- *Corresponding Author:
- V. F. Patel
Shri B. M. Shah College of Pharmaceutical Education and Research, Modasa-383 315, India.
E-mail: mviral27@yahoo.co.in
| Date of Submission | 14 November 2005 |
| Date of Revision | 14 June 2006 |
| Date of Acceptance | 25 January 2007 |
| Indian J Pharm Sci,2007, 69 (1): 51-57 |
Abstract
Present investigation describes statistically the influence of viscosity of hydroxypropyl methylcellulose and types of filler on dipyridamole release from floating matrix tablets using 3 2 full factorial design. Tablets were prepared using direct compression technique. Tablets were evaluated for in vitro floating ability and drug release study using USP 24 types II paddle apparatus using 0.1 N HCl (pH 1.2) at rotation of 100 rpm and temperature of 37±0.5°. Multiple regression analysis was performed for dependent variables studied and to evaluate contribution of factors with their levels two way ANOVA was performed followed by Tukey test. Polynomial equations and response surface plots were generated for all dependent variables. It was observed that both the factors had significant influence on all dependent variable studied (p<0.05). It was observed that as viscosity of polymer increases the release rate constant was decreased. Release rate obtained was highest when microcrystalline cellulose was employed as filler followed by dicalcium phosphate and lactose. Mechanism of drug release was anomalous types and depends upon viscosity of polymer and types of filler used. Microcrystalline cellulose gave release mechanism nearer to diffusion mechanism while dicalcium phosphate and lactose gave anomalous release. It was observed that both the factors had significant contribution on all dependent variables studied. A major controlling factor for kinetics of drug release was viscosity of polymer and it can be modified by incorporation of different types of filler. In initial phase of drug release viscosity of polymer and types of filler both governs the drug release while in later phase only the viscosity of polymer predominates.
Introduction
The cost involved both in terms of money and time in the development of a single new molecule has made it mandatory for pharmaceutical companies to reconsider their research focus. In an attempt to reduce the cost of drug development process and advantageously reap the benefits of patent regime, companies are now investing strategically in the development of new drug delivery systems [1]. Controlling the residence time of a drug delivery system in a particular region of gastrointestinal (GI) tract can be achieved via several approaches: intragastric floating system, high density system, mucoadhesive system, unfolding, extendable or expandable systems and superporous hydrogels [2]. From the technological point of view floating drug delivery system is a more convenient and logical approach to prolong gastric residence time.
Dipyridamole is poorly soluble weak base with pKa of 6.4. The solubility of dipyridamole has been reported to be altered to a considerable extent by the pH of different digestive fluid, i.e., dipyridamole dissolves readily in the stomach but incompletely in intestine [3] Miyazaki and co-workers [4] reported that the extent of absorption of dipyridamole is remarkably lower when gastric pH was continuously elevated to 6.0, whereas it was increased when gastric pH temporarily decreased to 1.8, which might be due to contribution of precipitation potential of drug when pH change from acidic to neutral region [5]. Due to the above reported facts about dipyridamole bioavailability, it would be beneficial to develop floating drug delivery system which prolongs the gastric residence time and releases drug in proximal GI tract where absorption of dipyridamole is more confined.
Nonionic cellulose ethers and most frequently hydroxypropyl methylcellulose (HPMC) have been widely utilized for their application in oral sustained drug delivery system. When in contact with aqueous fluid, HPMC hydrates rapidly and forms a gelatinous barrier layer around the tablet. The rate of drug release from HPMC matrix is dependent on various factors such as grade of polymer, solubility of drug, polymer content, particle size of drug and polymer as well as types and amount of filler used in the formulation [5]. The adjustment of polymer concentration, viscosity grade and addition of different types and levels of excipients to the HPMC matrix can modify the kinetics of drug release [6]. Hence in the present investigation, viscosity of HPMC and types of filler [lactose/microcrystalline cellulose (MCC)/dicalcium phosphate (DCP)] were used to evaluate statistically the influence of these parameters on kinetics of dipyridamole release from floating matrix tablet using 32 full factorial design.
