- *Corresponding Author:
- Yan Zhang
Department of Clinical Laboratory, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi Province 710061, China
E-mail: 76899846@qq.com
| Date of Received | 09 January 2023 |
| Date of Revision | 10 June 2023 |
| Date of Acceptance | 11 November 2023 |
| Indian J Pharm Sci 2023;85(6):1692-1701 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
P-glycoprotein, livin, and survivin are apoptosis inhibitory proteins. This study was to clarify the correlation between expression of P-glycoprotein, livin, survivin, and drug resistance in the human gastric cancer resistant cell line OCUM-2MD3/oxaliplatin. The highly invasive metastatic gastric cancer cell line OCUM- 2MD3 was utilized as experimental material. The oxaliplatin-resistant cell line, OCUM-2MD3/oxaliplatin, was established by inducing OCUM-2MD3 cells with oxaliplatin. Flow cytometry was employed to analyze the cell cycle and apoptosis of OCUM-2MD3/oxaliplatin cells. The sensitivity of OCUM-2MD3/oxaliplatin cells to drugs was determined using cell counting kit-8 assay. Real-time quantitative polymerase chain reaction and Western blot analysis were performed to determine the gene and protein expression levels of P-glycoprotein, livin, and survivin in OCUM-2MD3 and OCUM-2MD3/oxaliplatin cells, establishing the association between expression of these genes and proteins and drug resistance of OCUM-2MD3/oxaliplatin cells. Gene and protein expression levels of P-glycoprotein, livin, and survivin were markedly upregulated in OCUM-2MD3/ oxaliplatin cells relative to OCUM-2MD3 cells. P-glycoprotein and livin expression exhibited a positive correlation with cisplatin resistance in OCUM-2MD3/oxaliplatin cells, whereas survivin expression showed a negative correlation with resistance. The expression levels of P-glycoprotein, livin, and survivin are closely associated with drug resistance in the human gastric cancer resistant cell line OCUM-2MD3/oxaliplatin. A theoretical basis was provided for development of novel therapeutic strategies targeting these molecules and contributes to enhancing clinical efficacy in gastric cancer patients.
Keywords
P-glycoprotein, livin, survivin, human gastric cancer resistant cell line OCUM-2MD3/ oxaliplatin, drug resistance
Gastric Cancer (GC), a widely distributed and highly prevalent malignancy, ranks as the 3rd most common cancer globally, alongside lung cancer, liver cancer, breast cancer, and colorectal cancer, constituting the five major cancer types. According to the 2020 Global Cancer Statistics report, GC stands second in terms of new cases added to the global cancer burden, with its incidence steadily rising each year. In China, the incidence rate of GC was reported as high as 41 per 100 000 individuals in 2020[1]. The progression of GC typically occurs insidiously, with inconspicuous early symptoms, leading to diagnostic and therapeutic challenges. Currently, therapeutic modalities for GC mainly encompass surgical resection, chemotherapy, radiotherapy, and targeted therapy[2]. Surgical resection is commonly employed for the treatment of early-stage GC; however, it lacks inhibitory effects on cancer cell metastasis and local growth. Given the inconspicuous early symptoms of GC, most patients are diagnosed during advanced stages of the disease. Although surgical intervention is an option for treatment, its efficacy in advanced stage GC patient’s remains limited[3]. The majority of advanced- stage GC patients are prone to experience cancer recurrence following surgery. Therefore, surgical intervention alone is insufficient for complete recovery, necessitating adjuvant chemotherapy and radiotherapy for treatment[4]. Currently, with the clinical use of chemotherapy agents and the varying response of patients to these agents due to differences in individual constitution, some patients have exhibited decreasing sensitivity to chemotherapy-induced Drug Resistance (DR)[5], resulting in therapeutic failures. Hence, gaining a comprehensive understanding of the mechanisms underlying GC DR and identifying novel therapeutic targets are of paramount importance for enhancing the survival rate of GC patients.
Tumor Multidrug Resistance (MDR) refers to the phenomenon where tumor cells develop resistance to multiple anticancer drugs when administered concurrently or alternately, leading to a great reduction in the therapeutic efficacy of these agents and impeding the achievement of anticipated outcomes[6]. Furthermore, MDR may contribute to tumor recurrence and metastasis. For patients with tumors prone to frequent relapse or metastasis, MDR substantially diminishes long- term survival rates. Chemotherapeutic agents for GC include 5-fluorouracil and cisplatin, which have demonstrated favorable therapeutic effects[7]. With advances in medical technology, drugs such as paclitaxel, irinotecan, and oxaliplatin have been incorporated into clinical treatment regimens for GC[8,9]. P-glycoprotein (P-gp), livin, and survivin are proteins closely associated with DR, playing pivotal roles in GC. P-gp, a prevalent transmembrane protein, is widely regarded as a principal mechanism underlying MDR. Elevated Expression Levels (ELs) of P-gp in tumor cells and certain normal tissues are commonly observed, resulting in reduced therapeutic responses of these cells to various anticancer drugs[10]. P-gp facilitates drug efflux across cell membranes, actively transporting drugs out of cells, thereby reducing intracellular drug concentrations and diminishing their cellular effects[11]. The upregulation of P-gp often arises as an adaptive response following prolonged exposure of cells to drugs. Studies have revealed that mutations in the P-gp gene or alterations in its EL may contribute to the development of MDR. To overcome P-gp-mediated MDR, scientists propose the use of P-gp inhibitors to suppress its activity, thereby enhancing intracellular drug concentrations[12]. Another strategy involves designing drugs with structures distinct from P-gp substrates to circumvent its efflux function. Livin and survivin are two proteins capable of inhibiting cell apoptosis. Cell apoptosis is a normal physiological process that assists the body in eliminating aging, damaged, or aberrant cells. However, in GC, the overexpression of livin and survivin can inhibit cell apoptosis, leading to aberrant proliferation and survival of tumor cells. Livin, an anti-apoptotic protein, exerts its function by interacting with proteins involved in cell apoptosis, preventing the occurrence of apoptosis[13]. Notably, livin can interact with dominant members such as caspases, inhibiting their activity and thereby suppressing the cascade of apoptotic reactions[14]. Survivin is a member of the inhibitor of apoptosis family and is often overexpressed in GC cells. Survivin interacts with apoptotic regulatory proteins like Bcl-2-associated X protein (BAX) and Smac, preventing their activation and thus inhibiting cell apoptosis[15]. Furthermore, survivin regulates key factors in apoptotic signaling pathways, such as caspases and apoptotic kinases, thereby modulating cell survival and death signals, ultimately promoting the survival of cancer cells.
