K. Javidnia, E. Faghih-Mirzaei1*, R. Miri, M. Attarroshan and K. Zomorodian2
Medicinal and Natural Products Chemistry Research Center, Shiraz University of Medical Sciences.
1Department of Medicinal Chemistry, School of Pharmacy, Kerman University of Medical Sciences, Kerman, Iran
2Department of Mycology and Parasitology, Faculty of Medicine, Shiraz University of Medical Sciences, Shiraz
- Corresponding Author:
- E. Faghih-Mirzaei
Department of Medicinal Chemistry, School of Pharmacy, Kerman University of Medical Sciences, Kerman, Iran
E-mail: ehsancontact@kmu.ac.ir
| Date of Submission | 24 December 2014 |
| Date of Revision | 29 November 2015 |
| Date of Acceptance | 17 February 2016 |
| Indian J Pharm Sci 2016;78(1):73−79 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Chiral alcohols are the key chiral building blocks to many enantiomerically pure pharmaceuticals. The biocatalytic approach in asymmetric reduction of corresponding prochiral ketones to the preparation of these optically pure substances is one of the most promising routes. The stereoselective reduction of different kinds of prochiral ketones catalyzed by various plants and microorganisms was studied in this work. Benzyl acetoacetate, methyl 3-oxopentanoate, ethyl 3-oxopentanoate, and ethyl butyryl acetate were chosen as the model substrates for β-ketoesters. Benzoyl acetonitrile, 3-chloro propiophenone, and 1-acetyl naphthalene were chosen as aromatic aliphatic ketones. Finally, 2-methyl benzophenone and 4-chloro benzophenone were selected as diaryl ketones. Plant catalysis was conducted by Daucus carota, Brassica rapa, Brassica oleracea, Pastinaca sativa, and Raphnus sativus.For microbial catalysis, Aspergillus foetidus, Penicillum citrinum, Saccharomyces carlbergensis, Pichia fermentans, and Rhodotrula glutinis were chosen. Chiral alcohols were obtained in high yields and with optical purity. A superiority in the microorganisms' performance in the bioreduction of prochiral ketones was detected. Among microorganisms, Rhodotrula glutinis showed remarkable results with nearly all substrates and is proposed for future studies.
Keywords
Stereoselective, reduction, biocatalysts, prochiral ketones
The importance of obtaining enantiopure substances in the chemical or pharmaceutical industry has inspired considerable efforts to search for methods of constructing chiral precursors, especially optically active alcohols[1,2]. These alcohols are important intermediates and building blocks for the synthesis of pharmaceutically active compounds such as orphenadrine, fluoxetine, tomoxetine, cloperastine, neobenodine, and carbinoxamine[3].
Chiral alcohols with high optical purity can be obtained from the stereoselective reduction of prochiral ketones either by chemical or biological methods. Despite the vast progress of organic chemistry in chiral synthesis, biocatalysis as an alternative has attracted much attention recently from the viewpoint of green chemistry[4].
Biocatalysis performed by whole cell living organisms is regarded as one of the most effective and promising routes because of its regio- and stereoselectivity, mild and environment-friendly condition. This method appears advantageous over enzymatic reduction of carbonyl compounds using an isolated enzyme, since the oxido-reductase, cofactor (NAD(P)H) and its regenerating system are all located within the cell[5,6].
There are numerous works on the asymmetric reduction reaction catalyzed by whole cell biocatalysts with excellent enantioselectivity and yield. The previously reported works can be divided into two periods. Before the year 2000, the focus was on microorganisms as biocatalysts. Baker’s yeast was then the microorganism most widely used for the reduction of prochiral ketone[7]. At the turn of century, reports on the use of whole plant cells to reduce prochiral ketones began to appear[8]. Daucus carota has been the plant most frequently used to convert prochiral ketones to relevant chiral alcohols until now[9-11].
