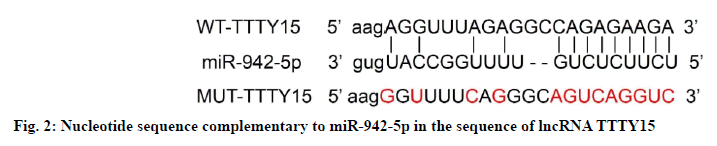- *Corresponding Author:
- L. Wang
Department of General Medicine, The Third Affiliated Hospital of Soochow University, Suzhou 215000, China
E-mail: scu_wangliangzhi@163.com
| Date of Received | 08 June 2020 |
| Date of Revision | 21 May 2021 |
| Date of Acceptance | 28 January 2022 |
| Indian J Pharm Sci 2022;84(1):108-114 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of long non-coding RNA TTTY15 on high glucose-induced renal tubular epithelial cell injury and its possible mechanism. Human renal tubular epithelial cells HK-2 were induced by high glucose to establish cell injury model. Small molecule interfering-negative control, small molecule interfering-RNA gene TTTY15, microRNA-negative control, miR-942-5p mimics, small molecule interfering-RNA gene TTTY15 and anti-microRNA-negative control, small molecule interfering-RNA gene TTTY15 and anti-miR-942-5p were transfected into HK-2 cells by liposome transfection method and then treated with 25 mmol/l glucose for 24 h. The expression of TTTY15 and microRNA-942-5p was detected by quantitative real time polymerase chain reaction; the levels of interleukin-6 and tumor necrosis factor-alpha in the supernatant were detected by enzyme-linked immunosorbent assay; apoptosis rate was detected by flow cytometry. Dual-luciferase report assay verified the targeted regulation relationship between TTTY15 and microRNA-942-5p; the expression of cleaved caspase-3 and cleaved caspase-9 proteins was detected by Western blot. The expression of TTTY15 increased (p<0.05) and microRNA-942- 5p decreased (p<0.05) in HK-2 cells induced by high glucose; transfection of small molecule interfering- RNA gene TTTY15 or microRNA-942-5p mimics could decrease the levels of interleukin-6, tumor necrosis factor-alpha (p<0.05), apoptosis, cleaved caspase-3 and cleaved caspase-9 proteins in HK-2 cells induced by high glucose (p<0.05); TTTY15 can regulate the expression of microRNA-942-5p; co-transfection of small molecule interfering-RNA gene TTTY15 and anti-miR-942-5p decreased the effect of small molecule interfering-RNA gene TTTY15 on inflammatory reaction and apoptosis induced by high glucose in HK-2 cells. Interference with TTTY15 expression may attenuate high glucose-induced tubular epithelial cell injury by targeting microRNA-942-5p expression and inhibiting the inflammatory reaction and apoptosis.
Keywords
Human renal tubular epithelial cell, high glucose, long non-coding RNA TTTY15, microRNA- 942-5p, apoptosis
Diabetic nephropathy is one of the common complications of diabetes mellitus. Persistent high glucose level in the body can cause damage of renal tubular epithelial cells and promote the development of diabetic nephropathy. Apoptosis and inflammatory reaction of renal tubular epithelial cells can cause damage of renal tubular epithelial cells. At present, the molecular mechanism of renal tubular epithelial cell injury has not been clarified. Therefore, how to alleviate the injury of renal tubular epithelial cells becomes the key to the treatment of diabetic nephropathy [1,2]. Long Non-Coding RNA (lncRNA) plays an important regulatory role in the development and progression of diabetic nephropathy and may serve as a potential target for the treatment of diabetic nephropathy [3,4].