In summary, the EL of P-gp, livin, and survivin is closely associated with DR in GC. A comprehensive investigation into the functional mechanisms of P-gp, livin, and survivin in GC can contribute to unraveling the molecular underpinnings of DR in this context, thereby offering novel targets and strategies for the treatment of GC.
Materials and Methods
Experimental materials and cell culture:
Human GC highly invasive metastatic Cell Line (CL) OCUM-2MD3 cells were utilized as experimental materials. These cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 15 % fetal bovine serum. Cell culture was maintained at 37° in a humidified atmosphere containing 5 % Carbon dioxide (CO2). For sub culturing, 0.25 % trypsin solution containing 0.53 mM Ethylene Diamine Tetra Acetic Acid (EDTA) was used to digest and passage the cells. During each passage, cells were seeded at 1×104 cells/well. To ensure cell viability, timely cryopreservation was conducted for long-term storage.
Establishment of OCUM-2MD3/Oxaliplatin (L-OHP) CL:
Human GC oxaliplatin-resistant CL OCUM- 2MD3/L-OHP was established through induction with L-OHP treatment on OCUM-2MD3 cells. An intermittent exposure experiment was employed using the Half Maximal Inhibitory Concentration (IC50) of L-OHP (1.92 μg/ml) for a 24 h duration. The experimental procedure was conducted as follows; logarithmic growth phase cells were exposed to DMEM culture medium containing 1.92 μg/ml L-OHP. After 24 h of exposure, the culture medium was discarded; cells were washed three times with 0.01 mol/l pH 7.4 Phosphate Buffer Saline (PBS) and subsequently replenished with fresh culture medium. Upon cellular recovery and regrowth, the culture medium containing L-OHP (1.92 μg/ml) was applied again for a 24 h incubation. This cycle was repeated iteratively. During the induction process, sensitive cells exhibited gradual cell death, while resistant cells continued to proliferate; following 6 mo of treatment, cells were maintained in drug-free culture medium for a duration of 2 w, after which the stable state of the half-maximal IC50 was assessed using the Cell Counting Kit-8 (CCK-8) assay and cells were further cultured in drug-free medium, and cryopreservation was performed using cell culture medium containing 10 % Dimethyl Sulfoxide (DMSO). Post-cryopreservation, IC50 values were reassessed, and the persistence of resistance were confirmed if the results remained consistent. All experiments assessing DR were conducted after a 2 w culture period in drug-free medium.
Cell observation of OCUM-2MD3/L-OHP CL:
When the OCUM-2MD3/L-OHP CL was cultured to logarithmic phase, its morphology was visualized with an inverted microscope.
Analysis of cell growth cycle and apoptosis of OCUM-2MD3/L-OHP CL by flow cytometry:
OCUM-2MD3/L-OHP CL was collected and subjected to flow cytometric analysis. Collected cells at 1×106 cells/ml were rinsed with PBS and subsequently fixed overnight in 70 % cold ethanol. After fixation, cells were washed with PBS, and 10 % chicken red blood cells were added as an internal reference for analysis. Subsequently, 1 ml of 50 mg/l Propidium Iodide (PI) was applied, and samples were incubated at 4° for staining. Following staining, the samples were filtered through a 500-mesh copper grid and then analyzed using flow cytometry to assess cell cycle progression and apoptosis.
Detection of drug sensitivity of OCUM-2MD3/L- OHP CL by CCK-8:
Cell sensitivity to the drug was determined using the CCK-8 assay. OCUM-2MD3/L-OHP cells cultured in normal cell culture dishes were digested, collected, and resuspended. Cell counting was performed by resuspending 10 μl of the cell suspension, and then cells were seeded into 96- well plates at a density of ≥5×104 cells/well, with approximately 100 μl of cell suspension applied to each well. Five replicate wells were established. Cells were cultured adherently in a cell culture incubator for 4 h. Subsequently, L-OHP was added to each well at final concentrations of 0.3, 0.6, 1.25, 2.5, 5, 10, and 20 μg/ml. Equal volumes of PBS were applied to designated wells as control groups, while wells without any substance added served as the blank group. The 96-well plate was then cultured in the cell culture incubator for 24 h. Following incubation, the supernatant was removed, and 10 μl of CCK-8 solution was applied to each well. The plate was then returned to the incubator and incubated for an additional 30 min for enzymatic reaction. Absorbance was measured at Optical Density (OD) (450-490) nm employing a microplate reader. After measurement, the plate was returned to the incubator for further incubation, and absorbance values were subsequently measured at 30 min intervals for a total of 6 times. The calculation equations are as follows:
Inhibition rate=(1-Amedication group/Acell control group)×100 % (1)
RI=IC50(resistant cells)/IC50(parental cells) (2)
Detection of DR in OCUM-2MD3/L-OHP CL:
A cell suspension with a density of 5×104 cells/ml (200 µl) was applied to each well of a 96-well plate. Subsequently, culture media containing seven chemotherapeutic agents, namely L-OHP, CBDCA, ADM, CDDP, 5-Fu, GEM, and IH, were applied to the culture wells. The final concentrations of these drugs in the medium were set as follows; 5.0, 680.0, 6.0, 13.0, 40.0, 66.0, and 74.0 µg/ml, with each concentration tested in five replicate wells.