Materials and Methods
Dipyridamole was received as a gift sample from Sun Pharmaceutical Ltd., Vadodara (India). Methocel K4M CR (4000 mPa.s), Methocel K15M CR (15 000 mPa.s) and Methocel K100M CR (100 000 mPa.s) were received as a gift samples from Colorcon Asia Pvt. Ltd., Goa (India). Tablet tose 80 (directly compressible lactose) was received as a gift sample from Meggle GMBH, Wasserberg (Germany). Avicel PH 102 (MCC) and dicalcium phosphate were obtained as a gift sample from Zydus Cadila Research Centre, Ahmedabad (India). All other ingredients were procured from Lesar chemicals, Vadodara (India) and were of analytical grade.
A 32 full factorial design:
Two factors were evaluated each at three levels and experimental trials were performed at all possible nine combinations. In the present investigation, viscosity of HPMC (X1) and types of filler (X) were selected as independent variables. The diffusion exponent (n), release rate constant (k), percentage drug release at 1 h (Q1), 6 h (Q6) and 12 h (Q12) were selected as dependent variables. The experimental design with corresponding formulations is outlined in Table 1. Viscosity of HPMC was evaluated at 4 000 mPa.s, 15 000 mPa.s and 100 000 mPa.s while types of filler evaluated were lactose (soluble), MCC (swellable) and DCP (insoluble).
| Batch Code | coded value | Diffusion exponent (n) | Release rate constant(k) | Percentage drug release | |||
|---|---|---|---|---|---|---|---|
| X1 | X2 | Q1 | Q6 | Q12 | |||
| B1 | -1 | 1 | 0.783 | 0.155 | 16 | 63.33 | 100 |
| B2 | 0 | 1 | 0.738 | 0.143 | 14.67 | 52.07 | 87.14 |
| B3 | 1 | 1 | 0.794 | 0.114 | 11.73 | 44.26 | 82.83 |
| B4 | -1 | 0 | 0.593 | 0.208 | 22.2 | 65.51 | 91.11 |
| B5 | 0 | 0 | 0.588 | 0.201 | 18.64 | 59.3 | 83.1 |
| B6 | 1 | 0 | 0.61 | 0.176 | 17.01 | 51.9 | 74.8 |
| B7 | -1 | -1 | 0.698 | 0.165 | 18.34 | 66.9 | 100 |
| B8 | 0 | -1 | 0.689 | 0.162 | 16.81 | 60.2 | 91.27 |
| B9 | 1 | -1 | 0.73 | 0.142 | 15.39 | 53.34 | 85.41 |
Coded values represent in terms of actual values, -1 represents X1- 4000 and X2- DCP, 0 represents X1-15 000 and X2- MCC and 1 represents X1- 100 000 and X2- Lactose. X1 is viscosity of HPMC (mPa.s) and X2 is types of filler. All batches contained 30% dipyridamole, 20% of HPMC, 10% sodium bicarbonate, 1% of magnesium stearate and quantity sufficient of filler
Table 1: Formulation And Dissolution Characteristics Of Batches In A 32 Fullfactorial Design
A statistical model incorporating interactive and polynomial terms was utilized to evaluate the response (Eqn. 1), Y=b0+b1X1+b2X2+b12 X1X2+b11X12+b22X22 - (1), where Y is the dependent variable, b0 is the arithmetic mean response of the 9 runs and bi is the estimated coefficients for the factor Xi. The main effect (X1 and X2) represents the average result of changing one factor at a time from its low to high value. The interaction term (X1X2) show how the response changes when two factors are changes simultaneously. The polynomial terms (X12, X22) are included to investigate nonlinearity.
Preparation of dipyridamole floating tablets
Dipyridamole (30%) was mixed with 20% of HPMC having different viscosity grade, sodium bicarbonate (10%) and filler by mixing in laboratory cube blender for 15 min. The powder blend was then lubricated with magnesium stearate (1%) for additional 3 min and compressed on 10 station rotary tablet machine (Rimek, Ahmedabad, India) using 12 mm standard flat face punch. Compression force was adjusted to obtain tablets with hardness in range of 5-6 kg/ cm2. The tablet weights were 500±2 mg and the tablets had a round flat face with average diameter of 12±0.1 mm and thickness of 4.5±0.2 mm.
