- *Corresponding Author:
- Z. F. Yu
Department of Intensive Care Unit, Hangzhou Traditional Chinese Medicine Hospital Affiliated to Zhejiang Chinese Medical University, Hangzhou, Zhejiang Province 310005,
China
E-mail: yepoyun078143@163.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “136-140” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the value and role of serum interleukin-35 and lymphocyte/monocyte ratio for the prognostic assessment of sepsis cases is the main objective of the study. 78 sepsis patients sera was collected from October 2019 to October 2021 in our intensive care unit and were classified as survival (n=45) and death (n=33) groups based on their prognosis. In addition, 50 normal healthy subjects were also enrolled as control. Then, interleukin-35 contents in serum and lymphocyte/monocyte ratio of different groups were measured separately. We also analyzed the association of serum interleukin-35 with lymphocyte/ monocyte ratio among 78 sepsis cases and performed survival analysis on the serum interleukin-35 levels. Interleukin-35 expression of survival group increased relative to normal group, with no significant difference (p>0.05). Death group had markedly increased interleukin-35 expression compared with survival and normal groups (p<0.05) and difference in the lymphocyte/monocyte ratio values between death and survival groups was of no statistical significance (p>0.05). Interleukin-35 was not evidently correlated with lymphocyte/monocyte ratio (p>0.05). Survival analysis showed that sepsis cases with high interleukin-35 expression had significantly decreased survival, relative to low interleukin-35 expression (p<0.05). Serum interleukin-35 is the prognostic biomarker for sepsis cases and high interleukin-35 expression during early sepsis may indicate poor prognosis and the concentration of interleukin-35 levels has a high prognostic assessment value for sepsis survival compared with lymphocyte/monocyte ratio levels.
Keywords
Sepsis, interleukin-35, prognosis, lymphocyte/monocyte ratio
Sepsis represents the complicated disease with the features of oxidative injury or even multi-organ dysfunction[1]. Its pathophysiology includes impaired epithelial, endothelial and immune cell function. The mortality rate of sepsis recently is 18 %-40 %, which is related to sepsis resulting from infection with sepsis-associated organisms (e.g., bacteria and fungi). Different factors have determined sepsis as well as its severity, such as pathogen type as well as immune status in the host[2].
The Lymphocyte/Monocyte Ratio (LMR) is a combination of two independent markers of inflammation in blood cells and LMR is now considered a new marker of systemic inflammation that is closely associated with the prognosis of most infectious diseases and malignancies[3]. In addition, the inflammatory response of the body activates immune cells to secrete cytokines such as Interleukin-35 (IL-35) [4]. As reported in studies, IL-35 up-regulation is related to severity of infectious diseases and the production of cytokine storms, which can be an important predictor of disease regression[5,6]. Consequently, the present work focused on investigating whether LMR and serum IL- 35 levels in sepsis blood had prognosis, prediction and significance, and by retrospectively analyzing them in order to provide a reference for clinical management.
Materials and Methods
Study objects:
This study include 78 patients diagnosed with sepsis between October 2019 and October 2021 from our center, including 59 male and 19 female patients, with the age of 25-90 y and with a mean age of 69.2 y. Exclusion criteria includes cancer patients; cases with severe congenital diseases or malformations; patients with a history of mental illness. 50 serum specimens from elderly people who were confirmed to be healthy in our hospital were enrolled for control, which include 28 male together with 22 female patients with the age of 20-80 y and with a mean age of 58.6 y. General data was comparable to both groups (p>0.05).
Specimen collection and testing of indexes:
3 ml of venous blood at fasting state was drawn in the morning on 1st, 3rd and 7th d of admission, and the serum was separated by centrifugation within 2 h, followed by preservation under -80°. IL-35 content in serum was measured by quantitative electrochemiluminescence analysis using an E170 electrochemiluminescence autoimmunoassay (Roche, Switzerland) and the IL-35 kit was purchased from Roche, Switzerland. The blood cells were counted using a fully automated hematology analyzer (Shenzhen Myriad Biomedical Electronics Co., Ltd., BC-5000) and the absolute values of lymphocytes, i.e. Absolute Lymphocyte Count (ALC) and monocytes, i.e. Absolute Monocyte Count (AMC) were recorded, and the LMR values were calculated.