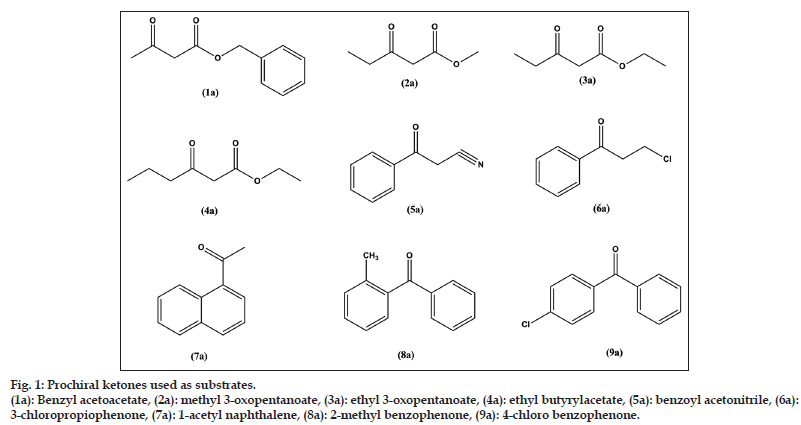
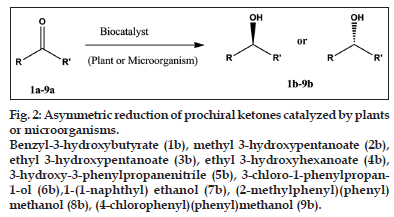
Progressive studies on the use of biocatalysts in chemicals and pharmaceuticals to obtain important optically active alcohols show the great value of this method. The objective of the current study was to explore the asymmetric reduction of some important prochiral ketones (fig. 1). For this purpose, five plants (D. carota, Brassica rapa, Brassica oleracea, Pastinaca sativa, and Raphnus sativus) and five microorganisms (Aspergillus foetidus, Penicillum citrinum, Saccharomyces carlbergensis, Pichia fermentans, and Rhodotrula glutinis) were chosen because of their efficiency and availability. In this paper, we report our results in the bioreduction of important prochiral ketones (fig. 2) from different categories using the aforementioned biocatalysts.
Materials and Methods
Optical rotations were measured with a Kruss P8100. The 1H NMR spectra was obtained with an Avance III Bruker 400 MHz instrument using TMS as the internal standard. Bioreduction reactions were monitored by GC-MS analysis (compound 1a-7a) equipped with an HP-5 Agilent capillary column (30 m×0.25 mm; 0.25 μm) and a CPChirasil- DEX CB Varian GC column. HPLC analysis (compound 8a, 9a) was carried out in a Knauer HPLC pump 1000, UV detector 2500 Knauer, and a 7725 injection valve with a 20 μl loop equipped with a Nucleocell Delta (4.6 mm×250 mm×5 μm) Macherey-Nagel, Germany. Preparative TLC was carried out on Silicagel 60 F254 Merck (Darmstadt, Germany).
The substrate 3-chloropropiophenone (6a) was purchased from Acros Organics. Other substrates were purchased from Merck.
Biocatalysts:
Fresh carrots (D. carota), turnips (Brassica rapa), cabbages (Brassica oleracea), parsnips (Pastinaca sativa), and radishes (Raphnus sativus) were obtained from a local market. Freeze-dried Aspergillus foetidus (PTCC 5099), Penicillum citrinum (PTCC 5304), Saccharomyces carlbergensis (PTCC 5051), Pichia fermentans (PTCC 5296), and Rhodotrula glutinis (PTCC 5256) were bought from the Persian Type Culture Collection (Iranian Research Organization for Science and Technology).
Figure 1: Prochiral ketones used as substrates.
Figure 2: Asymmetric reduction of prochiral ketones catalyzed by plants or microorganisms.
General procedure for the asymmetric reduction of ketones with plants
The plant roots of D. carota, B. rapa, R. sativus, P. sativa, and B. oleracea were excised. Their surfaces were disinfected with 70% EtOH for 5 min and 20% NaClO for 10 min, and then they were rinsed thoroughly with sterile distilled water. Roots were cut and put in 500 ml Erlenmeyer flasks (75 g per each). The substrate (50 mg) was dissolved in 5 ml of absolute ethanol, and then the solution was added to each Erlenmeyer flask. Incubation was continued for 144 h (6 days). Each experiment was repeated at least three times.
General procedure for the asymmetric reduction of ketones with microorganisms
The stock cultures were maintained on a Sabouraud dextrose agar medium at 4° and freshly sub-cultured before being used in transformation experiments. Ten 500 ml Erlenmeyer flasks, each containing 100 ml of Sabouraud broth medium, were inoculated with freshly obtained cells and incubated at 25° in a rotary shaker (150 rpm). The cultures were allowed to reach a constant cell density (detected by optical density or dry cell weight). The substrate (50 mg) was dissolved in 5 ml of absolute ethanol, and then solution was added to each Erlenmeyer flask. A higher concentration of substrate resulted in lower conversion and a decrease in yield. Incubation was continued for 144 h (6 days). Each experiment was repeated at least three times.