In vitro buoyancy study
The in vitro buoyancy was characterized by floating lag time and total floating time. The test was performed using USP 24 type II paddle apparatus using 900 ml of 0.1 N HCl at paddle rotation of 100 rpm at 37±0.5°. The time required for tablet to rise to surface of dissolution medium and duration of time the tablet constantly float on dissolution medium were noted as floating lag time and total floating time, respectively (n=3).
In vitro drug release study
The in vitro drug release was performed using USP 24 type II paddle apparatus using 900 ml of 0.1 N HCl at paddle rotation of 100 rpm at 37±0.5°. The samples were withdrawn at predetermined time intervals for period of 12 h and replaced with the fresh medium. The samples were filtered through 0.45 μm membrane filter, suitably diluted and analysed at 283 nm using double beam UV/ Vis spectrophotometer (Shimadzu Corporation, UV-1601,Japan). The content of drug was calculated using equation generated from standard calibration curve. The test was performed in triplicate. High reproducibility of data was obtained (SD<3%), hence only average value was considered.
Statistical analysis
The statistical analysis of the factorial design batches were performed by multiple regression analysis using Microsoft Excel®. To evaluate contribution of each factors with different levels on responses, two way analysis of variance (ANOVA) followed by Tukey test was performed using Sigma Stat software (Sigma stat 2.03, SPSS, USA). To graphically demonstrate the influence of each factor on responses, the response surface plots were generated using Sigma Plot Software (Sigma Plot Software 8.0, SPSS, USA). The P<0.05 was cobsider to be significant.
Results and Discussion
Requirement of low pH for optimum absorption of dipyridamole reflect its ideal candidature for development of floating drug delivery system [2]. An effervescent approach was utilized to prepare the floating system. It is known that viscosity HPMC significantly contributes to the overall kinetics of drug release as well as incorporation of different types of fillers can modify the drug release. So in the present study, the influence of viscosity of HPMC and types of fillers on drug release from floating matrix tablet was evaluated using 32 full factorial design.
Tablets of all formulation had desired floating lag time (<2 min) regardless of viscosity of HPMC and types of filler incorporated, mainly due to evolution of carbon dioxide entrapped inside the hydrated polymeric matrices resulting from interaction between gas generating agent (NaHCO3) and dissolution medium (0.1 N HCl, pH 1.2) that leads to lowering of density of matrices enabling the matrices to float. The floating lag time did not change with different viscosity grades and types of filler used because as for HPMC content of 20% or more, the particles of HPMC are close enough to permit a faster establishment of the gel layer, in a manner that the effect of different viscosity of HPMC and types of filler (soluble, swellable or insoluble) is minimized [7].
The tablet which is composed of a polymeric matrix on contact with water builds a gel layer around the tablet core, which governs the drug release. It is known that the drug release from HPMC matrices is controlled for water soluble drugs by diffusion through the gel layer or, for poorly soluble drugs, by erosion of the outer polymer chains [8]. HPMC hydrogels have several important characteristics that play an essential role in drug diffusion including swelling ratio and specific mesh or pore size. Swelling ratio describes the amount of water that is contained within the hydrogel at equilibrium and is a function of the network structure, hydrophilicity and ionization of the functional groups. The pore size is the space available for drug transport. The drug characteristics are as important as those of the gel. The size, shape and ionization of the drug affect its diffusion through the gel layer [9].
The drug diffusion through most types of polymeric systems is often best described by Fickian diffusion, but other processes in addition to diffusion are important. There is also a relaxation of the polymer chains, which influences the drug release mechanism. This process is described as non-Fickian or anomalous diffusion. Release from initially dry, hydrophilic glassy polymers that swell when added to water and become rubbery show anomalous diffusion as a result of the rearrangement of macromolecular chains. The thermodynamic state of the polymer and the penetrant concentration are responsible for the different types of the diffusion. A third class of the diffusion is Case II diffusion, which is a special case of non-Fickian diffusion [9]. To obtain kinetic parameters of dissolution profiles, data were fitted to Korsmeyer and Peppas power law model [10].
