- *Corresponding Author:
- AV. Bukkapatnam
Department of Pharmaceutical Analysis and Quality Assurance, GITAM Institute of Pharmacy, GITAM University, Visakhapatnam 530045, India
E-mail: venki.b11@gmail.com
| Date of Submission | 02 April 2020 |
| Date of Revision | 12 October 2021 |
| Date of Acceptance | 17 August 2022 |
| Indian J Pharm Sci 2022;84(4):1071-1082 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Abiraterone acetate is an androgen biosynthesis inhibitor used in the treatment of prostate cancer. This study focuses on a simple, economical and systematic liquid chromatographic and mass spectroscopic method to study the stress degradation behavior of abiraterone acetate under various stress conditions along with its validation. The chromatographic separation of abiraterone acetate and its major degradation product is achieved on Capcell PAK C18 MG-III (100×4.6 mm, 3 μm) column using combination of 0.1 % acetic acid and acetonitrile as mobile phase with a flow rate of 1.2 ml/min and the wavelength of detection is selected as 251 nm. The drug was degraded extensively in acidic and basic hydrolysis. A tentative degradation pathway of drug in acidic and alkaline condition was predicted. The developed analytical method was found selective, accurate, precise and robust in accordance with International Council for Harmonization guidelines. The method will also suffice the suitability for the assay of abiraterone acetate in bulk and finished products.
Keywords
Abiraterone acetate, anti-prostate cancer agent, liquid chromatographic and mass spectrophotometry, stability indicating, International Council for Harmonization guidelines
Metastatic Castration Resistant Prostate Cancer (MCRPC) is a major category of malignance, which is one of the most common cancers in the United States and the 2nd most common cause of cancer death among men. It causes 258 400 mortality rates yearly[1,2]. The therapy for metastatic prostate cancer involves suppressing testosterone production through androgen deprivation therapy. Abiraterone Acetate (ABT), the first compound that increases the overall survival in MCRPC patients, through the inhibition of adrenal gland production of testosterone. Abiraterone inhibits the enzymatic activity of steroid 17a-monooxygenase, a member of the cytochrome P450 family that catalyzes the 17a-hydroxylation of steroid intermediates involved in testosterone synthesis from the adrenal glands. ABT is an orally active acetate salt of the steroidal compound abiraterone with anti-androgen activity[3]. According to the available literature, abiraterone was determined using different techniques like spectrofluorimetry[4], High-Performance Liquid Chromatography (HPLC) fluorescence detection[5], Liquid Chromatography coupled to Electrospray Ionization Mass Spectrometry (LC-ESI-MS)[6], Reverse Phase-HPLC-Ultraviolet (RPHPLC/ UV)[7-10], Liquid Chromatography/ESI Tandem Mass Spectrometric (LC/ESI-MS/MS) LC-MS/MSESI[ 11,12] and Ultra Performance Liquid Chromatography (UPLC)-MS/MS in biological fluids[13,14]. As most of the methods available from literature are bioanalytical methods for biological fluids. Moreover, according to the International Council for Harmonization (ICH), Stress degradation studies for the compounds are mandatory not only to establish the inherent stability characteristics, but also to identify the degradation products. Therefore, there is a large scope for the development of a simple, short and better analytical method for the study of degradation behavior ABT and also identification of degradant production is very essential.
The main objective of this research work is to develop a fast and simple enough robust isocratic liquid chromatographic method that is compatible with LCMS, not only to quantify ABT in the presence of the degradation product under various stress degradation conditions but also, to identify the degradation product. This further facilitates to the prediction of the degradation pathway.
Materials and Methods
Chemicals and reagents:
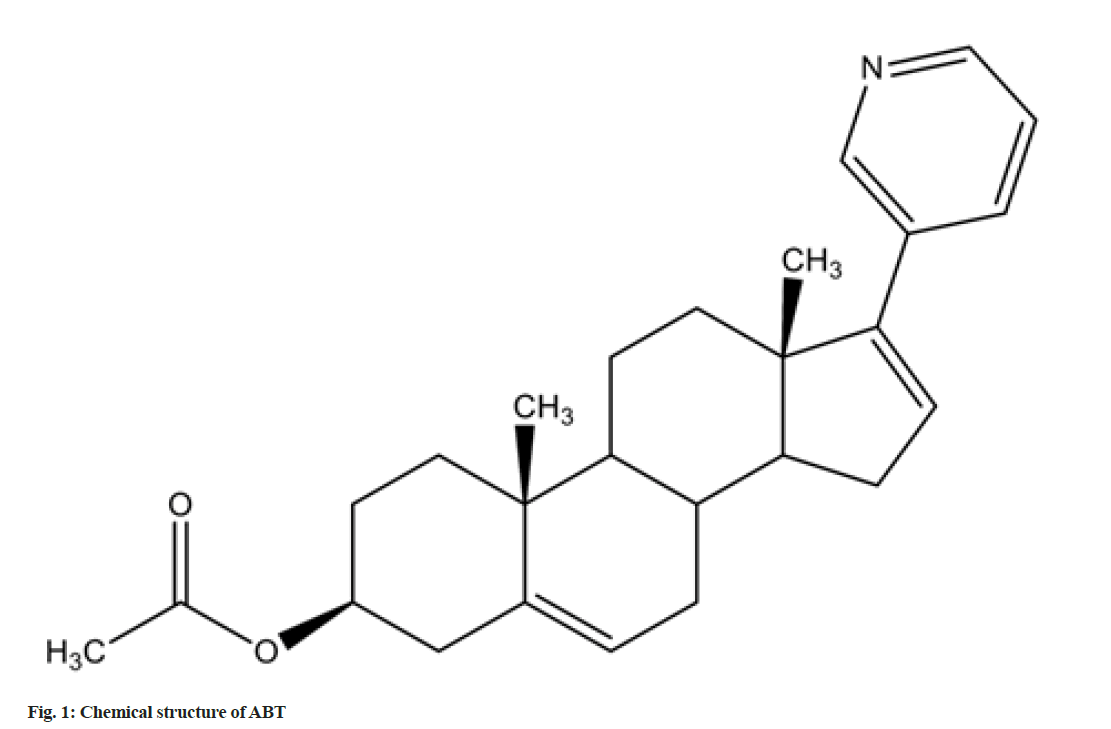
ABT standard (3S,8R,9S,10R,13S,14S)- 2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-10,13- dimethyl-17-(pyridin-3-yl)-1H-cyclopenta[a] phenanthren-3-yl acetate) (C26H33NO2; molecular weight-391.55 g/mol) (purity 99.8 %) was obtained as a gift sample from Dr. Reddy’s Ltd., India. The structure of the compound is shown in fig. 1. ABT is marketed as film coated tablets (label claim: 250 mg), with the brand names Zytiga® (Janssen-Cilag Ltd, India); Xibra (Cipla labs, India) and Abirapro (Glenmark Pharmaceuticals, India).