Statistical analysis:
This work employed Statistical Package for the Social Sciences (SPSS) 23.0 software in statistical processing. Measures were represented by mean±Standard Deviation (SD), while the Levene’s chi-square test for normal continuity was performed first and the results were represented by mean±SD. Data regarding general conditions, inflammatory indexes and serum growth chemokine IL-35 levels in each group were expressed using median and interquartile spacing. T-test was conducted for comparison of LMR levels between the survival and death groups. Correlation between serum IL-35 and LMR was analyzed through linear regression correlation. Differences were considered statistically significant at a two-sided p<0.05.
Results and Discussion
All cases were classified as death (n=33) and survival (n=45) groups based on the prognosis, sex, age and positive microbiological test rate were not significantly different between them (p>0.05), and death group had increased organ failure number relative to survival group (p=0.001) (Table 1).
| Grouping | Gender (Male/Female) | Age (y) | Number of organ failures | Number of positive microbial culture cases |
|---|---|---|---|---|
| Death group (n=33) | 22/11 | 63.1±7.4 | 3.1±0.9 | 16 |
| Survival group (n=45) | 26/19 | 64.4±6.9 | 1.3±0.2 | 14 |
| p value | 0.698 | 0.576 | 0.001 | 0.236 |
Table 1: General Conditions of the Survival and Death Groups
Comparison of inflammatory indexes between the survival and death groups was explained here. LMR, White Blood Cells (WBC), C-Reactive Protein (CRP) and temperature of the death group on d 1 of admission were not statistically different compared with survival group (p>0.05) (Table 2). Differences in LMR, CRP and body temperature were not significantly different between 2 groups on d 3 of admission (p>0.05) (Table 3).
| Test items | Survival group (n=45) | Death group (n=33) | p value |
|---|---|---|---|
| LMR | 5.5±0.9 | 5.7±0.6 | 0.244 |
| CRP (mg/dl) | 57.5 (24.1-113.8) | 61.7 (32.4-121.8) | 0.889 |
| WBC (×109/l)) | 14.3±9.6 | 14.6±6.5 | 0.944 |
| Body temperature (°) | 37.9±0.8 | 38.6±1.2 | 0.967 |
Table 2: Inflammatory Indexes of Survival and Death Groups on D 1 of Hospital Admission
| Test items | Survival group (n=45) | Death group (n=33) | p value |
|---|---|---|---|
| LMR | 5.5±0.8 | 5.4±0.9 | 0.563 |
| CRP (mg/dl) | 67.4 (21.5~153.8) | 70.1 (30.3~172.8) | 0.822 |
| WBC (×109/l)) | 13.4±6.1 | 14.6±5.8 | 0.569 |
| Body temperature (°) | 37.9±0.9 | 38.5±1.2 | 0.794 |
Table 3: Inflammatory Indexes of Survival and Death Groups on D 3 of Hospital Admission
Comparison of IL-35 expression between survival and death groups was explained here. Serum IL-35 concentration on d 1 of admission in the survival group was not significantly different from control (p=0.578), while death group had increased serum IL-35 concentration compared with control (p=0.004). Death group had remarkably elevated IL-35 concentrations in serum compared with survival groups at 1 d, 3 d and 7 d after admission (p=0.002, p=0.005, p=0.001) as shown in Table 4.
| Median (pg/ml) | Control | Survivors | p | Non-survivors | p |
|---|---|---|---|---|---|
| 1st d | 40.8 (20.3-51.1) | 57.2 (27.6-69.8) | 0.578 | 118.4 (45.6-134.8) | 0.004 |
| 1st d | 57.2 (27.6-69.8) | 118.4 (45.6-134.8) | 0.002 | ||
| 3rd d | 53.9 (23.4-61.2) | 238.9 (146.7-1659.4) | 0.005 | ||
| 7th d | 62.9 (32.4-79.2) | 489.4 (198.3-2123.1) | 0.001 |
Table 4: Changes of Serum IL-35 Concentration between 2 Groups
Comparison of LMR levels in the survival and death groups was clearly explained. The differences in LMR values on d 1, d 3 and d 7 of admission has no significance between death and survival groups (p>0.05) as shown in Table 5.