General procedure for preparation of racemic alcohols
Compounds 1a-9a were reduced to the corresponding racemic alcohol with NaBH4 in MeOH[12]. The reduced products were purified by preparative TLC, and their chemical structures were confirmed by 1H NMR.
Benzyl-3-hydroxybutyrate (1b): 1H NMR (400 MHz, CHCl3): δ 7.47-7.38 (m, 5H, CH-Ar), 5.18 (s, 2H, CH2O), 4.14-4.03 (m, 1H, CH), 3.58 (br s, 1H, OH), 2.58-2.54 (dd, 1H, CH2), 2.49-2.44 (dd,1H, CH2), 1.20-1.18 (d, 3H, CH3).
Methyl 3-hydroxypentanoate (2b): 1H NMR (400 MHz, CHCl3): δ 3.84-3.80 (m, 1H, CH), 3.71 (s, 3H, CH3), 3.55 (br s, 1H, OH), 2.52-2.48 (dd, 1H, CH2), 2.33-2.29 (dd, 1H, CH2), 1.48-1.45 (m, 2H, CH2), 0.85-0.79 (t, 3H, CH3).
Ethyl 3-hydroxypentanoate (3b): 1H NMR (400 MHz, CHCl3): δ 4.14-4.11 (q, 2H, CH2O), 3.89-3.85 (m, 1H, CH), 3.58 (br s, 1H, OH), 2.55-2.52 (dd, 1H, CH2), 2.31-2.28 (dd, 1H, CH2), 1.48-1.43 (m, 2H, CH2), 1.27-1.23 (t, 3H, CH3), 1.2-1.1 (t, 3H, CH3).
Ethyl 3-hydroxyhexanoate (4b): 1H NMR (400 MHz, CHCl3): δ 4.17-4.13 (q, 2H, CH2O), 3.91-3.85 (m, 1H, CH), 3.58 (br s, 1H, OH), 2.57-2.54 (dd, 1H, CH2), 2.29-2.26 (dd, 1H, CH2), 1.47-1.44 (m, 2H, CH2), 1.33-1.29 (m, 2H, CH2), 1.27-1.24 (t, 3H, CH3), 1.1-0.9 (t, 3H, CH3).
3-Hydroxy-3-phenylpropanenitrile (5b): 1H NMR (400 MHz, CHCl3): δ 7.34-7.19 (m, 5H, CH-Ar), 4.99-4.95 (m, 1H, CH), 3.19 (br s, 1H, OH), 2.70-2.69 (m, 2H, CH2).
3-Chloro-1-phenylpropan-1-ol (6b): 1H NMR (400 MHz, CHCl3): δ 7.30-7.18 (m, 5H, CH-Ar), 4.89-4.86 (m, 1H, CH), 3.7-3.6 (m, 2H, CH2-Cl), 3.66 (br s, 1H, OH), 2.21-2.13 (m, 1H, CH2), 2.06-1.98 (m, 1H, CH2).
1-(1-Naphthyl) ethanol (7b): 1H NMR (400 MHz, CHCl3): δ 7.72-7.27 (m, 7H, CH-Ar), 5.44-5.39 (q, 1H, CH), 2.44 (br s, 1H, OH), 1.47-1.45 (d, 3H, CH3).
(2-Methylphenyl)(phenyl)methanol (8b): 1H NMR (400 MHz, CHCl<sub>3</sub>): δ 7.41-7.02 (m, 9H), 5.85 (s, 1H, CH), 2.21 (br s, 1H, OH), 2.13 (s, 3H, CH3).
(4-Chlorophenyl)(phenyl)methanol (9b):1H NMR (400 MHz, CHCl<sub>3</sub>): δ7.26-7.17 (m, 9H, CH-Ar), 5.79-5.77 (m, 1H, CH), 2.23 (br s, 1H, OH).
Conversion rate analysis
After 2 and 4 days of incubation, biomass was separated from the media by filtration (plants) or centrifugation (microorganism). The media was extracted with EtOAc (×3). The solvent was dried over anhydrous Na2SO4 and removed in vacuo. The samples were analyzed by GC-MS (1a-7a) or HPLC (8a and 9a). The methods were established prior to sample analysis by use of racemic alcohols.