Acetonitrile (ACN), glacial acetic acid, Hydrochloric acid (HCl), Sodium hydroxide (NaOH), Hydrogen peroxide (H2O2) and HPLC water were all procured from Merck, India. All the reagents and chemicals used were all of HPLC grade.
Instrumentation and chromatographic conditions:
The analysis was carried out on UFLC Shimadzu Prominence System (CBM-20Alite) model equipped with SPD-M20A prominence Photodiode Array (PDA) detector. For the identification of major degrading product, the method is transferred to Agilent 1260 Series Infinity instrument equipped with 1260 Quad pump; 1260 auto-sampler device; 1260 thermostatted column compartment oven; 1260 variable wavelength detector; 6120 quadrupole mass spectrophotometer. The separation is achieved on Capcell PAK C18 MG-III (100×4.6 mm, 3 μm) column using combination of 0.1 % acetic acid and ACN (11:89, % v/v) in isocratic mode with a flow rate of 1.2 ml/min. The injection volume is 20 μl and wavelength of detection is set at 251 nm. The column is maintained at ambient temperature (25°). For identification of degradation product mass conditions are mass to charge (m/z) range 50-1000 m/z; nebulizer and curtain gas nitrogen; nebulizer gas pressure 35 psi; dry gas flow 12 l/min; dry gas temperature 250° and capillary voltage 4000 V.
Validation:
The method so optimized was validated as per ICH guidelines Q2(R1) guidelines, 2005[15]. The linearity (0.1-1000 μg/ml) and precision (50, 100 and 150 μg/ ml) studies were conducted. The accuracy studies were accomplished with three pre-delineates spiked concentration levels (80, 100 and 120 %). In robustness study the chromatographic conditions were marginally modified for flow rate (1.1 and 1.3 ml/min); percentage organic phase (87 and 91 % v/v) and detection wavelength (249 and 253 nm). The robustness study was carried at a concentration level of 10 μg/ml of the standard. All the validation data obtained was taken in triplicate.
Forced degradation studies:
To confirm that the analytical method is stability indicating, ABT was exposed to stress under various conditions to accomplish forced degradation studies[16]. As the present ICH guidelines did not make any statement regarding the detailed degradation conditions in the stress testing, the presently used forced degradation conditions were all based on trial and error. Acidic degradation was performed by refluxing stock solution (1 ml) with 1 ml of 0.1 N HCl for a time of 30 min at 80° in a thermostat, the solution was cooled, neutralised and then diluted as per requirement. Alkaline degradation was conducted by heating stock solution (1 ml) along with 1 ml of 0.1 N NaOH for a period of 30 min at 50°, later it was cooled, neutralised and then diluted as per requirement. During oxidative degradation, stock solution (1 ml) was left in a 10 ml volumetric flask with 1.0 ml of 30 % H2O2 for a period of 30 min at 40°, later cooled and then diluted. Thermal degradation was conducted by exposing a definite quantity of ABT standard in solution state to heat in a hot air oven maintained at 80° continuously for 7 d and the resultant was cooled and diluted before the study. Photolytic degradation was governed by exposing the solid drug sample to light source radiation with a wavelength range of 290-700 nm in the Ultra-Violet (UV) chamber, continuously for about 7 d. The sample was spread as a thin layer and sealed with a transparent cover to minimize the effects of the changes in the physical state. The solution was prepared with ACN and further dilution with diluent.
Assay of marketed formulations:
Twenty tablets of each available brands of ABT were procured from the local pharmacy store, weighed and crushed into fine powder. Powder equivalent to 25 mg of ABT was accurately weighed and extracted with mobile phase. The solution was filtered through 0.45 μm membrane prior analysing and the peak area was recorded from the respective chromatogram.
Results and Discussion
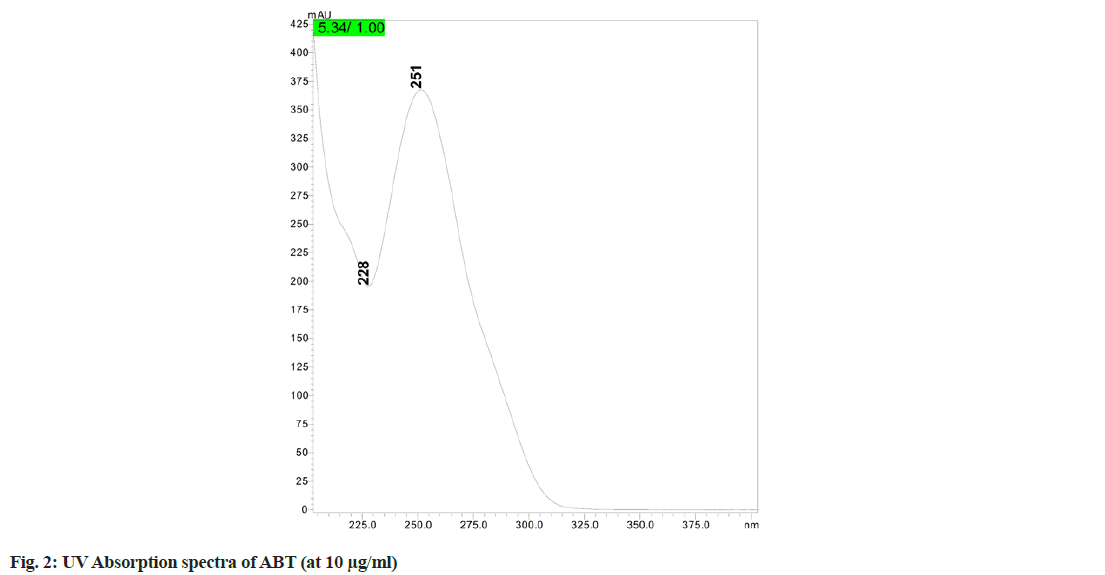
A comparative study of the previously published methods with the present method was summarized detail in Table 1. In the initial trials the experiments were conducted with different columns such as Sunfire C18 column (150×4.6 mm, 5 μm), Phenomenex Luna C18 column (250×4.6 mm, 5 μm) and Capcell PAK C18 MGIII (10×4.6 mm, 3 μm). After reviewing the results of column trials, it was found that the peak shape, retention time, tailing factor and column efficiency were found better with Capcell PAK C18 MG-III (100×4.6 mm, 3 μm), hence this column is selected for this method of analysis. In overall reviewing the results of mobile phases, their ratios and the flow rates, mobile phase comprising of 0.1 % acetic acid:ACN (11:89 v/v) and with a flow rate of 1.2 ml/min was selected as the optimum mobile phase for better peak shape, tailing factor and theoretical plates low retention time, etc. The overall analysis was performed with 251 nm as wavelength of detection ( fig.2).