lncRNA TTTY15 is up-regulated in Oxygen-Glucose Deprivation/Reperfusion (OGD/R) induced PC12 cell injury, and knockdown of its expression inhibits OGD/ R-induced Pheochromocytoma (PC12) cell injury [5]. However, the expression of TTTY15 in high glucoseinduced tubular epithelial cell injury and its effect on high glucose-induced tubular epithelial cell injury are unknown. StarBase prediction revealed complementary sequences between lncRNA TTTY15 and microRNA-942-5p (miR-942-5p), indicating reduced expression of miR-942-5p in lipopolysaccharide-induced tubular epithelial cells and up-regulation of its expression inhibits expression of inflammatory factors and apoptosis [6]. However, the expression of miR-942-5p in high glucose-induced tubular epithelial cell injury and its effect on high glucose-induced tubular epithelial cell injury are unknown. Thus, we used a model of high glucose-induced cell injury in HK-2 human renal tubular epithelial cells to investigate whether TTTY15 affects high glucose-induced cell injury in renal tubular epithelial cells by targeting miR-942-5p expression.
Materials and Methods
Materials and reagents:
Human renal tubular epithelial cells HK-2 were purchased from Shanghai Honsun Biological Technology Co., Ltd.; Dulbecco's Modified Eagle Medium (DMEM) culture medium was purchased from Gibco, USA; fetal bovine serum, Lipofectamine™ 3000 transfection reagent, was purchased from Thermo Fisher, USA; TTTY15 small molecule interfering RNA gene TTTY15 (si-TTTY15) and its Negative Control (si-NC), miR- 942-5p oligonucleotide mimics (miR-942-5p mimics) and Negative Control mimic NC sequence (miR-NC), miR-942-5p specific oligonucleotide inhibitor (antimiR- 942-5p) and its Negative Control (anti-miR-NC) were purchased from Guangzhou Ribobio Co., Ltd.; TTTY15 overexpression vector (pcDNA-TTTY15) and its control empty vector (pcDNA) were purchased from Invitrogen; RNA extraction reagent was purchased from TransGen Biotech Co., Ltd.; reverse transcription reagent and fluorescence quantitative Polymerase Chain Reaction (PCR) reagent were purchased from Tiangen Biotech (Beijing) Co., Ltd.; Interleukin-6 (IL- 6) and Tumor Necrosis Factor-alpha (TNF-α) detection kits were purchased from Shanghai Mlbio Co., Ltd.; apoptosis detection kit was purchased from Beijing Solarbio Science and Technology Co., Ltd.; rabbit antihuman cleaved caspase-3, cleaved caspase-9 antibodies and Horseradish Peroxidase (HRP) labeled goat antirabbit Immunoglobulin G (IgG) secondary antibodies were purchased from Abcam, USA.
Methods:
Experimental treatment and grouping: HK-2 cells were cultured in DMEM medium containing 10 % fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. When the cell growth reached 80 %, the cells were digested (3×104 cells/ml) and inoculated into a 96-well plate (100 μl/well). The cells were treated with medium containing 25 mmol/l glucose for 24 h [7], which was recorded as High Glucose Group (HG). Treat with culture medium containing 5.5 mmol/l glucose for 24 h and record it as normal Control (Con) group. According to the instructions of Lipofectamine™ 3000 transfection reagent, si-NC, si-TTTY15, miR-NC, miR-942-5p mimics, si-TTTY15 and anti-miR-NC, si-TTTY15 and anti-miR-942-5p were transfected into HK-2 cells by liposome transfection. After successful transfection, they were treated with 25 mmol/l glucose medium for 24 h and were recorded as HG+si-NC group, HG+si- TTTY15 group, HG+miR-NC group, HG+miR-942- 5p group, HG+si-TTTY15+anti-miR-NC group and HG+si-TTTY15+anti-miR-942-5p group.
Expression levels of TTTY15 and miR-942-5p in cells by quantitative Real Time Polymerase Chain Reaction (qRT-PCR): Total RNA of HK-2 cells was extracted by RNA extraction kit, RNA concentration was detected by NanoDrop 2000c ultra-micro spectrophotometer, cDNA was synthesized by reverse transcription, PCR amplification reaction system: SYBR Green Master Mix 10 μl, positive and negative primers 0.8 μl, cDNA 2 μl, double distilled Water (ddH2O) complement system to 20 μl; the reaction conditions were as follows: Pre-denaturation at 95° for 2 min, denaturation at 95° for 15 s, annealing at 60° for 30 s and extension at 72° for 30 s, with total of 40 cycles. The relative expression of TTTY15 and miR- 942-5p was calculated using the 2-ΔΔCt method.