| Time | Survivors (n=25) | Non-survivors (n=30) | t | p |
|---|---|---|---|---|
| 1st d | 9.3±2.5 | 11.7±2.6 | 0.824 | 0.444 |
| 3rd d | 8.1±1.3 | 12.9±2.2 | 0.394 | 0.728 |
| 7th d | 7.8±1.1 | 13.3±4.2 | 0.324 | 0.726 |
Table 5: Comparison of LMR Levels on D 1, D 3 and D 7 of Onset in the Sepsis Survival and Death Groups
Association of serum IL-35 expression with LMR value was clearly explained. Pearson correlation analysis revealed that IL-35 was not related to LMR levels on d 1, d 3 and d 7 of admission (p>0.05) as shown in Table 6.
| Time | IL-35 (pg/ml) | LMR (ng/ml) | r | p |
|---|---|---|---|---|
| 1st d | 90.5 | 4.6 | -0.199 | 0.812 |
| 3rd d | 129.8 | 6.5 | -0.167 | 0.688 |
| 7th d | 346.4 | 9.5 | -0.288 | 0.422 |
Table 6: Correlation between Serum IL-35 and LMR on D 1, D 3 and D 7 in the Survival Group and Death Group
Survival analysis of patients with sepsis was clearly explained. 78 sepsis cases were classified as high- IL-35 (n=46) or low-IL-35 (n=32) group based on their median expression (56.01 (pg/ml)) and survival analysis showed that the survival of sepsis patients with high-IL-35 expression markedly decreased compared with patients with low-IL-35 expression (p=0.009). As for 28 d survival rates, they were 57.5 % and 26.4 % in low-IL-35-expression and high-IL-35-expression groups, separately (fig. 1).
Sepsis is a systemic disease caused by infection, also known clinically as systemic inflammatory syndrome and is one of the common critical emergencies in intensive care units, which is characterized by variable disease, rapid progression and high mortality[7]. Since the early symptoms of the disease are not obvious and although it can be diagnosed by blood culture, it is time-consuming and labor-intensive and it is more difficult for the early diagnosis of sepsis[8]. Therefore, it is important to find an early diagnosis method with high sensitivity and specificity for improving sepsis prognosis, which is also a hot spot and focus of clinical research at present.
LMR indicates the ALC to AMC, where lymphocytes reflect immune system regulation, while monocytes reflect the non-specific inflammatory response of the body, linking the intrinsic and adaptive immunity through antigen presentation. As a novel inflammatory marker, LMR is tightly related to development and progression of diverse disorders[9,10]. Low LMR is associated with the prognosis of tuberculosis and malignancy. LMR predicts survival and aggressiveness in patients with endometrial cancer[11]. According to our findings, sepsis group had reduced lymphocyte number compared to the non-sepsis group, monocytes were increased and LMR was reduced; in addition, death group had decreased LMR compared with survival group. Decrease in LMR was associated with sepsis development and progression.
IL-35 belongs to IL family, mainly produced via T regulatory cells (Treg), with stable expression in the normal organism and mainly exerts a negative regulatory effect on suppressing immune factor activity and inflammatory responses[12]. It has been suggested that IL-35 can suppress inflammatory responses and prevents the development of excessive autoimmune reactions. The animal studies have shown that IL-35 is produced after dendritic cell stimulation and can suppress Cluster of Differentiation 4 (CD4) and Cluster of Differentiation 8 (CD8) positive T cell responses in peripheral blood and inhibits the progression of allergic lesions. Normal IL-35 expression has a negative regulatory effect on the immune system and immune responses, and can suppress excessive inflammatory responses and excessive autoimmune responses[13]. Treg number within organisms with reduced IL-35 expression is significantly lower than that in normal organisms. Therefore, the amount of Treg cells in patients with sepsis has a direct effect on IL-35 expression and it is speculated that Treg cells in patients with sepsis may be damaged to some extent. According to our findings, death group did not show statistical significance in LMR, CRP and WBC on d 1 and d 3 of admission, suggesting that LMR, CRP and WBC can only be used as diagnostic indicators of sepsis, reflecting the inflammatory status of the organism and not reflecting the prognosis of sepsis patients.