The results of diffusion exponent (n), release rate constant (k) and percentage drug release at 1 h (Q1), 4 h (Q4) and 12 h (Q12), showed wide variation (Table 1). The high values of correlation co-efficients clearly indicate the responses are strongly dependent on variables studied. The fitted equations (full model) relating the responses to the transformed factor are shown in Eqn. 2 to Eqn. 6. The polynomial equations can be used to draw conclusion after considering the magnitude of co-efficient and the mathematical sign it carries (positive or negative). n=0.577+0.012X1+0.033X2–0.005X1X2+0.029X12+0.142X22, (r2=0.995) —(2), k=0.201– 0.016X1–0.010X2–0.004X1X2–0.009X 12–0.049X22, (r2=0.979) — (3), Q1=19.24–2.067X1–1.355X2–0.330X1X2+0.067X12–3.796X22, (r2=0.986) —(4), Q6=58.72–7.620X1–3.380X2–1.510X1X2– 0.267X12–2.306X22, (r2=0.995) —(5), Q12=81.76–8.010X1– 1.117X2–0.644X1X2+1.856X12+ 8.107X22, (r2=0.994) —(6)
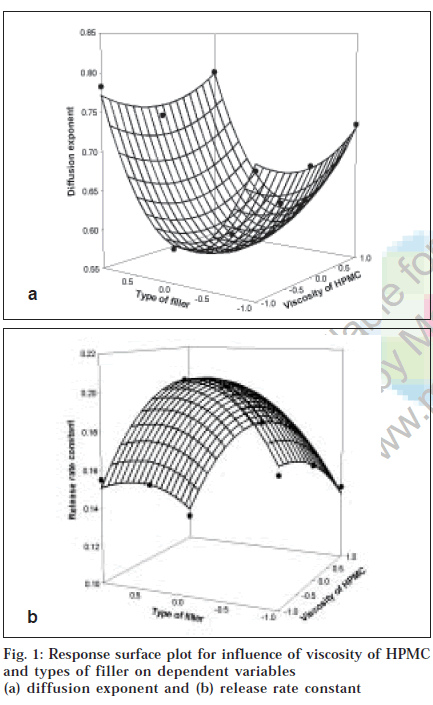
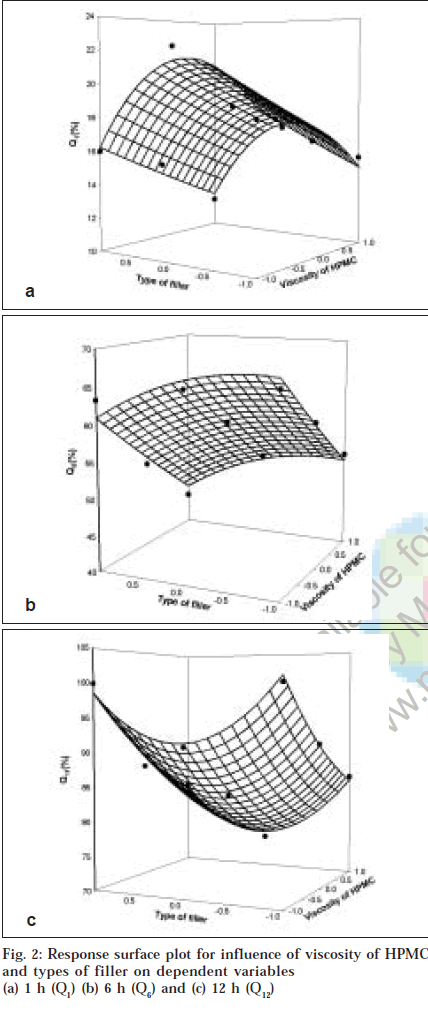
To demonstrate graphically the effect of viscosity of HPMC and types of filler used, the response surface plots were generated (figs. 1 and 2) for the dependent variables. From results of multiple regression analysis, it was found that both factors had statistically significant influence on all dependent variables (p<0.05, Table 2), while their interaction terms appeared to be insignificant (p>0.05).