Detection of IL-6 and TNF-α by Enzyme-Linked Immunosorbent Assay (ELISA): The supernatant of HK-2 cell culture was collected from each group and the levels of IL-6 and TNF-α were detected by ELISA. The operation was carried out in strict accordance with the instructions for use of the kit.
Apoptosis rate by flow cytometry: Each group of HK-2 cells was washed with precooled Phosphate- Buffered Saline (PBS), centrifuged at 1000 r/min for 6 min, the supernatant was discarded and the cell pellet was collected. Cells were resuspended with 500 μl binding buffer, adding 5 μl Annexin V-FITC and 5 μl Propidium Iodide (PI) respectively and incubated with shaking at room temperature for 10 min. The apoptosis rate was measured by FACSCalibur™ flow cytometer.
Targeting relationship between TTTY15 and miR- 942-5p detected by dual-luciferase reporter assay: The wild type vector WT-TTTY15 and mutant vector MUT-TTY15 were designed and synthesized by Promega Company of USA. miR-NC/WT-TTTY15,miR-NC/MUT-TTTY15, miR-942-5p mimics/WTTTTY15, miR-942-5p mimics/MUT-TTTY15 were co-transfected into HK-2 cells by liposome transfection method. The cells were cultured in incubator for 48 h and their luciferase activity was detected according to the instructions of luciferase activity detection kit produced by Promega Company of USA. pcDNA, pcDNA-TTTY15, si-NC and si-TTTY15 were transfected into HK-2 cells by lipofectamine and cultured in culture chamber for 48 h. The expression of miR-942-5p in cells was detected by qRT-PCR after cell collection.
The cleaved caspase-3 and cleaved caspase-9 proteins expression detected by Western blot: Collect HK-2 cells from each group, add proper amount of Radioimmunoprecipitation Assay (RIPA) buffer lysate to extract total cell protein. Protein concentration was determined by Bicinchoninic acid (BCA) method. 5×SDS loading buffer was added to the protein samples and boiled at 100° for 10 min to denaturate the protein. Protein was denatured with 10 % Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE). The separated protein gel was transferred to Polyvinylidene Difluoride (PVDF) membrane, sealed with 5 % skim milk powder for 2 h, incubated with cleaved caspase-3 (1:1000), cleaved caspase-9 (1:1000) and GAPDH (1:3000) diluents at 4° overnight, washed with Tris-Buffered Saline with 0.1 % Tween® 20 Detergent (TBST), incubated with secondary antibodies (1:5000) at room temperature for 1 h, washed with TBST and developed with Enhanced Chemiluminescence (ECL) dropwise. ImageJ software was used to analyze the gray value of each band.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 21.0 statistical software was used to analyze the data. The measurement data were expressed as (x̄±s) and were in normal distribution. Independent sample t test was used for the comparison between the two groups. Oneway analysis of variance was used for the comparison between the two groups. p<0.05 was used for the difference.
Results and Discussion
Compared with Con group, the expression of TTTY15 was increased (p<0.05) and the expression of miR-942- 5p was decreased (p<0.05) in HG group as shown in Table 1.
| Grouping | TTTY15 | miR-942-5p |
|---|---|---|
| Con | 1.00±0.00 | 1.00±0.00 |
| HG | 2.84±0.24* | 0.37±0.04* |
| t | 23.000 | 47.250 |
| p | 0.000 | 0.000 |
Note: Compared with control group, *p<0.05, Con: Control group; HG: High Glucose group
Table 1: Expression of Lncrna Ttty15 And Mir-942-5p In High Glucose-Induced Tubular Epithelial Cell Injury (x̄±s, n =9)
Compared with Con group, the levels of IL-6 and TNF-α in HG group were increased (p<0.05); compared with HG+si-NC group, the levels of IL-6 and TNF-α in HG+si-TTTY15 group were decreased (p<0.05), as shown in Table 2.