| Method | Reagent/Mobile phase (% v/v) | Flow rate (ml/min) | Column | Remarks | Reference |
|---|---|---|---|---|---|
| LC-MS/MS | 2 % Propan-2-ol:ACN:Ammonium acetate (gradient mode) | - | Luna C5 (50 mm×2.1 mm, 5 μm) | Human plasma | [10] |
| LC-MS | Ammonium acetate:CAN (10:90) | 0.7 | Atlantis d C18 | Rat and human plasma | [8] |
| UPLC-MS/MS | ACN:Water:Formic acid (90:10:0.1) | 0.3 | UPLC BEH™ C18 | Human plasma | [9] |
| LC-UV-ESI-MS | 0.1 % Formic acid:ACN (70:30) | 0.5 | Agilent Zorbax Extend-C18 (150 mm×4.6 mm, 5 µm) | Stability indicating | [6] |
| HPLC | ACN:Water:Potassium dihydrogen phosphate (pH 3.0), (55:5:40) | 1 | Betasil C18 | Rat plasma | [8] |
| HPLC | ACN:Glycine buffer (pH 9.0) (60:40) | 0.9 | C8 Xterra® MS | Fluorescent detection | [5] |
| LC-MS | Ammonium acetate (pH 3.5):ACN (10:90) | 0.6 | Kromasil C18 (250 mm×4.6 mm, 4.0 µm) | Stability indicating | [15] |
| HPLC | Potassium phosphate buffer: ACN (40:60) | 1 | Hypersil ODS C18 (150 mm×4.6 mm, 5 µm) | Stability indicating | [15] |
| LC-MS-NMR | Water:ACN (gradient) | 1 | Waters X-terra MC C18 (250 mm×4.6 mm, 5 µm) | Related substance | [16] |
| HPLC | ACN:0.1 % Orthophosphoric acid (15:85) | 1 | Hypersil BDS C18 (250 mm×4 mm, 5 μm) | Stability indicating | [14] |
| HPLC | Potassium phosphate buffer:ACN (40:60) | 1 | Hypersil ODS C18 (150 mm×4.6 mm, 5 μm) | Stability indicating | [13] |
| LC-UV-ESI-MS | 0.1 % Acetic acid:ACN (11:89) | 1.2 | Capcell PAK C18 MG-III (100×4.6 mm, 3 µm) | Stability indicating | Present method |
Table 1: Comparison of the Previously Reported Methods with the Present Method
The method was validated as per ICH guidelines. The method shows a linearity range of 0.1-1000 μg/ml, with their percentage Relative Standard Deviation (% RSD) in limits. The linearity data with their linear regression equations and their correlation coefficients, limit of detection and limit of quantitation were clearly presented in Table 2. The method accuracy was demonstrated by the recovery test at three dissimilar concentrations in which known amount of standard was supplemented to aliquots of sample solutions and then diluted to yield the total concentrations of 80, 100 and 120 μg/ml (80, 100 and 120 %) for which percentage recovery was found to be 97.96 %-99.48 % (Table 3).
| Parameter | Result |
|---|---|
| Calibration curve | y=28388.67x+110388.00 |
| Slope | 28388.67 |
| Intercept | 110388 |
| R2 | 0.9994 |
| Limit of detection (μg/ml) | 0.03275 |
| Limit of quantitation (μg/ml) | 0.0992 |
| Range (μg/ml) | 0.1-1000 |
Table 2: Linearity of Abt
| Concentration (µg/ml) | Intraday precision | Interday precision | Accuracy | Overall % recovery | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *Measured concentration (µg/ml)±SD | % RSD | SEM | *Measured concentration (µg/ml)±SD | % RSD | SEM | Spiked concentration (µg/ml) | Total concentration (µg/ml) | *Concentration found (µg/ml)±SD | % RSD | SEM | % Recovery | |||
| 50 | 49.76±0.19 | 0.39 | 0.11 | 49.48±0.50 | 1.01 | 0.29 | 80 % | 80 | 79.58±0.42 | 0.47 | 0.3 | 99.48 | Mean | 98.9 |
| 100 | 98.08±0.66 | 0.67 | 0.38 | 100.05±0.46 | 0.46 | 0.27 | 100 % | 100 | 97.96±0.40 | 0.41 | 0.2 | 97.96 | SD | 0.8 |
| 150 | 149.44±0.25 | 0.16 | 0.14 | 149.36±0.69 | 0.46 | 0.4 | 120 % | 120 | 118.98±0.27 | 0.25 | 0.1 | 99.15 | % RSD | 0.81 |
Note: *Mean of three replicates
Table 3: Precision and accuracy studies of Abt
For precision studies conducted at three concentrations (i.e., 50, 100 and 150 μg/ml) for both intraday and interday precision for which the % RSD was found to be was found to be 0.16 %-0.67 % (intraday precision) and 0.46 %-1.01 % (interday precision) (Table 3). The robustness of the method was estimated by assaying the sample in diverse analytical conditions by deliberately making slight fluctuations the original condition. From the results (Table 4), it was shown that the system suitability parameters, retention times and the assays for the test solution were not much affected there by signifying that the method is robust.