The experimental results suggested that survival group had slightly elevated serum IL-35 content on d 1 of disease onset compared to the healthy controls. However, sepsis with an aggressive prognosis showed a significant high expression of IL-35 at an early stage and the experiment confirmed that, higher the IL-35 level, the worse the prognosis. High levels of IL-35 showed significantly lower survival days, providing a new method for clinical judgment of the severity and prognosis of patients.
According to our experimental results, serum IL-35 is the biomarker reflecting sepsis prognosis, while IL-35 up-regulation in early sepsis may suggest that patients have a poor prognosis and IL-35 level has a great prognostic assessment value for sepsis survival compared with LMR level.
In addition, this study further demonstrated that the survival rate of sepsis patients with high-IL-35 expression significantly decreased relative to those with low-IL-35 expression by Kaplan-Meier survival curves. The 28 d survival rates were 57.5 % and 26.4 % for low and high-IL-35 expression groups, separately, suggesting that IL-35 has a good predictive value for survival assessment in patients with sepsis[14-16].
In conclusion, according to our results, IL-35 combined with LMR shows a higher significance in predicting sepsis development and prognosis from the perspective of LMR and pro-inflammatory cytokine IL-35, and that dynamic monitoring of the changes of these two indicators is important for timely assessment of patient’s conditions and prognosis, early clinical intervention and improvement of the treatment rate. In addition, certain limitations should be noted in this work, including its retrospective nature and small sample size. Large, prospective clinical research should be conducted to guarantee our result accuracy.
Conflict of interests:
The authors declared no conflict of interest.
References
- Wentowski C, Ingles DP, Nielsen ND. Sepsis 2021: A review. Anaesth Intensive Care Med 2021;22(11):676-84.
- Nedeva C. Inflammation and cell death of the innate and adaptive immune system during sepsis. Biomolecules 2021;11(7):1-15.
[Crossref] [Google scholar] [PubMed]
- Pan YC, Jia ZF, Cao DH, Wu YH, Jiang J, Wen SM, et al. Preoperative lymphocyte-to-monocyte ratio (LMR) could independently predict overall survival of resectable gastric cancer patients. Medicine 2018;97(52):1-7.
[Crossref] [Google scholar] [PubMed]
- Li X, Fang P, Sun Y, Shao Y, Yang WY, Jiang X, et al. Anti-inflammatory cytokines IL-35 and IL-10 block atherogenic lysophosphatidylcholine-induced, mitochondrial ROS-mediated innate immune activation, but spare innate immune memory signature in endothelial cells. Redox Biol 2020;28:1-9.
[Crossref] [Google scholar] [PubMed]
- Egwuagu CE, Yu CR, Sun L, Wang R. Interleukin 35: Critical regulator of immunity and lymphocyte-mediated diseases. Cytokine Growth Factor Rev 2015;26(5):587-93.
[Crossref] [Google scholar] [PubMed]
- Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014;507(7492):366-70.
[Crossref] [Google scholar] [PubMed]
- da Silva Ramos FJ, de Freitas FG, Machado FR. Sepsis in patients hospitalized with coronavirus disease 2019: How often and how severe? Curr Opin Crit Care 2021;27(5):474.
[Crossref] [Google scholar] [PubMed]
- Feichtinger S, de Man A, Dalia AA, Groose MK, Long MT. Sepsis and resuscitation: The importance of time. Crit Care Med 2022;50(6):e615-6.
[Crossref] [Google scholar] [PubMed]
- Li J, Jiang R, Liu WS, Liu Q, Xu M, Feng QS, et al. A large cohort study reveals the association of elevated peripheral blood lymphocyte-to-monocyte ratio with favorable prognosis in nasopharyngeal carcinoma. PLoS One 2013;8(12):1-10.