The experimental procedure followed the same steps as described in the aforementioned CCK-8 assay.
Western blot analysis:
Protein collection was as follows; OCUM-2MD3 and OCUM-2MD3/L-OHP cells were lysed using a cell lysis buffer containing 20 mM Tris (pH 7.5), 150 mM Sodium chloride (NaCl), and 1 % Triton X-100. Protein extraction of P-gp, livin, and survivin from cells was implemented using a protein extraction kit (PROTTOT-1KT), following the manufacturers protocol rigorously. The tumor-related proteins extracted in the previous step were loaded onto polyacrylamide gel electrophoresis gels. Each well was loaded according to concentration, with a minimum of 40 µg of protein per well. After electrophoresis, the gel was transferred onto a membrane, followed by membrane washing (3×Tris Buffered Saline Tween-20 (TBST)). Subsequently, the membrane was blocked using 5 % skim milk, and then incubated with primary antibodies against P-gp, livin, and survivin at 4°. After incubation, membrane was rinsed (3×TBST) and incubated with a secondary antibody labeled with horseradish peroxidase at room temperature. Following further washing, infrared detection was applied to the membrane, and the grayscale values were analyzed.
Data collection and analysis:
The data from this experiment were organized using Microsoft Excel, and subsequent analysis was conducted using Statistical Package for the Social Sciences (SPSS) 22.0. Results were presented as mean±standard deviation. Inter-group comparisons were performed using a t-test, with statistical significance set at p<0.05.
Results and Discussion
Observation of OCUM-2MD3 and OCUM-2MD3/ L-OHP cells was conducted using an inverted microscope. When cultured in suspension, both CLs displayed a rounded morphology, with drastic variations in cell size. Under adherent conditions, the cells exhibited a spindle-shaped morphology, arranged in a monolayer within the culture dish. Transmission electron microscopy revealed that OCUM-2MD3 CL exhibited normal organelle morphology and distinct nuclei, whereas the OCUM-2MD3/L-OHP CL displayed evident apoptotic changes. The surface microvilli of OCUM-2MD3/L-OHP cells were reduced in length and density. Numerous vacuoles were observed in the cytoplasmic matrix, and ribosomes were observed to detach from the rough endoplasmic reticulum.
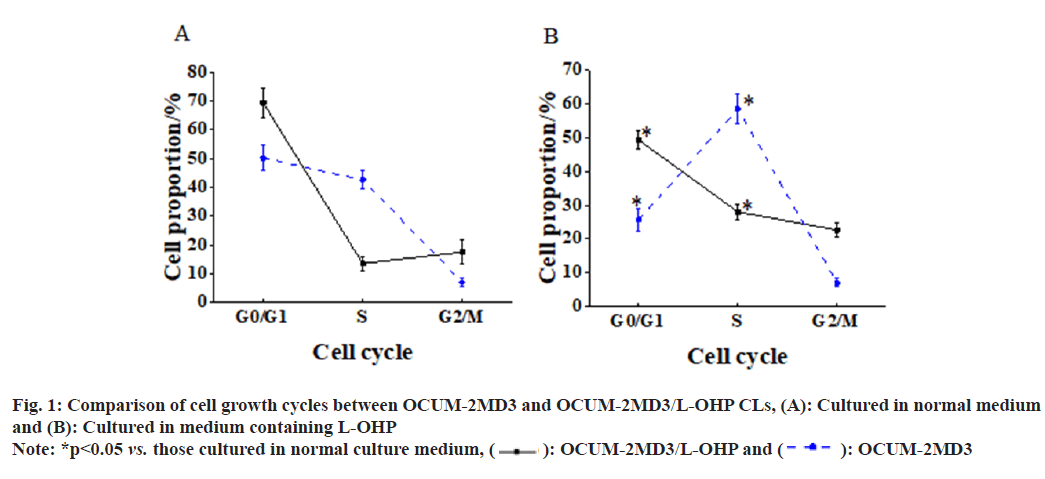
Under normal culture conditions, relative to OCUM-2MD3 CL, the OCUM-2MD3/L-OHP CL exhibited a distribution of cells in the cell cycle phases as follows; (69.34±5.23) % in G0/G1 phase, (13.56±2.36) % in S phase, and (17.53±4.15) % in G2/M phase. Following a 24 h culture in L-OHP- containing medium, a distinct shift in cell cycle distribution was observed in the OCUM-2MD3/ L-OHP CL vs. cells cultured in normal medium. A notable reduction in cells in G0/G1 phase [(49.24±2.68) % vs. (69.34±5.23) %, p<0.05], a notable increase in cells in S phase [(28.02±2.35) % vs. (13.56±2.36) %, p<0.05], and an elevated proportion of cells in G2/M phase [(22.63±2.19) % vs. (17.53±4.15), p<0.05]. In comparison to the CL cultured in normal medium, the OCUM- 2MD3 CL exhibited a distinct alteration in cell cycle distribution. A considerable decrease in cells in G0/G1 phase [(25.63±3.26) % vs. (50.24±4.37) %, p<0.05], a notable increase in cells in S phase [(58.52±4.24) % vs.(42.62±3.26)%, p<0.05], and a similar elevation in the proportion of cells in G2/M phase [(7.03±1.24)% vs. (7.03±1.24), p<0.05] as shown in fig. 1.