| Parameters | Co-efficients of regression parameters | ||||||
|---|---|---|---|---|---|---|---|
| b0 | b1 | b2 | b11 | b22 | r2 | P | |
| Diffusion exponent (n) | 0.577 | 0.022 | 0.033 | 0.029 | 0.141 | 0.995 | 0.0032 |
| Release rate constant (k) | 0.201 | -0.016 | -0.01 | - | -0.049 | 0.979 | 0.0264 |
| Q1 | 19.23 | -2.067 | -1.355 | - | -3.796 | 0.986 | 0.015 |
| Q6 | 58.72 | -7.623 | -3.378 | - | - | 0.995 | 0.003 |
| Q12 | 81.76 | -8.009 | - | - | 8.1 | 0.994 | 0.004 |
Table 2: Summary Of Regression Output Of Significant Factors For The Measured Responses
For diffusion exponent although the viscosity of HPMC and types of filler had significant influence (p<0.05), the diffusion exponent ranges from 0.588 to 0.794 indicating anomalous drug release involving combination of swelling, diffusion and/or erosion of matrices. This might be due to poor water solubility of dipyridamole as well as difference in characteristics of filler employed. Results of Tukey test (Table 3) showed that all levels of types of filler had significant influence on mechanism of drug release while in case of viscosity of HPMC only difference was significant between matrices prepared with either HPMC K15M (15 000 mPa.s) or HPMC K100M (100 000 mPa.s) at p<0.05. Among the overall effect of both the factors, it p<0.05. Among the overall effect of both the factors, it dependent upon the types of filler employed compared to viscosity of HPMC. The value of diffusion exponent for formulation containing MCC as filler regardless of viscosity of HPMC was found to be nearer to diffusion mechanism than either lactose or DCP which gave diffusion exponent value nearer to erosion mechanism. The observed effect might be due to synergistic interaction between molecules of MCC and HPMC resulting in a stronger gel layer formation alongwith opening of channels for penetration of dissolution fluid. It can leads to more availability of dissolution fluid for drug to get dissolved resulting in dominance of diffusion mechanism for drug release from swollen matrices compared to lactose and DCP. The observed effect is evident from response surface plot (fig. 1a) as the curvature in middle portion on axis of types of fillers stating decrease in value of diffusion exponent indicates diffusion based drug release from formulation containing MCC as filler (0.588 to 0.610). Similar observation was also reported by Xu and Sunada [11]. They reported that indomethacin, a poorly soluble drug, released via diffusion mechanism from HPMC matrices when employing MCC as filler while zero order release was obtained in case of formulation prepared with either lactose or corn starch as filler.