[Crossref] [Google scholar] [PubMed]
- Wei X, Huang F, Wei Y, Jing H, Xie M, Hao X, et al. Low lymphocyte-to-monocyte ratio predicts unfavorable prognosis in non-germinal center type diffuse large B-cell lymphoma. Leuk Res 2014;38(6):694-8.
[Crossref] [Google scholar] [PubMed]
- Eo WK, Kwon S, Koh SB, Kim MJ, Ji YI, Lee JY, et al. The lymphocyte-monocyte ratio predicts patient survival and aggressiveness of endometrial cancer. J Cancer 2016;7(5):538-45.
[Crossref] [Google scholar] [PubMed]
- Xin PL, Jie LF, Cheng Q, Bin DY, Dan CW. Pathogenesis and function of interleukin-35 in rheumatoid arthritis. Front Pharmacol 2021;12:1-12.
[Crossref] [Google scholar] [PubMed]
- Chen C, Xu H, Peng Y, Luo H, Huang GX, Wu XJ, et al. Elevation in the counts of IL-35-producing B cells infiltrating into lung tissue in mycobacterial infection is associated with the downregulation of Th1/Th17 and upregulation of Foxp3+Treg. Sci Rep 2020;10(1):1-4.
[Crossref] [Google scholar] [PubMed]
- Lin S, Fang Y, Mo Z, Lin Y, Ji C, Jian Z. Prognostic value of lymphocyte to monocyte ratio in pancreatic cancer: A systematic review and meta-analysis including 3338 patients. World J Surg Oncol 2020;18(1):1-11.
- Abushouk A, Nasr A, Masuadi E, Allam G, Siddig EE, Fahal AH. The role of interleukin-1 cytokine family (IL-1β, IL-37) and interleukin-12 cytokine family (IL-12, IL-35) in eumycetoma infection pathogenesis. PLoS Negl Trop Dis 2019;13(4):e0007098.
[Crossref] [Google scholar] [PubMed]
- Dixon KO, van der Kooij SW, Vignali DA, van Kooten C. Human tolerogenic dendritic cells produce IL‐35 in the absence of other IL‐12 family members. Eur J Immunol 2015;45(6):1736-47.
[Crossref] [Google scholar] [PubMed]
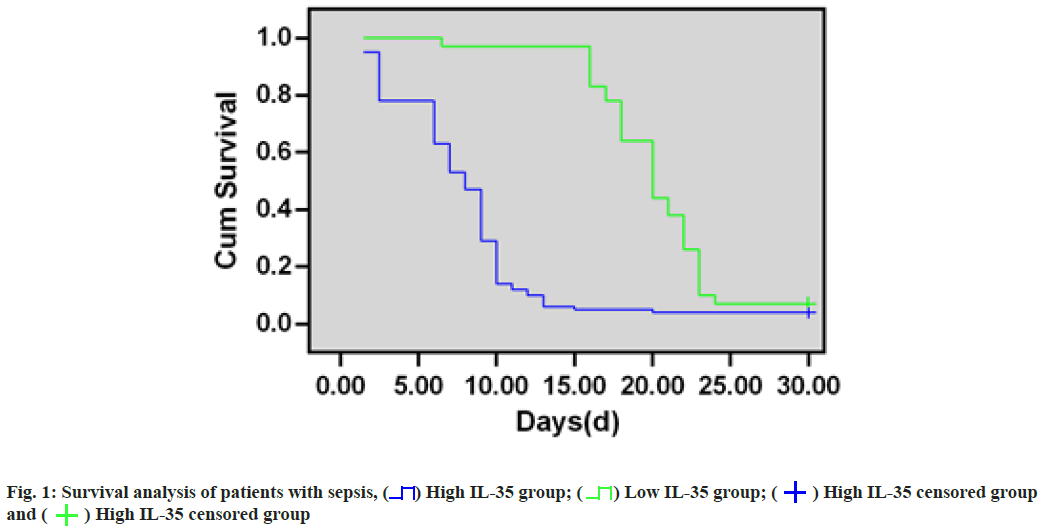
 High IL-35 group;
High IL-35 group;  Low IL-35 group;
Low IL-35 group;  High IL-35 censored group
and
High IL-35 censored group
and  High IL-35 censored group
High IL-35 censored group