The apoptotic rates of both CLs were observed after 24 h of culture in L-OHP containing medium. The results revealed that, in comparison to the CL cultured in normal medium, both CLs exhibited an increase in apoptotic rates. Notably, the apoptotic rate of the OCUM-2MD3/L-OHP CL was notably inferior to that of the OCUM-2MD3 CL [(27.24±1.24) % vs. (36.62±1.35) %, p<0.05] as shown in fig. 2. +
Testing with varying concentrations of L-OHP revealed that higher concentrations of L-OHP markedly enhanced the growth inhibition of both OCUM-2MD3 and OCUM-2MD3/L-OHP CLs. Additionally, it was observed that at the same concentration of L-OHP, the growth inhibition rate was lower for the OCUM-2MD3/L-OHP CL versus the OCUM-2MD3 CL as shown in fig. 3.
Based on the calculation, the IC50 values of OCUM- 2MD3 and OCUM-2MD3/L-OHP CLs for L-OHP were determined to be 2.02 µg/ml and 8.63 µg/ml, respectively. Therefore, the RI of OCUM-2MD3/ L-OHP CL was calculated to be 4.77 as shown in fig. 4.
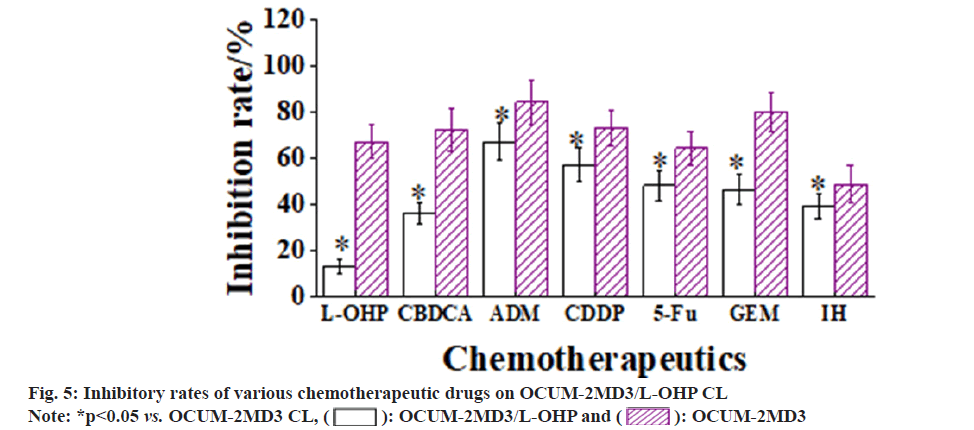
The resistance rates of two CLs were assessed using a culture medium containing seven chemotherapy drugs; L-OHP, CBDCA, ADM, CDDP, 5-Fu, GEM, and IH. The results revealed that the inhibitory rates of all chemotherapy drugs were markedly higher for the OCUM-2MD3 CL vs. OCUM- 2MD3/L-OHP CL. The parent CL exhibited resistance to IH, and the OCUM-2MD3/L-OHP CL demonstrated strong resistance to L-OHP. Moreover, the sensitivity of OCUM-2MD3/L-OHP cells to CBDCA, ADM, CDDP, 5-Fu, GEM, and IH chemotherapy drugs was markedly reduced.
Notably, the OCUM-2MD3/L-OHP CL exhibited cross-resistance to CBDCA, 5-Fu, GEM, and IH drugs as shown in fig. 5.
The results revealed that, when cultured in normal medium, the EL of P-gp in OCUM-2MD3/L-OHP CL was greatly superior to that in OCUM-2MD3 CL. The EL of livin showed no considerable difference between two CLs. However, the EL of survivin in OCUM-2MD3/L-OHP CL was notably inferior to that in OCUM-2MD3 CL. After 24 h of culture in L-OHP-containing medium, both CLs exhibited reduced ELs of P-gp, livin, and survivin. In OCUM-2MD3/L-OHP CL, the ELs of P-gp and livin remained markedly higher than OCUM- 2MD3 CL, while the EL of survivin was notably lower in OCUM-2MD3/L-OHP CL relative to OCUM-2MD3 CL as shown in fig. 6-fig. 8.
Despite great advancements in recent decades, the treatment of GC continues to face numerous challenges. MDR is a phenomenon frequently observed after prolonged chemotherapy, leading to refractory cancers and tumor recurrence. MDR in patients can be categorized into two types; inherent resistance to specific drugs and acquired resistance that develops during chemotherapy. Studies have indicated that functional proteins in the cell membrane and nuclear envelope undergo aberrant expression in MDR patients. Furthermore, certain proteins or enzymes within the cytoplasm and nucleus experience altered expression and activity, potentially triggering the activation of oncogenes or causing tumor suppressor genes to lose their function, ultimately leading to overexpression of anti-apoptotic genes[16].