| Parameter (condition) | *% Assay±SD | % RSD | SEM | *Retention time±SD | % RSD | SEM |
|---|---|---|---|---|---|---|
| Mobile phase flow rate (±0.1 ml/min) | ||||||
| 1.1 ml/min | 100.22±0.21 | 0.21 | 0.12 | 5.94±0.01 | 0.09 | 0 |
| 1.3 ml/min | 99.34±0.31 | 0.31 | 0.18 | 4.94±0.02 | 0.42 | 0.01 |
| Detection wavelength (±2 nm) | ||||||
| 249 nm | 98.74±0.59 | 0.6 | 0.34 | 5.39±0.00 | 0.04 | 0 |
| 253 nm | 98.54±0.16 | 0.16 | 0.09 | 5.39±0.00 | 0.03 | 0 |
| Mobile phase composition (% ACN) (±2 % v/v) | ||||||
| 09:91 v/v | 98.53±0.15 | 0.16 | 0.09 | 5.294±0.01 | 0.09 | 0 |
| 13:87 v/v | 99.76±0.28 | 0.28 | 0.16 | 5.434±0.01 | 0.23 | 0.01 |
Note: *Mean of three replicates
Table 4: Robustness Study of Abt
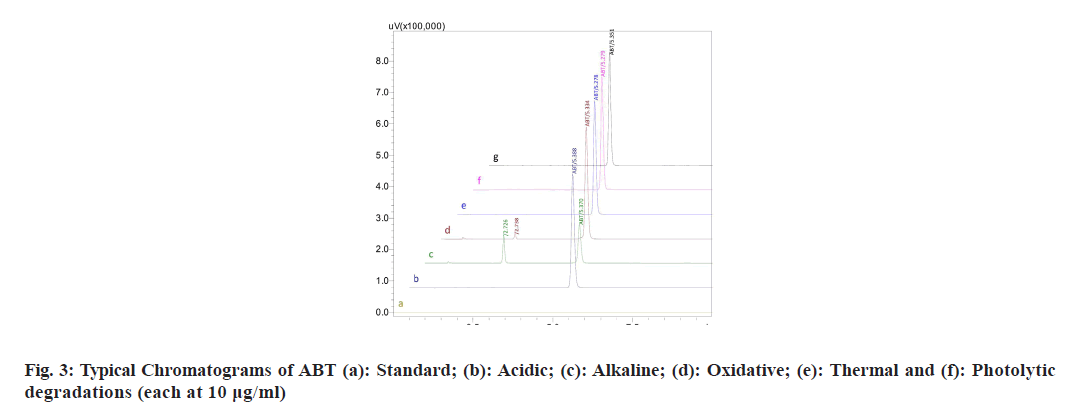
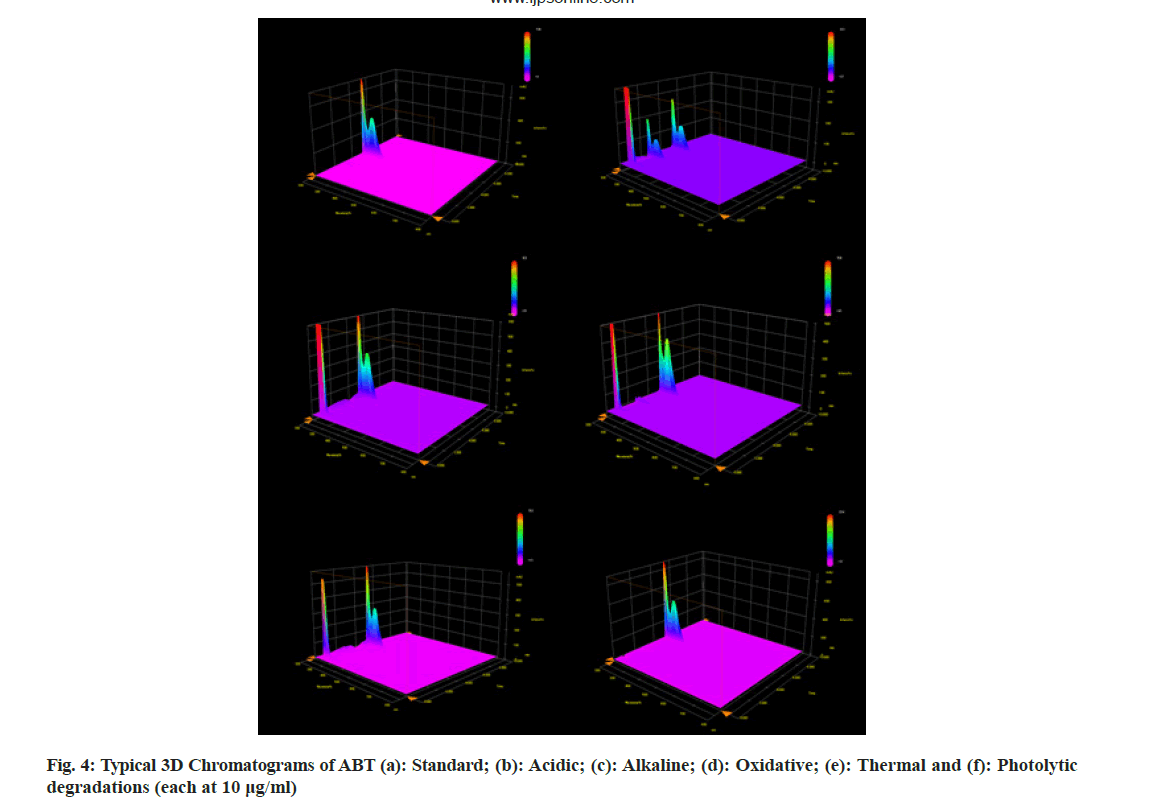
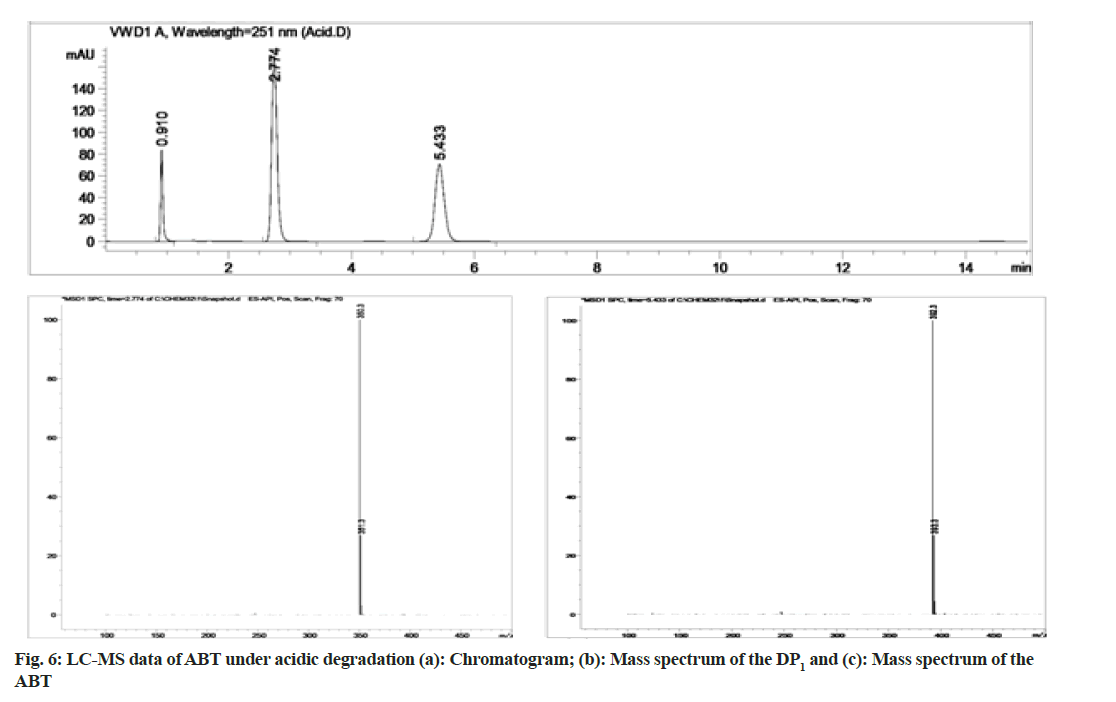
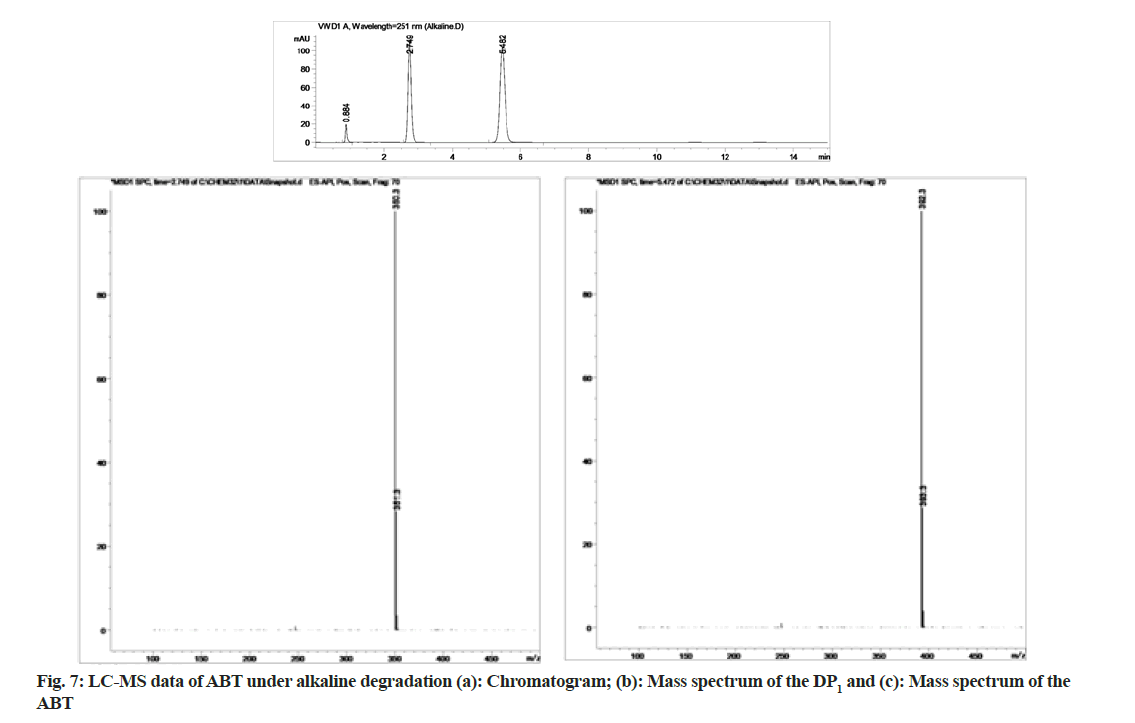
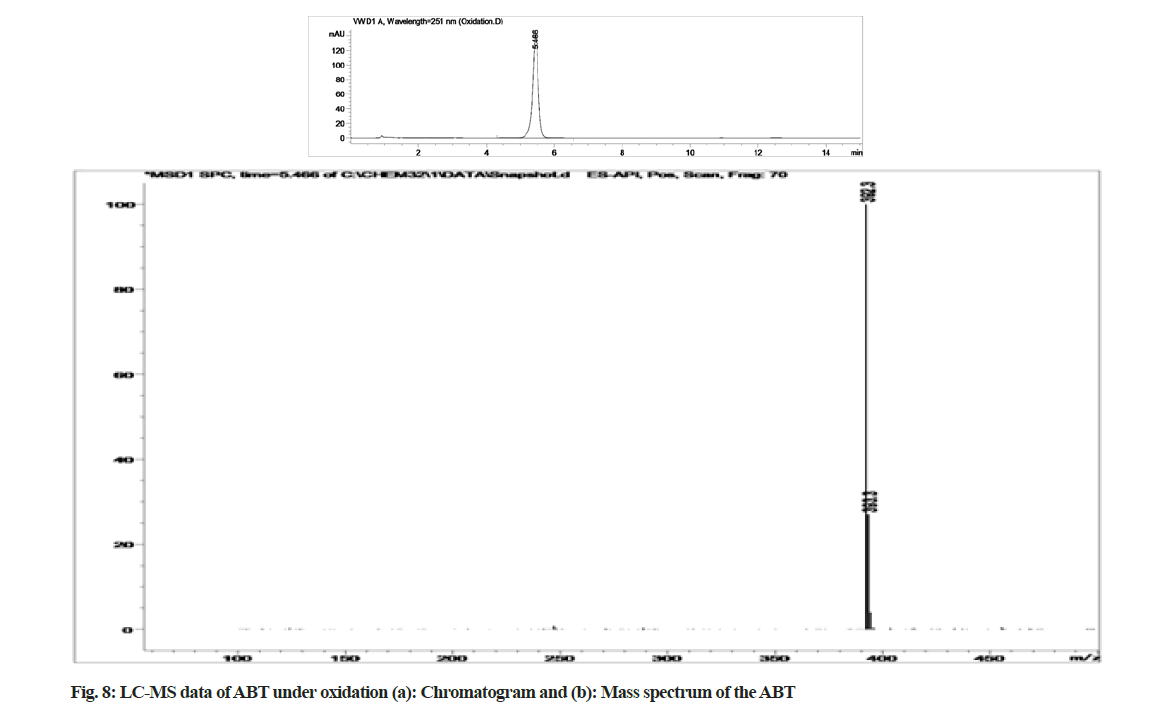
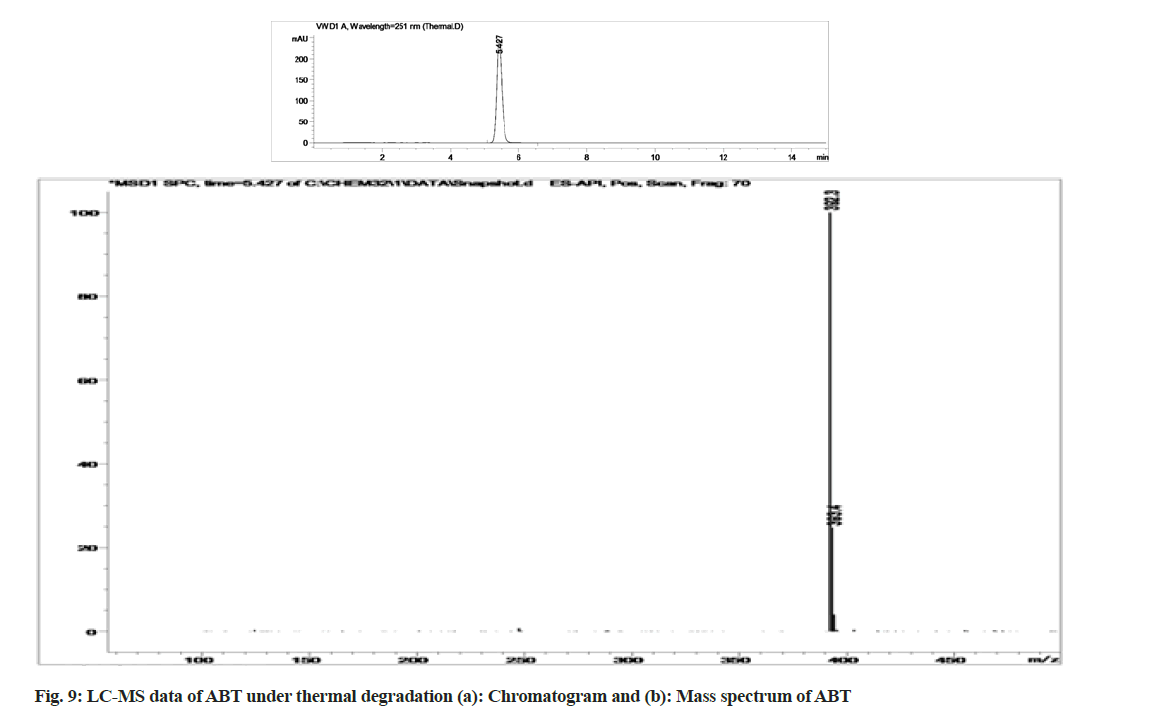
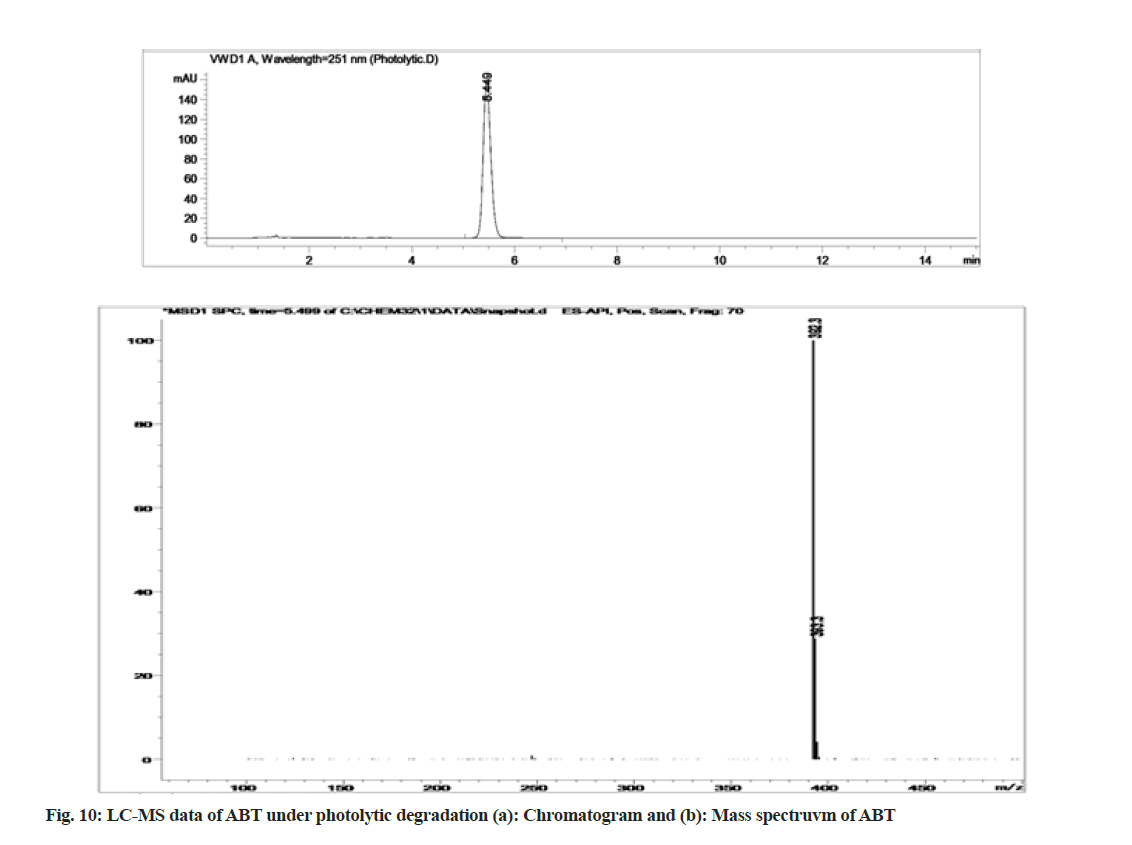
The stability indicating capability of the method was established from the separation of ABT peak from the degradation peaks of degraded samples. Typical chromatograms obtained following the assay of stressed samples are shown in fig. 3. The drug shows degradation in acidic (62.72 %) and alkaline (16.99 %) conditions. Whereas, it shows almost no decomposition when exposed to thermal (1.09 %), photolytic (0.34 %) and oxidative degradation (0.26 %) (Table 5). The Three- Dimensional (3D) chromatographs for the degradation studies were obtained from the PDA data which shows the selectivity of the wavelength and the degradation peaks at the wide range of wavelength (fig. 4). The system suitability parameters for all the degradation studies supporting the selectivity of the method were shown in Table 5.