Enantiomeric excess analysis
For the reduction of (1a-7a), enantiomeric separation was achieved on a Chirasil-Dex CB Varian GC column. For the reduction of (8a and 9a), enantiomer separation was achieved on a Nucleocell delta MN column (4.6mm×250mm, 5μm) n-Hexan/2-propanol 95/5 flow: 0.5 ml/min.
Assignment of absolute configuration for alcohols
Optical rotations of the purified alcohols (1b-9b) obtained by bioreductions were measured and compared with the literature: Benzyl (S)-(+)-3- hydroxybutyrate (1b) [α]D 25=+29.0 (CHCl3, c=l)[13]; methyl (R)-(−)-3-hydroxypentanoate (2b) [α]D 25=-35.7 (CHCl3, c=l)[14]; ethyl (R)-(−)-3-hydroxypentanoate (3b) [α]D 25=-34.6 (CHCl3, c=5)[14]; ethyl (R)-(−)- 3-hydroxyhexanoate (4b) [α]D 25=-24.3 (CHCl3, c=0.7)[15]; (S)-(−)-3-hydroxy-3-phenylpropanenitrile (5b)[α]D 20=-57.0 (EtOH, c=1.1)[16]; (R)-(+)-3-chloro- 1-phenylpropan-1-ol (6b)[α]D 22=+24.3 (CHCl3, c=1)[17]; (S)-(−)-1-(1-naphtyl)ethanol (7b) [α]D 20=-78.0 (MeOH, c=1)[18]; (S)-(+)-(2-methylphenyl) (phenyl) methanol (8b)) [α]D 22=+6.38 (CHCl3, c=0.9)[19]; (S)- (+)-(4-chlorophenyl) (phenyl)methanol (9b) [α]D 22=+22 (CHCl3, c=0.48).[20]
Results and Discussion
Among the plants tested, B. oleracea, D. carota, and P. sativa reduced benzyl acetoacetate (1a) with a high conversion rate, and all plants produced benzyl (S)-(+)-3-hydroxybutyrate (S-1b). B. oleracea achieved the highest stereoselectivity (>99% ee) (Table 1). All microorganisms except P. citrinum could produce (S)-alcohol with a high conversion rate; using S. carlbergensis and A. foetidus as biocatalysts resulted in a higher optical purity. (R)-Alcohol was not produced in any bioreduction.
| Compound 1a | Biocatalyst | Time | % | Enantiomeric |
|---|---|---|---|---|
| (days) | conversion | excess% | ||
| Plants and | D. carota | 2 | 91 | 38 (S) |
| microorganisms | 4 | 93 | 70 (S) | |
| B. oleracea | 2 | 98 | >99 (S) | |
| 4 | 100 | >99 (S) | ||
| P. sativa | 2 | 45 | 70 (S) | |
| 4 | 100 | 70 (S) | ||
| S. carlbergensis | 3 | 84 | >99 (S) | |
| 6 | 100 | 96 (S) | ||
| P. fermentans | 2 | 100 | 75 (S) | |
| 4 | 100 | 75 (S) | ||
| R. glutinis | 2 | 85 | 76 (S) | |
| 4 | 85 | 76 (S) | ||
| P. citrinum | 2 | 13 | 73 (S) | |
| 4 | 13 | 73 (S) | ||
| A. foetidus | 2 | 96 | 73 (S) | |
| 4 | 96 | >99 (S) |
Table 1: Results for bioreduction of benzyl acetoacetate (1a)
B. oleracea, R. sativus, and P. sativa could reduce methyl 3-oxopentanoate (2a) when it was used as the substrate. The stereoselectivity of bioreduction with B. oleracea was quite good (>76%ee S-2b), although the conversion rate was highest with R. sativus. All microorganisms except P. citrinum managed to reduce R. sativus and P. sativa could accomplish the bioreduction of ethyl 3-oxopentanoate (3a), but the stereoselectivity was achieved with P. sativa rather than R. sativus. Almost all microorganisms could reduce 3a to (R-3b) with high yield and stereoselectivity (Table 3).