| Grouping | TTTY15 | IL-6 (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|
| Con | 1.00±0.00 | 34.06±3.09 | 27.24±2.17 |
| HG | 2.91±0.25* | 161.23±13.79* | 67.84±5.53* |
| HG+si-NC | 2.99±0.28 | 166.09±15.21 | 69.38±6.04 |
| HG+si-TTTY15 | 1.47±0.13# | 46.11±4.24# | 37.34±3.43# |
| F | 232.310 | 410.353 | 196.992 |
| p | 0.000 | 0.000 | 0.000 |
Note: Compared with miR-con group, *p<0.05; compared with the anti-miR-con group, #p<0.05, Con: Control group; HG: High Glucose group; si-NC: small molecule interfering-Negative Control; si-TTTY15: small molecule interfering-RNA gene; IL-6: Interleukin-6; TNF-α: Tumor Necrosis Factor-alpha
Table 2: Effect of Interference With Lncrna Ttty15 Expression On High Glucose-Induced Expression of Inflammatory Cytokines In Renal Tubular Epithelial Cells (x̄±s, n=9)
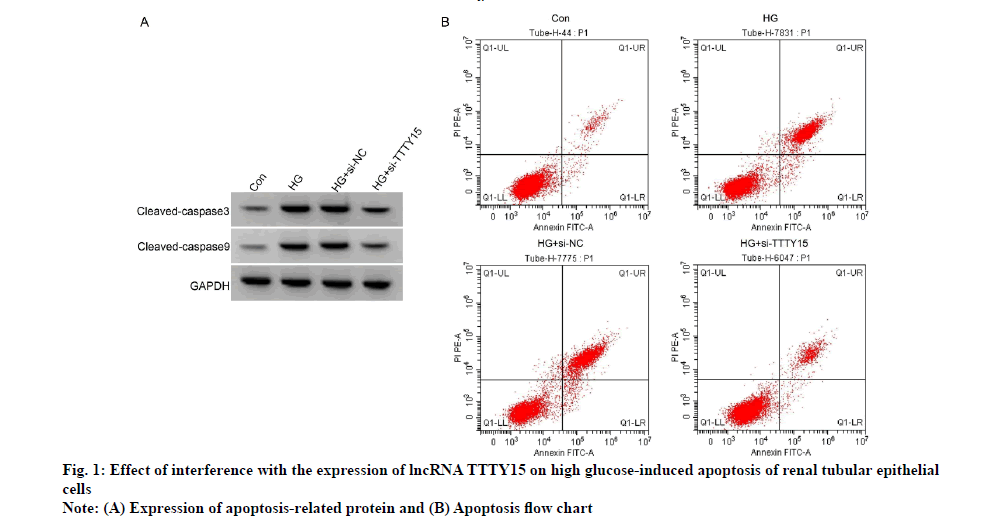
Compared with Con group, apoptosis rate and levels of cleaved caspase-3 and cleaved caspase-9 proteins were increased in HG group (p<0.05); compared with HG+si-NC group, apoptosis rate and levels of cleaved caspase-3 and cleaved caspase-9 proteins were decreased in HG+si-TTTY15 group (p<0.05 ), as shown in fig. 1 and Table 3.