P-gp, encoded by the human ABCB1 gene, is an efflux transporter protein belonging to the Adenosine Triphosphate (ATP)-binding cassette membrane transporter superfamily. It is intricately linked to the pharmacokinetics of various compounds. Research has revealed elevated ELs of P-gp in multiple normal tissues, including the apical membrane of proximal tubules in the kidney[17]. The functions of P-gp encompass include restricting the absorption of orally administered drugs from the intestine into systemic circulation, limiting drug penetration through the blood- brain barrier, and facilitating the hepatic, biliary, and renal excretion of drugs[18]. These activities are reliant on the hydrolytic energy of ATP and constitute an active, energy-consuming process. P-gp exhibits broad substrate specificity, capable of transporting diverse chemically distinct drugs, including antibiotics, anticancer agents, hormones, and antiepileptic drugs, among others[19]. The EL of P-gp is associated with tissue and cellular metabolism, proliferation, and differentiation functions. It has the potential to alleviate caspase- dependent apoptosis induced by death receptor Fas-Ligand (Fas-L), radiation, and Tumor Necrosis Factor (TNF), suggesting a close correlation between P-gp and cellular apoptosis mechanisms. In this study, after administering L-OHP to OCUM-2MD3/L-OHP and OCUM-2MD3 CLs for 24 h, a decrease in P-gp expression was observed in both CLs, accompanied by evidence of tumor cell apoptosis. These findings suggest that inhibiting P-gp expression can promote tumor cell apoptosis. Furthermore, it was noted that the EL of P-gp in OCUM-2MD3/L-OHP CL was higher versus OCUM-2MD3 CL, and the apoptotic rate in OCUM-2MD3/L-OHP cells exposed to L-OHP was notably inferior to that of OCUM-2MD3 cells. According to this study and previous studies, elevated levels of P-gp can inhibit tumor cell apoptosis. This aligns with the findings of Jiang et al.[20], who demonstrated that reducing P-gp expression in tumor cells enhances the sensitivity of GC cells to chemotherapy drugs.
Livin, an apoptosis inhibitor protein, can bind to caspases, thereby inhibiting caspase activity and subsequently blocking the transmission of the Fas/Fas-L signaling. This inhibition of apoptotic processes leads to abnormal cell numbers and contributes to tumorigenesis. In the study by Chung et al.[21], it was demonstrated that livin promotes proliferation of ovarian cancer cells by activating the transcriptional co-activator YAP, a critical component of the Hippo signaling. This suggests a role for livin in the growth and invasion of cancer cells both in vitro and in vivo. Other research has indicated that knockdown of the livin gene may result in decreased levels of cell cycle proteins cyclin D1, cyclin-dependent kinases 4 and 6, and increased EL of p21 and p27, inducing cell cycle arrest. Additionally, livin knockdown can inhibit the ERK1/2 and JNK pathways, implying an upregulation of Livin at both mRNA and protein levels in cancerous tissues[22]. In this study, it was observed that both OCUM-2MD3/L- OHP and OCUM-2MD3 CLs exhibited EL of livin, with similar levels of expression in both CLs. This suggests a potential role of the livin gene in the mechanism of MDR in OCUM-2MD3 cells. In a study focusing on the prognosis of stage IIb GC, it was indicated that modulation of livin expression could enhance apoptosis in GC cells. Following 24 h treatment with L-OHP in both OCUM-2MD3/L- OHP and OCUM-2MD3 CLs, the results revealed a remarkable increase in apoptotic rates in both CLs, consistent with the findings reported by Qi et al.[23]. The EL of livin in OCUM-2MD3/L-OHP cells was greatly superior to OCUM-2MD3 cells, and the apoptotic rate was also markedly reduced. This suggests that upregulation of livin expression may inhibit apoptosis in GC cells. While no marked difference was indicated in livin expression between the drug-resistant CL and the parental CL under normal culture conditions, livin ELs markedly increased in the drug-resistant CL after treatment with L-OHP-containing medium. This elevation in livin expression might be attributed to the higher EL of P-gp in OCUM-2MD3/L-OHP cells, which could lead to reduced intracellular levels of L-OHP. As a result, the EL of livin was not suppressed, potentially contributing to an elevated level of livin expression within the cells.
Survivin is also a member of the apoptosis inhibitor protein family; however, survivin is scarcely detectable in normal tissues except for placenta, thymus and germ cells. Nevertheless, it is found to be overexpressed in all malignant tumors. Survivin can inhibit apoptosis and promote cell proliferation, and its anti-apoptotic function appears to be linked to caspases[24]. Survivin ELs were examined in drug-resistant and parental CLs without exposure to L-OHP-containing medium. In comparison to OCUM-2MD3 cells, EL of survivin was markedly reduced in OCUM-2MD3/L-OHP cells, suggesting an association between survivin and the mechanism of multi-DR in OCUM-2MD3 cells. In a study investigating DR in osteosarcoma, it was found that survivin expression was markedly elevated in tumor tissues of chemoresistant osteosarcoma patients compared to chemo sensitive patients. Additionally, the knockout of survivin in osteosarcoma cells markedly inhibited cell proliferation and chemo resistance both in vitro and in vivo, indicating that survivin expression contributes to DR in osteosarcoma[25,26]. In this study, the apoptosis rate of OCUM-2MD3/L-OHP cells was greatly superior to OCUM-2MD3 cells, indicating that low levels of survivin can promote apoptosis in GC cells. Research has shown that overexpression of survivin can induce the transition of the cell cycle to the S phase, while knocking out the survivin gene can induce cell cycle arrest and apoptosis[27]. Following treatment with L-OHP for 24 h in both CLs, the EL of survivin decreased in both CLs, accompanied by a notable increase in apoptosis rate. This finding further supports the notion that low levels of survivin can enhance cell apoptosis. This phenomenon may also be related to the elevated EL of P-gp; the high intracellular EL of P-gp could lead to reduced levels of L-OHP within the cells, thereby diminishing the inhibitory effect on the survivin gene and consequently suppressing apoptosis.