| Stress Condition/media/duration | % Recovery* | % Drug degradation* | Theoretical plates (N) | Tailing factor | Resolution (R) | Peaks (min) | m/z values | Peak purity |
|---|---|---|---|---|---|---|---|---|
| Standard | 100 | - | 11398.5 | 1.25 | - | 5.43 | 392.3 | 1 |
| Acidic/0.1 N HCl/80°/1 h | 37.28 | 62.72 | 11591.64 | 1.12 | 16.15 | 5.43 | 392.3 | 1 |
| 2.77 | 350.3 | |||||||
| Alkaline/0.1 N NaOH/80°/1 h | 83.01 | 16.99 | 11251.61 | 1.23 | 15.718 | 5.47 | 392.3 | 0.9999 |
| 2.75 | 350.3 | |||||||
| Oxidative/30 % H2O2/40°/1 h | 99.74 | 0.26 | 9338.09 | 1.27 | - | 5.47 | 392.3 | 0.9999 |
| Thermal/80°/7 d | 98.91 | 1.09 | 9348.4 | 1.24 | - | 5.42 | 392.3 | 1 |
| Photolytic/7 d | 99.66 | 0.34 | 10861.28 | 1.31 | - | 5.45 | 392.3 | 0.9999 |
Note: *Mean of three replicates
Table 5: Forced degradation studies of Abt
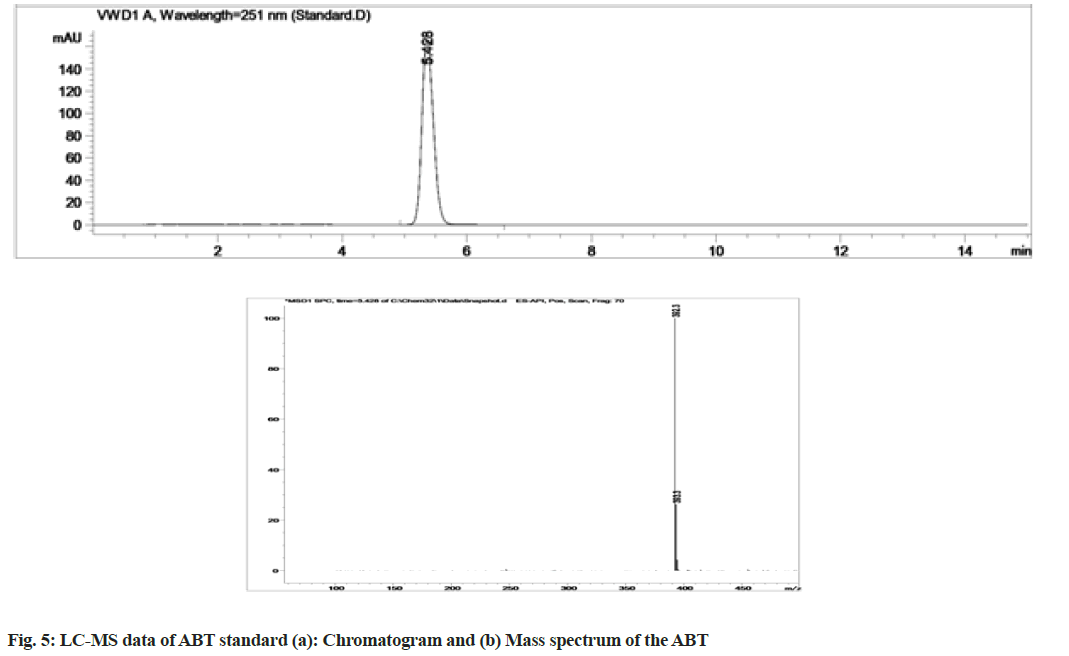
Stress degradation studies for ABT were conducted with different conditions such as acidic, alkaline, oxidative, thermal and photolytic degradations. After the degradations the separation was achieved using HPLC and further the samples were processed for LC-MS study for the characterization of ABT and its degradants (fig. 5-fig. 10). The positive ESI-MS of ABT shows an abundant [M+H]+ ion at m/z 392.3 (fig. 5).
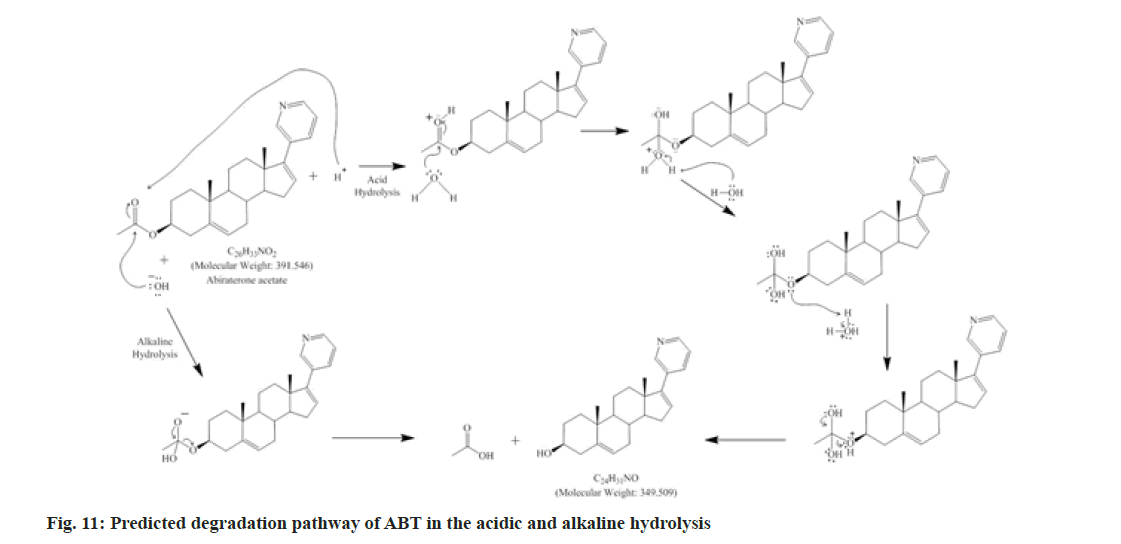
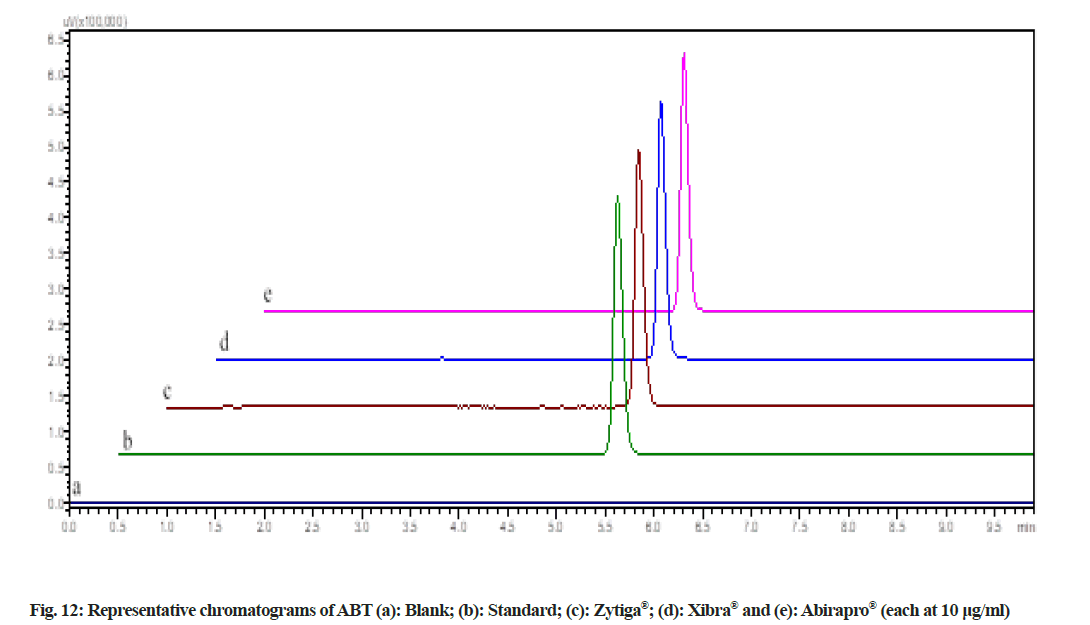
During acidic and alkaline degradations ABT shows sufficient degradation forming a single degradation peak (DP)1 with the retention time at 3.5 min as depicted in fig. 6 and fig. 7. The degradation product DP1 shows an abundant [M+H]+ ion at m/z 350.3 [M+H]+; C24H31NO, from the structure of ABT, DP1 can be assigned with the structure (3S,8R,9S,10R,13S,14S)- 2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-10,13-dimethyl-17-(pyridin-3-yl)-1H-cyclopenta[a] phenanthren-3-ol. The degradation pathway for the acidic/alkaline hydrolysis of ABT was predicted as shown in fig. 11. From this it was predicted that, the degradation product might be formed by preferably cleaved ABT, which lose one acetyl group.The proposed method was applied to the available marketed formulations (Zytiga®, Xibra® and Abirapro®) for the determination of ABT. The percentage recovery was found to be 97.54 %-98.87 % (Table 6). The resultant chromatograms obtained from the extraction of marketed formulations were shown in fig. 12.