| Response | Comparison for levels P | Viscosity of HPMC(X1) | Types of filler (X2) |
|---|---|---|---|
| Diffusion exponent (n) | -1 vs 1 | 0.226 | 0.006 |
| -1 vs 0 | 0.234 | 0.001 | |
| -0 vs 1 | 0.035 | 0.001 | |
| Release rate constant (k) | -1 vs 1 | 0.002 | 0.012 |
| -1 vs 0 | 0.043 | 0.001 | |
| 0 vs 1 | 0.005 | 0.001 | |
| Q1 | -1 vs 1 | 0.006 | 0.003 |
| -1 vs 0 | 0.056 | 0.037 | |
| 0 vs 1 | 0.068 | 0.026 | |
| Q6 | -1 vs 1 | 0.001 | 0.023 |
| -1 vs 0 | 0.013 | 0.765 | |
| 0 vs 1 | 0.017 | 0.04 | |
| Q12 | -1 vs 1 | 0.001 | 0.256 |
| -1 vs 0 | 0.003 | 0.003 | |
| 0 vs 1 | 0.014 | 0.009 |
Table 3: Results Of Tukey Test Performed Using Two Way Anova For Comparison Of Levels
In case of the poorly water-soluble drug, amount of dissolution fluid entering into matrices significantly contribute on overall kinetics of drug release. In case of formulation prepared using MCC as filler, due to its high
swelling capability and disintegration properties, it can allowed faster penetration of dissolution medium. It can create such an environment that more amount of drug gets dissolved within gel matrix and subsequently behave as soluble component and release from matrix via diffusion mechanism.Formulations containing lactose or DCP as filler showed value of diffusion exponent from 0.738 to 0.794 and 0.689 to 0.730, respectively. It was known that lactose was water-soluble filler, while DCP was water- insoluble filler, but in case of DCP, this property does not exist when pH of the medium was acidic. As floating drug delivery system should remain for long period of time, so the drug release kinetics should be important in acidic medium. Solubility of DCP was 17.39 mg/ml at pH of 1.2 while it was only 0.068 mg/ml at pH of 7.4 [12]. Thus, although DCP was characterized as a water-insoluble filler, it acts as soluble component at low pH. Diffusion exponent value obtained for formulation containing DCP was lower compared to lactose because the lactose and DCP are soluble in acidic medium, hence diffusivity depend upon its molecular weight. As DCP being a smaller molecule compared to lactose it diffused more readily giving lower release restriction leading to lowering of diffusion exponent. Compared to DCP, lactose produce decreased in tortuosity leading to increase in value of diffusion exponent resulting in shifting of drug release towards case-II transport.
Fig. 1b showed the influence of viscosity of HPMC and types of filler on release rate constant. Multiple regression analysis of release rate constant showed that both factors had statistically significant influence with their quadratic terms (p<0.05). Results of Tukey test (Table 3) showed that all levels of both the factors significantly contribute on release rate constant (p<0.05). Increasing the viscosity of HPMC from 4000 mPa.s to 15 000 mPa.s and 100 000 mPa.s, the release rate was declined as indicated by negative co-efficient in regression analysis. Following the assumption made before and supposing that HPMC particles of increasing viscosity grades swell slower and produce swollen particles of smaller volume, then matrices made up of particles of HPMC with higher viscosity will contain pores of smaller diameter and will show slower release rate than those made up of HPMC particles with lower viscosity grade. As viscosity of polymeric matrices increases, matrices will decrease the tortuosity and subsequently formation of tightly swollen gel layer leads to decreased mobility of insoluble particles in swollen matrices resulting in decreased release rate8. Amongst the types of fillers evaluated, the drug release rate was higher in order of MCC, DCP and lactose. Release rate from formulation containing MCC as filler was highest which might be due to swelling and disintegration properties of MCC leading to increase in rate of penetration of dissolution fluid giving more fluid to enter within matrix to dissolve drug particles resulting in both of them act as soluble components in acidic medium they exhibited slower release rate compared to MCC. The release rate constant obtained with lactose was low compared to DCP and might be due to fact that besides significant contribution on diffusivity of penetrates. DCP of dissolution fluid due to its smaller molecular volume compared to lactose resulting in increase in diffusivity. Lactose in aqueous solution plays a role as important physical barrier affecting the release kinetics by reducing tortuosity of diffusion pattern of the drug [13]. The results obtained are in good agreement with study reported by Xu and co-workers [11] as well as Bravo and co-workers6. Xu and co-workers [11] reported higher release rate obtained by replacing lactose with MCC in formulation prepared with HPMC as polymeric matrices. Bravo and co-workers6 reported increase in release rate of diclofenac sodium upon addition of MCC in formulation containing lactose and starch as filler due to swelling behavior of MCC allowed further penetration of dissolution medium resulting in rapid drug release rate.