The above findings indicate a close association between the upregulation of P-gp, livin, and survivin expression and the DR of human GC CL OCUM- 2MD3/L-OHP. P-gp functions by increasing the efflux of platinum-based chemotherapeutic agents from cells, leading to insufficient drug accumulation within the cells and consequently enhancing DR. The elevated EL of livin and survivin may contribute to DR by inhibiting apoptosis, promoting tumor cell proliferation, and enhancing cell survival. This contributes to a deeper understanding of underlying mechanisms of GC development and offers potential targets for novel therapeutic strategies.
The notable upregulation of P-gp, livin, and survivin expression is closely associated with the DR of human GC CL OCUM-2MD3/L-OHP. A theoretical foundation was laid for finding novel targeted therapeutic strategies against P-gp, livin, and survivin, thereby potentially enhancing clinical outcomes for GC patients. In clinical practice, considering the ELs of P-gp, livin, and survivin may aid in predicting patient responses and resistance to chemotherapy drugs. Further research will contribute to a deeper understanding of the mechanistic roles of these molecules in GC DR.
Conflict of interests:
The authors declared no conflict of interests.
References
- Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J 2021;134(7):783-91.
[Google Scholar] [PubMed] [Crossref]
- Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci 2020;21(11):4012.
[Google Scholar] [PubMed] [Crossref]
- Douda L, Cyrany J, Tachecí I. Early gastric cancer. Vnitr Lek 2022;68(6):371-5.
- Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 vs. adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): An open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol 2021;22(8):1081-92.
[Google Scholar] [PubMed] [Crossref]
- Huang WJ, Ruan S, Wen F, Lu XN, Gu SP, Chen XX, et al. Multidrug resistance of gastric cancer: The mechanisms and chinese medicine reversal agents. Cancer Manag Res 2020;12:12385-94.
[Google Scholar] [PubMed] [Crossref]
- Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 2020;21(9):3233.
- Coen JJ, Zhang P, Saylor PJ, Lee CT, Wu CL, Parker W, et al. Bladder preservation with twice-a-day radiation plus fluorouracil/cisplatin or once daily radiation plus gemcitabine for muscle-invasive bladder cancer: NRG/RTOG 0712-A randomized phase ii trial. J Clin Oncol 2019;37(1):44-51.
[Google Scholar] [PubMed] [Crossref]
- Li WZ, Lv X, Hu D, Lv SH, Liu GY, Liang H, et al. Effect of induction chemotherapy with paclitaxel, cisplatin, and capecitabine vs. cisplatin and fluorouracil on failure-free survival for patients with stage iva to ivb nasopharyngeal carcinoma: A multicenter phase 3 randomized clinical trial. JAMA Oncol 2022;8(5):706-14.
[Google Scholar] [PubMed] [Crossref]
- Cui Z, Zhang J, Zhang J, Xu L. Evaluation of IgG, IgM, CD4+ and CD8+ T cells during neoadjuvant chemotherapy with Tezio and Apatinib in gastric cancer patients. Cell Mol Biol 2020;66(3):113-8.
[Google Scholar] [PubMed]
- Cabaud O, Berger L, Crompot E, Adélaide J, Finetti P, Garnier S, et al. Overcoming resistance to anti-nectin-4 antibody-drug conjugate. Mol Cancer Ther 2022;21(7):1227-35.
[Google Scholar] [PubMed] [Crossref]
- Robinson K, Tiriveedhi V. Perplexing role of P-glycoprotein in tumor microenvironment. Front Oncol 2020;10:265.
- Zhang H, Xu H, Ashby CR Jr, Assaraf YG, Chen ZS, Liu HM. Chemical molecular-based approach to overcome multidrug resistance in cancer by targeting P-glycoprotein (P-gp). Med Res Rev 2021;41(1):525-55.
[Google Scholar] [PubMed] [Crossref]
- Jin Q, Lin C, Zhu X, Cao Y, Guo C, Wang L. 125I seeds irradiation inhibits tumor growth and induces apoptosis by Ki-67, P21, survivin, livin and caspase-9 expression in lung carcinoma xenografts. Radiat Oncol 2020;15(1):238.
[Google Scholar] [PubMed] [Crossref]
- Wu SQ, Xu QB, Sheng WY, Su LY, Zhu LW. A novel role for Livin in the response to ultraviolet B radiation and pterygium development. Int J Mol Med 2020;45(4):1103-11.
[Google Scholar] [PubMed] [Crossref]
- Ismail Z, Dam J, Penny C, de Koning CB, Harmse L. Copper-imidazo[1,2-a]pyridines differentially modulate pro- and anti-apoptotic protein and gene expression in HL-60 and K562 leukaemic cells to cause apoptotic cell death. Biochim Biophys Acta Mol Cell Res 2022;1869(1):119160.
