Directorate; 1992. p. 122.
- Anonymous. The Ayurvedic Formulary of India, New Delhi: Govt. of India, Ministry of Health and Family Welfare; 1976.
- Siddiqui A, Hakim MA. Format for the pharmacopoeial analytical standards of compound formulation, wokshop on standardization of unani drugs, (appendix), New Delhi: Central council for research in unani medicine; 1995. p. 25.
- Anonymous. Pharmacopeial standards for Aurvedic formulations, Central council for research in Ayurveda and Siddha, New Delhi: Govt. of India, Ministry of Health and Family Welfare; 1987. p. 85.
- Mukerjee PK. Quality control of herbal drugs. Mumbai: Business Horizons Pharmaceutical Publisher; 2002. p. 192.
- Lachman L, Liberman HA, Kanig JL. The theory and practice of industrial Pharmacy, Mumbai: Varghese Publishing House; 1987.
- Aulton ME. Phamaceutics, The science of dosage forms design. New Delhi: Churchill Livingstone; 2002. p. 205.
- Skoog AD, West DM, Holler FJ. Fundamentals of analytical chemistry. New York: Saunders College Publishing; 1991. p. 613.
- Mendham J, Denney RC, Barnes JD, Thomas M. Vogel?s text book of quantitative chemical analysis. Singapore: Pearson Education Pvt. Ltd; 2002. p. 605.
- Anonymous. Quality standards of Indian medicinal plants. Vol. 1. New Delhi: Indian Council of Medical Research; 2003. p. 10.
- Sante. Organization Mondiale De La., Quality control methods for medicinal plant mateials, World Health Organization, 559, Rev. 1, Original English, 1992. p. 159.
- Bose A, Mondal S, Gupta JK, Dash GK, Ghosh T, Si S. Studies on Diuretic and Laxative activity of ethanolic extract and its fractions of Cleome rutidospermaaerial parts. Phcog Mag 2006;2:178-82.