To describe the entire dissolution profile, three time level points were selected. Percentage drug release at 1 h (Q1),6 h (Q6) and 12 h (Q12) were selected to describe the entire dissolution profile. Q1 represents the initial phase of drug release profile, Q6 represents the phase of mid portion describing completion of gel layer formation and stating of erosion of fully swollen matrices while Q12 represents end phase of drug release profile, an indicative of overall release profile. Fig. 2a showed influence of viscosity of HPMC and types of filler on percentage drug release at 1 h. Multiple regression analysis for Q1 showed that both factors had significant effect (p<0.05). The interaction term of both the factors and quadratic term of factor X1 were found to be insignificant (p>0.05). For percentage drug release at 1 hr, Tukey test revealed that all levels of types of filler and drug release from matrices prepared with 4000 mPa.s and 100 000 mPa.s were found to be significant. It was found that drug release in initial phase termed as burst effect depended more on types of filler evaluated. Co-efficients obtained for both factors were negative indicating antagonistic effect as viscosity of HPMC increase and when filler changed from DCP to MCC and lactose, the burst effect was minimized might be due to difference in solubility and molecular size of the filler employed.
Multiple regression analysis for Q6 showed that both the factors had statistically significant effect (p<0.05). Tukey test showed significant difference amongst all levels of viscosity of HPMC and types of filler employed except the difference was not observed between formulation containing MCC and DCP. Also it was observed that Q6 was more dependent on viscosity of HPMC compared to types of filler employed (fig. 2b).Q6 depends on main factors only as interaction term and quadratic terms were found to be insignificant (p>0.05). Multiple regression analysis for percentage drug release at 12 h showed that only viscosity of HPMC had statistically significant influence (p<0.05). Tukey test showed that Q12 was more dependent on viscosity of HPMC regardless of types of filler employed (fig. 2c, Table 3).
Overall results explained that the viscosity of HPMC had a dominant role as controlling factors on kinetics of drug release while types of filler contribute equally but in initial period only. From results of Tukey test, we found that dependency of drug release in middle and end phase was more dependent on viscosity of HPMC compared to types of fillers employed. From overall discussion it was concluded that the kinetics of drug release mainly dependent on viscosity of HPMC and it was modified by incorporation of different types of fillers.
Acknowledgements
Authors thank Sun Pharmaceutical Ltd., Vadodara (India) for providing gift sample of dipyridamole, Colorcon Asia Pvt. Ltd., Goa (India), Meggle GMBH, Wasserberg (Germany) and Zydus Cadila Research Centre, Ahmedabad (India) for providing gift sample of excipients.
References
- Colombo, P., Bettini, R., Santi, P. and Peppas, N.A., Pharm. Sci. Technol. Today , 2000, 3, 1.
- Yeole, P.G., Khan, S. and Patel, V.F., Indian J. Pharm. Sci. , 2005, 67, 265.
- Kohri, N., Miyata, N., Takechi, S. and Nomura, A., Int. J. Pharm. , 1992, 81, 49.
- He, X., Kadomura, S., Takekuma, Y., Sugawara, M. and Miyazaki, K., J. Pharm. Sci. , 2004, 93, 71.
- Levina, M. and Rajabi-Siahboomi, A.R., J. Pharm. Sci. , 2004, 93, 2746.
- Bravo, S.A., Lamas, M.C. and Salomon, C.J., J. Pharm. Pharm. Sci. , 2002, 5, 213.
- Alderete, M.E. and Villafuerte-Robles, L., Eur. J. Pharm. Biopharm. , 1997, 43, 173.
- Mitchell, K., Ford, J.L., Armstrong, D.J., Elliott, P.N.C., Rostron, C. and Hogan, J.E., Int. J. Pharm. , 1993, 100, 155.
- Peppas, N.A. and Wright, S.L., Eur. J. Pharm. Biopharm. , 1998, 46, 15.
- Peppas, N.A., Pharm. Acta. Helv. , 1985, 60, 110.
- Xu, G. and Sunada, H., Chem. Pharm. Bull. , 1995, 43, 483.
- Bryan, J.W. and McCallister, J.D., Drug Develop. Ind. Pharm. , 1992, 18, 2029.
- Gao, P. Nixon, P. and Skoug, J., Pharm. Res. , 1995, 12, 965.