[Google Scholar] [PubMed] [Crossref]
- Zhang C, Sui X, Jiang Y, Wang X, Wang S. Antitumor effects of icaritin and the molecular mechanisms. Discov Med 2020;29(156):5-16.
[Google Scholar] [PubMed]
- Elmeliegy M, Vourvahis M, Guo C, Wang DD. Effect of P-glycoprotein (P-gp) inducers on exposure of P-gp substrates: Review of clinical drug-drug interaction studies. Clin Pharmacokinet 2020;59(6):699-714.
[Google Scholar] [PubMed] [Crossref]
- Ahmed Juvale II, Abdul Hamid AA, Abd Halim KB, Che Has AT. P-glycoprotein: New insights into structure, physiological function, regulation and alterations in disease. Heliyon 2022;8(6):e09777.
[Google Scholar] [PubMed] [Crossref]
- Angelini A, Di Ilio C, Castellani ML, Conti P, Cuccurullo F. Modulation of multidrug resistance p-glycoprotein activity by flavonoids and honokiol in human doxorubicin-resistant sarcoma cells (MES-SA/DX-5): Implications for natural sedatives as chemosensitizing agents in cancer therapy. J Biol Regul Homeost Agents 2010;24(2):197-205.
[Google Scholar] [PubMed]
- Jiang L, Zhang Y, Guo L, Liu C, Wang P, Ren W. Exosomal microRNA-107 reverses chemotherapeutic drug resistance of gastric cancer cells through HMGA2/mTOR/P-gp pathway. BMC Cancer 2021;21(1):1290.
[Google Scholar] [PubMed] [Crossref]
- Chung CY, Park YL, Kim N, Park HC, Park HB, Myung DS, et al. Expression and prognostic significance of livin in gastric cancer. Oncol Rep 2013;30(5):2520-8.
[Google Scholar] [PubMed] [Crossref]
- Gao J, Han W, He Y, Zhou J, Miao J, Zhang G. Livin promotes tumor progression through YAP activation in ovarian cancer. Am J Cancer Res 2020;10(10):3179-93.
[Google Scholar] [PubMed]
- Qi J, Xie FJ, Liu S, Yao CY, Liu WH, Cai GQ, et al. Huaier granule combined with tegafur gimeracil oteracil potassium promotes stage iib gastric cancer prognosis and induces gastric cancer cell apoptosis by regulating livin. Biomed Res Int 2020;2020:2403595.