For the compound ethyl butyrylacetate (4a), only R. sativus could produce 4b in high optical purity and yield. All microorganisms except R. glutinis accomplished the bioreduction with high optical purity and yield, and all produced (R-4b, Table 4).
Among plants and microorganisms, only R. glutinis and A. foetidus could reduce benzoyl acetonitrile (5a). Using R. glutinis as the biocatalyst resulted in an outstanding conversion yield and optical purity with (S)-(−)-3-hydroxy-3-phenyl propanenitrile (S-5b) as the main product, which is a useful precursor in the synthesis of (S)-fluoxetine, the active form of fluoxetine (Table 5).
| Compound 2a | Biocatalyst | Time | % | Enantiomeric |
|---|---|---|---|---|
| (days) | conversion | excess % | ||
| Plants an | B. oleracea | 2 | 44 | - |
| microorganisms | 4 | 52 | 76 (S) | |
| R. sativus | 2 | 0 | - | |
| 4 | 91 | 34 (R) | ||
| P. sativa | 2 | 71 | <2 | |
| 4 | 73 | low | ||
| S. carlbergensis | 2 | 79 | - | |
| 4 | 100 | 92 (R) | ||
| P. fermentans | 2 | 100 | 99 (R) | |
| 4 | 100 | 99 (R) | ||
| R. glutinis | 2 | 100 | 72 (R) | |
| 4 | 100 | 72 (R) | ||
| A. foetidus | 2 | 100 | 98 (R) | |
| 4 | 100 | 98 (R) |
Table 2: Results for bioreduction of methyl 3 oxopentanoate (2a)
| Compound 3a | Biocatalyst | Time | % | Enantiomeric |
|---|---|---|---|---|
| (days) | conversion | excess % | ||
| Plants and | R. sativus | 2 | 0 | - |
| microorganisms | 4 | 100 | 6 (R) | |
| P. sativa | 2 | 65 | 82 (S) | |
| 4 | 80 | 82 (S) | ||
| S. carlbergensis | 2 | 100 | 95 (R) | |
| 4 | 100 | 95 (R) | ||
| P. fermentans | 2 | 100 | 99 (R) | |
| 4 | 100 | 99 (R) | ||
| R. glutinis | 2 | 100 | 72 (R) | |
| 4 | 100 | 72 (R) | ||
| P. citrinum | 2 | 100 | 98 (R) | |
| 4 | 100 | 98 (R) | ||
| A. foetidus | 2 | 100 | 98 (R) | |
| 4 | 100 | 98 (R) |
Table 3: Results for bioreduction of ethyl 3 oxopentanoate (3a)
| Compound 4a | Biocatalyst | Time | % | Enantiomeric |
|---|---|---|---|---|
| (days) conversion | excess % | |||
| Plants and | B. oleracea | 2 | 0 | - |
| microorganisms | 4 | 17 | - | |
| R. sativus | 2 | 30 | 64 (R) | |
| 4 | 100 | 90 (R) | ||
| P. sativa | 2 | 72 | 14 (S) | |
| 4 | 72 | 14 (S) | ||
| S. carlbergensis | 2 | 100 | 98 (R) | |
| 4 | 100 | 98 (R) | ||
| P. fermentans | 2 | 100 | >99 (R) | |
| 4 | 100 | >99 (R) | ||
| P. citrinum | 2 | 100 | >99 (R) | |
| 4 | 100 | >99 (R) | ||
| A. foetidus | 2 | 100 | 99 (R) | |
| 4 | 100 | 99 (R) | ||
Table 4: Results for bioreduction of ethyl butyrylacetate (4a)
| Biocatalyst | Time | % | Enantiomeric | |
| (days) conversion | excess % | |||
| Compound 5a | ||||
| Microorganisms R. glutinis | 2 | 99 | 96 (S) | |
| 4 | 99 | 96 (S) | ||
| A. foetidus | 2 | 15 | - | |
| 4 | 70 | 7 (S) | ||
| Compound 6a | ||||
| Plants and | D .carota | 2 | 0 | >99 (S) |
| microorganisms | 4 | 25 | ||
| P. sativa | 2 | 30 | 90 (S) | |
| 4 | 32 | 90 (S) | ||
| S. carlbergensis | 2 | 29 | 65 (S) | |
| 4 | 29 | 65 (S) | ||
| R. glutinis | 2 | 70 | 93 (S) | |
| 4 | 70 | 93 (S) | ||
| P. citrinum | 2 | 40 | 40 (S) | |
| 4 | 40 | 40 (S) | ||
Table 4: Results for bioreduction of ethyl butyrylacetate (4a)
In the case of 3-chloropropiophenone (6a), the plants D. carota and P. sativa could reduce the ketone with low yield but high optical purity. Among microorganisms, S. carlbergensis, P. citrinum, and R. glutinis were capable of producing 3-chloro-1- phenyl propanol (6b). R. glutinis could produce (S)-(−)-3-chloro-1-phenylpropanol, a useful precursor for the synthesis of (S)-fluoxetine, with acceptable yield (70%) and high optical purity (93% ee). As could be expected, elimination and dehalogenation products such as propiophenone, 1-phenyl-1-propanol, and phenyl vinyl ketones were also observed in the reduction of (6a) (Table 5).