| Grouping | Apoptosis rate (% ) | Cleaved-caspase3 protein | Cleaved-caspase9 protein |
|---|---|---|---|
| Con | 5.53±0.49 | 0.21±0.03 | 0.13±0.02 |
| HG | 33.61±3.19* | 0.72±0.05* | 0.66±0.04* |
| HG+si-NC | 36.18±3.58 | 0.74±0.06 | 0.67±0.05 |
| HG+si-TTTY15 | 10.91±1.04# | 0.29±0.03# | 0.25±0.03# |
| F | 359.953 | 355.139 | 517.500 |
| p | 0.000 | 0.000 | 0.000 |
Note: Compared with miR-con group, *p<0.05; compared with the anti-miR-con group, #p<0.05, Con: Control group; HG: High Glucose group; si-NC: small molecule interfering-Negative Control; si-TTTY15: small molecule interfering-RNA gene
Table 3: Effect of Interference With The Expression of Lncrna Ttty15 on High Glucose-Induced Apoptosis of Renal Tubular Epithelial Cells (x̄±s, n=9)
StarBase prediction showed that there was a binding site between lncRNA TTTY15 and miR-942-5p, as shown in fig. 2. In cell experiments co-transfected with wild-type vector WT-TTTY15, compared with miR-NC group, the relative luciferase activity in miR-942-5p group was lower than that in miR-NC group (p<0.05); in the cell experiment of co-transfection of mutant vector MUT-TTTY15, the relative luciferase activity of renal tubular epithelial cells in miR-942-5p group was not significantly different from that in miR-NC group, as shown in Table 4. Compared with pcDNA group, the expression of miR-942-5p in pcDNA-TTTY15 group decreased (p<0.05); the expression of miR-942-5p in si-TTTY15 group was higher than that in si-NC group (p<0.05), as shown in Table 5.
| Grouping | WT-TTTY15 | MUT-TTTY15 |
|---|---|---|
| miR-NC | 1.00±0.05 | 1.01±0.06 |
| miR-942-5p | 0.45±0.04* | 0.96±0.05 |
| t | 25.769 | 1.921 |
| p | 0.000 | 0.073 |
Note: Compared with miR-NC group, *p<0.05
Table 4: Dual-Luciferase Reporter Assay (x̄±s, n=9)
| Grouping | miR-942-5p |
|---|---|
| pcDNA | 1.00±0.00 |
| pcDNA-TTTY15 | 0.35±0.03* |
| si-NC | 1.02±0.07 |
| si-TTTY15 | 2.68±0.22# |
| F | 657.493 |
| p | 0.000 |
Note: Compared with pcDNA group, *p<0.05; compared with the si-NC group, #p<0.05
Table 5: Expression of miR-942-5p Regulated By Lncrna Ttty15 (x̄±s, n=9)
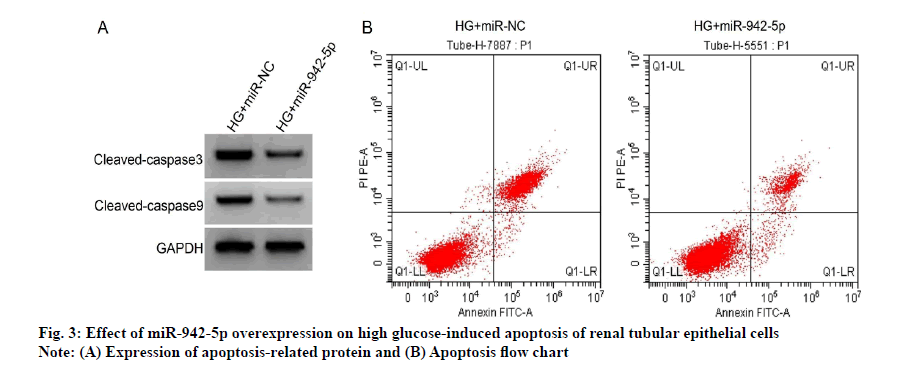
Compared with HG+miR-NC group, HG+miR-942-5P group had lower levels of IL-6 and TNF-α (p<0.05), lower rates of apoptosis and cleaved caspase-3 and cleaved caspase-9 protein (p<0.05), see fig. 3 and Table 6.