[Google Scholar] [PubMed] [Crossref]
- Shojaei F, Yazdani-Nafchi F, Banitalebi-Dehkordi M, Chehelgerdi M, Khorramian-Ghahfarokhi M. Trace of survivin in cancer. Eur J Cancer Prev 2019;28(4):365-72.
[Google Scholar] [PubMed] [Crossref]
- Wei D, Li C, Ye J, Xiang F, Xu Y, Liu J. Codelivery of survivin inhibitor and chemotherapeutics by tumor-derived microparticles to reverse multidrug resistance in osteosarcoma. Cell Biol Int 2021;45(2):382-93.
[Google Scholar] [PubMed] [Crossref]
- Hu Y, Wang H, Zhang J, Wu J. microRNA-218 inhibits the growth and invasion of osteosarcoma cells by targeting survivor. Acta Med Mediterr 2020;36(3):1427-33.
- Chen Y, Bieerkehazhi S, Li X, Ma L, Yibulayin W, Ran J. Survivin regulates bad gene expression by binding to its promoter and modulates cell cycle and apoptosis in esophageal carcinoma cell. J Oncol 2021;2021:1384289.
[Google Scholar] [PubMed] [Crossref]
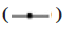 : OCUM-2MD3/L-OHP and
: OCUM-2MD3/L-OHP and  : OCUM-2MD3
: OCUM-2MD3
 : Normal culture medium and
: Normal culture medium and  : L-OHP containing medium
: L-OHP containing medium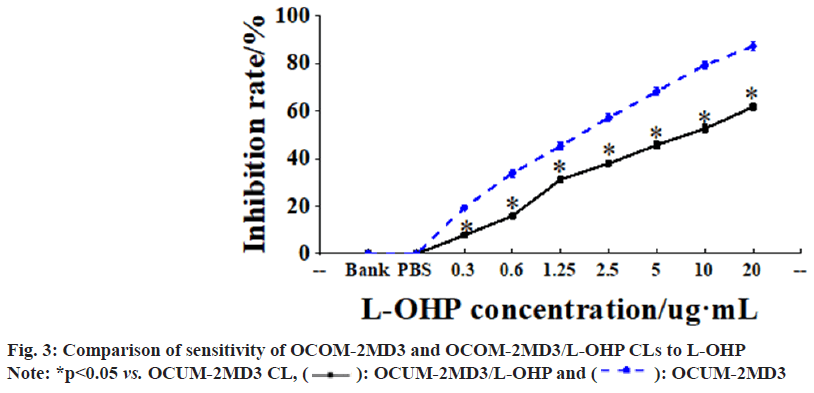
 : OCUM-2MD3/L-OHP and
: OCUM-2MD3/L-OHP and  : OCUM-2MD3
: OCUM-2MD3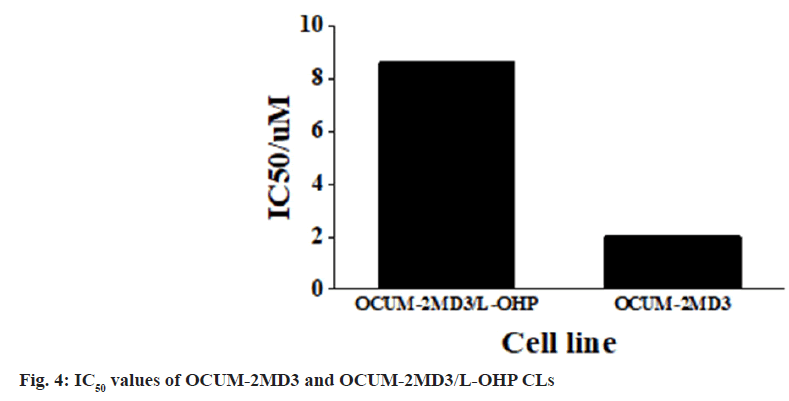
 : OCUM-2MD3/L-OHP and
: OCUM-2MD3/L-OHP and  : OCUM-2MD3
: OCUM-2MD3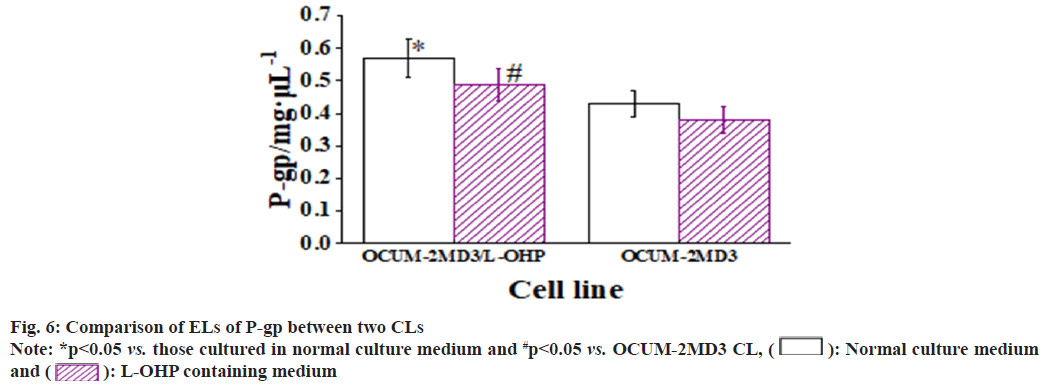
 : Normal culture medium and
: Normal culture medium and  : L-OHP containing medium
: L-OHP containing medium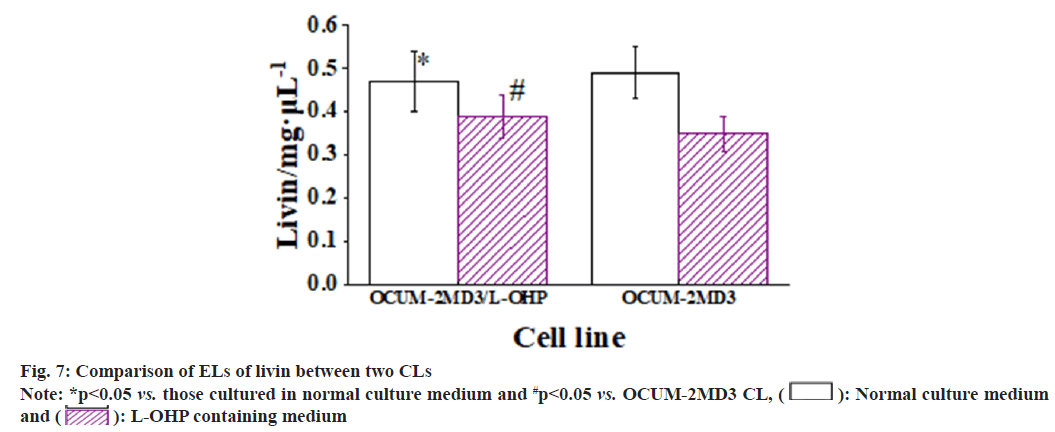
 : Normal culture medium and
: Normal culture medium and  : L-OHP containing medium
: L-OHP containing medium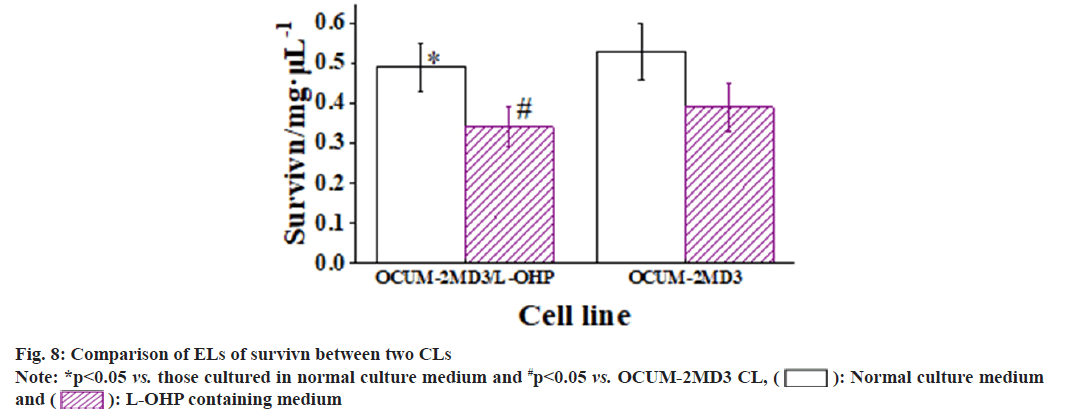
 : Normal culture medium and
: Normal culture medium and  : L-OHP containing medium
: L-OHP containing medium