For compound (7a), the conversion rate when using plants as the biocatalysts was very low and insignificant. Among microorganisms, only R. glutinis could produce optically active alcohol (S-7b) with high yield (94%) and stereoselectivity (>96%). (S)-(−)-1-1-(naphtyl)ethanol (S-7b) is an important intermediate in the synthesis of mevinic acid which has an HMG-Co-A reductase inhibitory effect (Table 6).
Diaryl ketones like 2-methyl benzophenone (8a) and 4-chloro benzophenone (9a) are important prochiral ketones for the production of diarylmethanols which are important intermediates for the synthesis of pharmaceutically interesting molecules, such as (S)-cloprastine, (S)- carbinoxamine, (R)-orphenadrine, (R)-neobenodine. Among plants and microorganisms, only R. glutinis could produce the relevant chiral alcohol (S-8b) for compound (8a) with 45% conversion rate and more than 99% optical purity. In the case of compound (9a), only S. carlbergensis and R. glutinis could produce chiral alcohol (R-9b). The conversion rate (>99%) and optical purity (>99% ee) were very high when R. glutinis was used as the biocatalyst (Table 7).
In view of these results, it can be concluded that plant and microbial biocatalysis is an efficient, easy, green procedure and an alternative to chemical methods for the production of enantiopure chiral alcohols. Overall, our results show the superiority of microorganisms over plants in the bioreduction of prochiral ketones. This may be attributed to the presence of more dehydrogenases in microbes than in plants. However, in some cases and in the production of a special enantiomer, some plants were more successful. Among microorganisms, R. glutinis showed a noteworthy performance and could reduce nearly all prochiral ketones with high conversion rates and optical purity. Thus, R. glutinis can be recommended as a promising microorganism for yeast mediated stereoselective production of the aforementioned chiral alcohols.
| Compound 7a | Biocatalyst | Time | % | Enantiomeric |
|---|---|---|---|---|
| (days) | conversion | excess % | ||
| Plants and | D. carota | 2 | 6 | >99 (S) |
| microorganisms | 4 | 3 | >99 (S) | |
| B. rapa | 2 | 3 | >99 (S) | |
| 4 | 3 | >99 (S) | ||
| B. oleracea | 2 | 3 | >99 (S) | |
| 4 | 3 | >99 (S) | ||
| P. sativa | 2 | 3 | >99 (S) | |
| 4 | 3 | >99 (S) | ||
| S. carlbergensis | 2 | 5 | >99 (S) | |
| 4 | 5 | >99 (S) | ||
| R. glutinis | 2 | 25 | 96 (S) | |
| 4 | 94 | 96 (S) |
Table 6: Results for bioreduction of 1 acetyl naphtalene (7a).
| Biocatalyst | Time | % | Enantiomeric | |
|---|---|---|---|---|
| (days) | conversion | excess % | ||
| Compound 8a | ||||
| Micoorganisms | R. glutinis | 2 | 38 | >99 (S) |
| 4 | 45 | >99 (S) | ||
| Compound 9a | ||||
| Micoorganisms | S. carlbergensis | 2 | 26 | >99 (R) |
| 4 | 34 | >99 (R) | ||
| R. glutinis | 2 | 100 | >99 (R) | |
| 4 | 100 | >99 (R) |
Table 7: Results for bioreduction of 2 methyl benzophenone (8a) and4 chlorobenzophenone (9a)
Acknowledgements
We are grateful to the Alexander von Humboldt Foundation for supporting our institute by donating a Krüss Automatic Polarimeter P8100 under a Research Group Linkage Project (3.4-IRN/1101775).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Muñoz Solano D, Hoyos P, Hernáiz MJ, Alcántara AR, Sánchez-Montero JM. Industrial biotransformations in the synthesis of building blocks leading to enantiopure drugs. BioresourTechnol 2012;115:196-207.