| Grouping | miR-942-5p | IL-6 (pg/ml) | TNF-α (pg/ml) | Apoptosis rate (%) | Cleaved caspase-3 protein | Cleaved caspase-9 protein |
|---|---|---|---|---|---|---|
| HG+miR-NC | 1.00±0.00 | 170.13±11.85 | 71.14±7.01 | 34.57±3.23 | 0.75±0.05 | 0.68±0.05 |
| HG+miR-942-5p | 3.31±0.22* | 58.44±5.19* | 36.27±3.14* | 14.91±1.23* | 0.35±0.03* | 0.32±0.03* |
| t | 31.500 | 25.901 | 13.619 | 17.065 | 20.580 | 18.522 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with HG+miR-NC group, *p<0.05, IL-6: Interleukin-6; TNF-α: Tumor Necrosis Factor-alpha
Table 6: Effect of miR-942-5p Overexpression On High Glucose-Induced Tubular Epithelial Cell Injury (x̄±s, n=9)
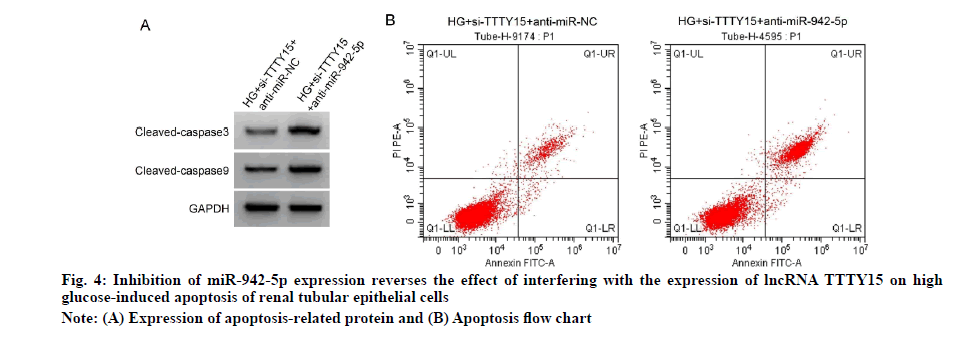
Compared with HG+si-TTTY15+anti-miR-NC group, HG+si-TTTY15+anti-miR-942-5p group had higher levels of IL-6 and TNF-α (p<0.05) and higher rates of apoptosis and cleaved caspase-3 and cleaved caspase-9 proteins (p<0.05), see fig. 4 and Table 7.
| Grouping | miR-942-5p | IL-6 (pg/ml) | TNF-α (pg/ml) | Apoptosis rate (%) | Cleaved caspase-3 protein | Cleaved caspase-9 protein |
|---|---|---|---|---|---|---|
| HG+si-TTTY15+anti-miR-NC | 1.00±0.00 | 44.32±4.41 | 36.94±3.14 | 9.78±0.88 | 0.26±0.03 | 0.23±0.02 |
| HG+si-TTTY15+anti-miR-942-5p | 0.28±0.03* | 126.04±12.14* | 55.72±5.14* | 27.57±2.64* | 0.65±0.05* | 0.56±0.05* |
| t | 72.000 | 18.981 | 9.354 | 19.178 | 20.065 | 18.384 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with HG+si-TTTY15+anti-miR-NC group, *p<0.05; IL-6: Interleukin-6; TNF-α: Tumor Necrosis Factor-alpha
Table 7: Inhibition of miR-942-5p Expression Reverse The Effect of Interfering With Lncrna Ttty15 Expression on High Glucose-Induced Tubular Epithelial Cell Injury (x̄±s, n=9)
By competitive binding to microRNAs (miRNAs), lncRNA can participate in the process of high glucoseinduced tubular epithelial cell injury, modulate biological behavior such as apoptosis and potentially serve as a potential target for the treatment of diabetic nephropathy [8-10].