| Formulation | Manufacturer | Labelled claim (mg) | Amount found* (mg) | % Recovery*±SD (% RSD) |
|---|---|---|---|---|
| Zytiga | Janssen-Cilag Ltd, India | 250 | 243.85 | 97.54±0.44 (0.45) |
| Xibra | Cipla labs, India | 250 | 247.18 | 98.87±0.13 (0.13) |
| Abirapro | Glenmark Pharmaceuticals, India | 250 | 244.63 | 97.85±0.28 (0.29) |
Note: *Mean of three replicates
Table 6: Analysis of abiraterone acetate in commercial formulations
In this work, an attempt made to develop a fast simple and robust stability-indicating RP-HPLC compatible with LC-ESI/MS for the determination of ABT in the presence of degradation product which was validated as per ICH guidelines and applied for the determination of ABT in pharmaceutical dosage form (film coated tablets). In the forced degradation studies, it was observed that ABT is more sensitive towards the acidic and slightly towards alkaline environment.
From the LC-ESI/MS study both the acidic and alkaline degradation show the same degradant and were identified thereby providing a prospect for the prediction of degradation pathway. This method can be successfully applied to perform long-term and accelerated stability studies of ABT formulations. This study might also be helpful in designing of the formulations.
Acknowledgments:
The authors are grateful to University Grants Commission (UGC), New Delhi, India for the financial support (UGC Ref No: 42-674/2013(SR), 2013) and also GITAM University, Visakhapatnam for providing research facilities.
Conflict of interest:
The authors have no conflict of interest.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61(2):69-90.
[Crossref] [Google Scholar] [PubMed]
- Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008;26(7):1148-59.
[Crossref] [Google Scholar] [PubMed]
- De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364(21):1995-2005.
[Crossref] [Google Scholar] [PubMed]
- Gong A, Zhu X. β-cyclodextrin sensitized spectrofluorimetry for the determination of abiraterone acetate and abiraterone. J Fluoresc 2013;23(6):1279-86.
[Crossref] [Google Scholar] [PubMed]
- Belleville T, Noé G, Huillard O, Thomas-Schoemann A, Vidal M, Goldwasser F, et al. A HPLC-fluorescence method for the quantification of abiraterone in plasma from patients with metastatic castration-resistant prostate cancer. J Chromatogr B Analyt Technol Biomed Life Sci 2015;989:86-90.
[Crossref] [Google Scholar] [PubMed]
- Khedr A, Darwish I, Bamane F. Analysis of abiraterone stress degradation behavior using liquid chromatography coupled to ultraviolet detection and electrospray ionization mass spectrometry. J Pharm Biomed Anal 2013;74:77-82.
[Crossref] [Google Scholar] [PubMed]
- Kumar SV, Rudresha G, Gurav S, Zainuddin M, Dewang P, Kethiri RR, et al. Validated RP?HPLC/UV method for the quantitation of abiraterone in rat plasma and its application to a pharmacokinetic study in rats. Biomed Chromatogr 2013;27(2):203-7.
[Crossref] [Google Scholar] [PubMed]
- Kavitapu D, Maruthapillai A, Mahapatra S, Selvi JA. New stability indicating RP-HPLC method for the determination of abiraterone acetate, its related substances and degradation products in bulk and dosage form. Mater Today Proc 2021;34:469-78.
- Beg S, Malik AK, Afzal O, Altamimi AS, Kazmi I, Al-Abbasi FA, et al. Systematic development and validation of a RP-HPLC method for estimation of abiraterone acetate and its degradation products. J Chromatogr Sci 2021;59(1):79-87.
[Crossref] [Google Scholar] [PubMed]
- Chandra Reddy BJ, Sarada NC. Development and validation of a novel RP-HPLC method for stability-indicating assay of abiraterone acetate. J Liq Chromatogr Relat Technol 2016;39(7):354-63.
- Gurav S, Punde R, Farooqui J, Zainuddin M, Rajagopal S, Mullangi R. Development and validation of a highly sensitive method for the determination of abiraterone in rat and human plasma by LC?MS/MS?ESI: Application to a pharmacokinetic study. Biomed Chromatogr 2012;26(6):761-8.
[Crossref] [Google Scholar] [PubMed]
- Hu C, Zhang H, Zhang M, Mi Z, Wang J, Lu W, et al. Identification, characterization and high-performance liquid chromatography quantification for process-related impurities in abiraterone acetate bulk drug. J Chromatogr Sci 2018;56(9):802-11.
- Wani TA. Highly sensitive ultra-performance liquid chromatography–tandem mass spectrometry method for the determination of abiraterone in human plasma. Anal Method 2013;5(15):3693-9.
- Martins V, Asad Y, Wilsher N, Raynaud F. A validated liquid chromatographic–tandem mass spectroscopy method for the quantification of abiraterone acetate and abiraterone in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2006;843(2):262-7.
[Crossref] [Google Scholar] [PubMed]
- Stability testing of new drug substances and products Q1A (R2), International Conference on Harmonization, IFPMA, Geneva; 2003.
- Validation of analytical procedures: Text and methodology Q2 (R1), International Conference on Harmonization, Geneva; 2005.