- Habulin M, Knez Z. Optimization of (R,S)-1-phenylethanol kinetic resolution over Candida antarctica lipase B in ionic liquids. J MolCatal B Enzym 2009;58:24-8.
- Li H, Zhu D, Hua L, Biehl ER. Enantioselective reduction of diaryl ketones catalyzed by a carbonyl reductase from sporobolomycessalmonicolor and its mutant enzymes. Adv Synth Catal 2009;351:583-8.
- Ran N, Zhao L, Chen Z, Tao J. Recent applications of biocatalysisin developing green chemistry for chemical synthesis at the industrial scale. Green Chem 2008;10:361-72.
- Yang ZH, Zeng R, Yang G, Wang Y, Li LZ, Lv ZS, et al.Asymmetricreduction of prochiral ketones to chiral alcohols catalyzed by plants tissue. J IndMicrobiolBiotechnol 2008;35:1047-51.
- Wohlgemuth R. Biocatalysis − key to sustainable industrial chemistry. CurrOpinBiotechnol 2010;21:713-24.
- Csuk R, Glaenzer BI. Baker’s yeast mediated transformations in organic chemistry. Chem Rev 1991;91:49-97.
- Baldassarre F, Bertoni G, Chiappe C, Marioni F. Preparative synthesis of chiral alcohols by enantioselective reduction with Daucuscarota root as biocatalyst. J MolCatal B Enzym 2000;11:55-8.
- Yadav JS, Nanda S, Reddy PT, Rao AB. Efficient enantioselective reduction of ketones with Daucuscarota root. J Org Chem 2002;67:3900-3.
- Blanchard N, van de Weghe P. Daucuscarota L. mediated bioreduction of prochiral ketones. Org BiomolChem 2006;4:2348-53.
- Cordell GA, Lemos TL, Monte FJ, de Mattos MC. Vegetables as chemical reagents. J Nat Prod 2007;70:478-92.
- Vieira GA, Araujo DM, Lemos TL, Mattos MC, Oliveira MD, Melo VM, et al. Candida tropicalis CE017: A new Brazilian enzymatic source for the bioreduction of aromatic prochiral ketones. J BrazChemSoc 2010;21:1509-16.
- Medson C, Smallridge AJ, Trewhella MA. The stereoselectivepreparation of ß-hydroxy esters using a yeast reduction in an organic solvent. Tetrahedron Asymmetry 1997;8:1049-54.
- Seebach D, Züger MF. On the preparation of methyl and ethyl (R)- (-)-3-hydroxy-valerate by depolymerization of a mixed PHB/PHV biopolymer. Tetrahedron Lett 1984;25:2747-50.
- Dahl AC, Madsen J. Baker’s yeast: Production of d-and l-3-hydroxy esters. Tetrahedron Asymmetry 1998;9:4395-417.
- Florey P, Smallridge AJ, Ten A, Trewhella MA. Chemo- and stereoselective reduction of an alpha-cyanoketone by bakers’ yeast at low temperature. Org Lett 1999;1:1879-80.
- Liu HL, Hoff BH, Anthonsen T. Chemoenzymatic synthesis of the non-tricyclic antidepressants fluoxetine, tomoxetine and nisoxetine. J Chem Soc Perkin 1 2000;11:1767-9.
- Sigma-Aldrich. St. Louis, 70693-(S)-(-)-1-(1-Naphthyl)ethanol. Available from: http://www.sigmaaldrich.com/catalog/product/ fluka/70693lang=en®ion=IR. [Last cited on 2014 Dec 23].
- Ohkuma T, Koizumi M, Ikehira H, Yokozawa T, Noyori R. Selective hydrogenation of benzophenones to benzhydrols. Asymmetric synthesis of unsymmetrical diarylmethanols Org Lett 2000;2:659-62.
- Wu X, Liu X, Zhao G. Catalyzed asymmetric aryl transfer reactions to aldehydes with boroxines as aryl source. Tetrahedron Asymmetry 2005;16:2299-305.