TTTY15 is highly expressed in hypoxia-induced endothelial cell injury and interference with its expression attenuates hypoxia-induced endothelial cell injury [11]. TTTY15 is up-regulated in patients with acute myocardial infarction and hydrogen peroxide-induced myocardial cell injury and knockdown of its expression reduces hydrogen peroxide-induced cardiomyocyte injury [12]. Inhibition of TTTY15 expression reduces hypoxia-induced cardiomyocyte injury [13]. However, relatively few studies on TTTY15 and diabetic nephropathy have been reported. The results of this study showed that the levels of IL-6 and TNF-α in the culture supernatant of renal tubular epithelial cells induced by high glucose were increased, similar to those reported in previous studies [14], suggesting the successful establishment of a model of renal tubular epithelial cell injury. Furthermore, increased expression of TTTY15 in high glucose-induced renal tubular epithelial cells interferes with reduced levels of IL-6 and TNF-α in culture supernatant of high glucose-induced renal tubular epithelial cells after TTTY15 expression. Interference with TTTY15 expression was suggested to suppress the high glucose-induced inflammatory reaction in renal tubular epithelial cells. The results of this study showed that the rates of apoptosis and the levels of cleaved caspase-3 and cleaved caspase-9 proteins in renal tubular epithelial cells induced by high glucose were increased, similar to those reported in previous studies [15]. Further studies showed that the rates of apoptosis and the levels of cleaved caspase-3 and cleaved caspase-9 proteins induced by high glucose were decreased after interference with the expression of TTTY15, suggesting that interference with the expression of TTTY15 could inhibit the apoptosis of renal tubular epithelial cells induced by high glucose, thus reducing cell damage.
This study demonstrates that TTTY15 targets miR-942- 5p and negatively regulates miR-942-5p expression. It is suggested that TTTY15 may be involved in the pathogenesis of diabetic nephropathy by targeting and regulating the expression of miR-942-5p. miR- 942-5p has been shown to be poorly expressed in oxidative-Low Density Lipoprotein (ox-LDL) induced endothelial cell injury and up-regulation of miR-942- 5p expression reduces ox-LDL-induced endothelial cell injury [16]. miR-942-5p is poorly expressed in adriamycin-induced cardiomyocytes and up-regulation of miR-942-5p expression inhibits doxorubicin-induced cardiomyocyte apoptosis [17]. Our results suggest that high glucose-induced miR-942-5p expression in renal tubular epithelial cells is reduced and overexpression of miR-942-5p inhibits high glucose-induced inflammatory factor expression and apoptosis in renal tubular epithelial cells, whereas inhibition of its expression reverses the effects of TTTY15 expression on high glucose-induced inflammatory reaction and apoptosis in renal tubular epithelial cells, suggesting that TTTY15 targeting miR-942-5p promotes high glucose-induced inflammation and apoptosis so as to aggravate the cell injury.
To sum up, the expression of TTTY15 was up-regulated and miR-942-5p was down-regulated in renal tubular epithelial cells induced by high glucose. TTTY15 could regulate the expression of miR-942-5p and interfering with TTTY15 could inhibit inflammatory reaction and apoptosis of renal tubular epithelial cells induced by high glucose by up-regulating miR-942-5p expression, thus alleviating cell injury. TTTY15/miR-942-5p may be a potential biomarker for diagnosis of diabetic nephropathy and a potential target for treatment. However, the specific mechanism of action needs to be further explored.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Zhang LC, Wei ZB, Tang SF. Knockdown of the Long Noncoding RNA LUCAT1 Inhibits High-Glucose-Induced Epithelial-Mesenchymal Transition through the miR-199a-5p–ZEB1 Axis in Human Renal Tubular Epithelial Cells. Biomed Res Int 2020;2020.
[CrossRef] [Google Scholar] [Pub Med]
- Zhu B, Cheng X, Jiang Y, Cheng M, Chen L, Bao J, et al. Silencing of KCNQ1OT1 decreases oxidative stress and pyroptosis of renal tubular epithelial cells. Diabetes Metab Syndr Obes 2020;13(1):365-75.
[CrossRef] [Google Scholar] [Pub Med]
- Liu C, Zhuo H, Ye MY, Huang GX, Fan M, Huang XZ. LncRNA MALAT1 promoted high glucose-induced pyroptosis of renal tubular epithelial cell by sponging miR-30c targeting for NLRP3. Kaohsiung J Med Sci 2020;36(9):682-91.
[CrossRef] [Google Scholar] [Pub Med]
- Wang R, Yan Y, Li C. LINC00462 is involved in high glucose‐induced apoptosis of renal tubular epithelial cells via AKT pathway. Cell Biol Int 2020;44(1):286-94.
[CrossRef] [Google Scholar] [Pub Med]
- Hao C, Chen S. Knockdown of lncRNA TTTY15 alleviates ischemia/reperfusion‑induced inflammation and apoptosis of PC12 cells by targeting miR-766‑5p. Exp Ther Med 2021;21(5):1-9.
[CrossRef] [Google Scholar] [Pub Med]
- Luo N, Gao HM, Wang YQ, Li HJ, Li Y. MiR-942-5p alleviates septic acute kidney injury by targeting FOXO3. Eur Rev Med Pharmacol Sci 2020;24(11):6237-44.
[CrossRef] [Google Scholar] [Pub Med]
- Xu W, Mou S, Wang Q. Protective effect of astragaloside A on renal tubular epithelial cell injury induced by high glucose. Chin J Integr Tradit West Med 2012;13(9):765-9.
- Zhou L, Xu DY, Sha WG, Shen L, Lu GY. Long non-coding RNA MALAT1 interacts with transcription factor Foxo1 to regulate SIRT1 transcription in high glucose-induced HK-2 cells injury. Biochem Biophys Res Commun 2018;503(2):849-55.
[CrossRef] [Google Scholar] [Pub Med]
- Li Y, Ren D, Xu G. Long noncoding RNA MALAT1 mediates high glucose‐induced glomerular endothelial cell injury by epigenetically inhibiting klotho via methyltransferase G9a. IUBMB Life 2019;71(7):873-81.
[CrossRef] [Google Scholar] [Pub Med]
- Wang J, Zhao SM. LncRNA-antisense non-coding RNA in the INK4 locus promotes pyroptosis via miR-497/thioredoxin-interacting protein axis in diabetic nephropathy. Life Sci 2021;264:118728-38.
[CrossRef] [Google Scholar] [Pub Med]
- Zheng J, Zhuo YY, Zhang C, Tang GY, Gu XY, Wang F. LncRNA TTTY15 regulates hypoxia-induced vascular endothelial cell injury via targeting miR-186-5p in cardiovascular disease. Eur Rev Med Pharmacol Sci 2020;24(6):3293-301.
[CrossRef] [Google Scholar] [Pub Med]
- Ma R, Gao L, Liu Y, Du P, Chen X, Li G. LncRNA TTTY15 knockdown alleviates H2O2-stimulated myocardial cell injury by regulating the miR-98-5p/CRP pathway. Mol Cell Biochem 2021;476(1):81-92.
- Huang S, Tao W, Guo Z, Cao J, Huang X. Suppression of long noncoding RNA TTTY15 attenuates hypoxia-induced cardiomyocytes injury by targeting miR-455-5p. Gene 2019;701:1-8.
[CrossRef] [Google Scholar] [Pub Med]
- Song D, Du S, Peng J. LncRNA SNHC7 modulates miR-10a-5p to affect high glucose-induced tubular epithelial cell injury. Chin J Integr Tradit West Med 2020;21(11):978-82.
- Bao F, Song J, Dai Z. Astragalus polysaccharides inhibit apoptosis of renal tubular epithelial cells induced by high glucose through inactivation of Wnt signaling pathway. Chin Herb Med 2019;42(2):414-7.
- Wan H, You T, Luo W. circ_0003204 regulates cell growth, oxidative stress and Inflammation in ox-LDL-induced vascular endothelial cells via regulating miR-942-5p/HDAC9 axis. Front Cardiovasc Med 2021;8:240.
[CrossRef] [Google Scholar] [Pub Med]
- Wang H, Lin X, Li J, Zeng G, Xu T. Long noncoding RNA SOX2-OT aggravates doxorubicin-induced apoptosis of cardiomyocyte by targeting miR-942-5p/DP5. Drug Des Devel Ther 2021;15(1):481-92.
[CrossRef] [Google Scholar] [Pub Med]