- *Corresponding Author:
- X. Hu
Department of Pharmacy,
Affiliated Hangzhou Chest Hospital,
Zhejiang University School of Medicine,
Zhejiang 310053,
China
E-mail: huxu1992@yeah.net
| Date of Received | 24 August 2020 |
| Date of Revision | 03 June 2021 |
| Date of Acceptance | 16 March 2022 |
| Indian J Pharm Sci 2022;84(2):311-318 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Based on plasma endotoxin/toll-like receptor-4/nuclear factor-kappa B signal pathway to investigate the protective effect of soothing liver mixture on mice with liver injury induced by antituberculosis drugs. A total of 72 Sprague Dawley male rats were randomly divided into blank control group, model group, silymarin group and soothing liver mixture group (low, medium and high dosage), with 12 rats in each group. 16 h after the last administration, the pathological changes of liver tissue were observed by hematoxylin eosin staining; blood samples were taken from eyeball to detect liver function indexes (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase) oxidative stress indexes (superoxide dismutase, malondialdehyde, glutathione peroxidase) and inflammatory factors (interleukin- 1beta, interleukin-6, tumor necrosis factor-alpha) and the content of plasma endotoxin in plasma; the microRNA expression levels of toll-like receptor-4 and nuclear factor-kappa B in rat liver were detected by real time reverse transcription–polymerase chain reaction. Soothing liver mixture can alleviate hepatocyte injury, effectively improve liver function in rats, attenuate oxidative stress and inflammatory reactions in the body, reduce plasma endotoxin content and inhibit hepatic toll-like receptor-4 and nuclear factorkappa B microRNA and protein expression, the higher the drug dose, the better the silymarin was similar to the high dose soothing liver mixture efficacy. Soothing liver mixture has a better protective effect on rats with liver injury induced by antituberculosis drugs; the mechanism may involve the regulation of plasma endotoxin/toll-like receptor-4/nuclear factor-kappa B signaling pathway the alleviation of the body's oxidative stress and inflammatory reaction.
Keywords
Plasma endotoxin, soothing liver mixture, antituberculosis drugs, liver injury, oxidative stress, inflammatory reaction
Drug Induced Liver Injury (DILI) refers to liver injury caused by the drug itself or its metabolites during drug administration. DILI is one of the most common adverse drug reactions and survey statistics show that DILI accounts for 3.0 %-9.0 % of all adverse drug reactions[1]. In China, liver injury caused by antituberculosis drugs is the most common, accounting for 21.99 %[2]. The incidence of DILI caused by firstline antituberculosis drugs in China is about 8 % -30 % and the higher DILI incidence seriously limits the clinical application of antituberculosis drugs[3]. Current research argues that Lipopolysaccharide (LPS)/ Toll-Like Receptor-4 (TLR4)/Nuclear Factor Kappa B (NF-κB) signal pathway plays a key role in the development of DILI and LPS can exacerbate hepatocyte injury by activating inflammatory factors and inducing oxidative stress and block TLR4/NF-κB signal pathway to inhibit oxidative stress and inflammatory reaction, becoming a research hotspot for intervention in the progression of DILI[4]. As Traditional Chinese Medicine (TCM) researches progress continuously, more and more scholars have found that TCMs can regulate TLR4/NF-κB signal pathway and play a therapeutic role, which has a high clinical value. This study aimed to observe the protective effect of soothing liver mixture on rats with liver injury induced by antituberculosis drugs and explore its mechanism, which may provide a certain reference value for clinical intervention in DILI.
Materials and Methods
Experimental animal:
Seventy-two Specific Pathogen Free (SPF) grade Sprague Dawley (SD) male rats with a body mass (200±20) g were purchased from Liaoning Changsheng Biotechnology Co., Ltd. (production license: SCXY (Liao) 2015-0001; use license: SYXK (Ji) 2016-0001). The rats were adaptively housed in an environment of room temperature (20°±2°), relative humidity 50 % to 60 % for 1 w with 12 h alternating light, during which time the rats were given free access to water. The experimental procedures strictly followed the "Guide for the Care of Laboratory Animals".
Drugs and reagents:
Soothing liver mixture formula: Radix bupleuri (R. bupleuri) 6 g, Sedum sarmentosum (S. sarmentosum) 20 g, Bran Paeonia lactiflora (P. lactiflora) Pallas 10 g, pericarpium citri reticulatae 10 g, Rhizoma Atractylodes macrocephalae (A. macrocephalae) fried with bran 10 g, fructus aurantii fried with bran 10 g, Salvia miltiorrhiza (S. miltiorrhiza) 15 g and Glycyrrhiza 3 g; the medicinal materials were Soothing liver mixture formula: Radix bupleuri (R. bupleuri) 6 g, Sedum sarmentosum (S. sarmentosum) 20 g, Bran Paeonia lactiflora (P. lactiflora) Pallas 10 g, pericarpium citri reticulatae 10 g, Rhizoma Atractylodes macrocephalae (A.macrocephalae) fried with bran 10 g, fructus aurantii fried with bran 10 g, Salvia miltiorrhiza (S. miltiorrhiza) 15 g and Glycyrrhiza 3 g; the medicinal materials were
Rifampicin (Guangdong South China Pharmaceutical Group Co., Ltd., Saudi Food and Drug Authority (SFDA) Approval No. H44020771, specification: 0.15 g×100 s); isoniazid (Shanxi Fenhe Pharmaceutical Co., Ltd. SFDA Approval No. H14022402, specification: 0.1 g×100 s); silymarin (Madaus GmbH (Germany), Approval No. H20181067, specification: 140 mg×10 s); Hematoxylin Eosin (HE) staining kit (Solarbio, Cat. No: G1120); Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Alkaline Phosphatase (ALP), Superoxide Dismutase (SOD), Malondialdehyde (MDA), Glutathione Peroxidase (GSH-Px) kits were all purchased from Nanjing Jiancheng Bioengineering Institute (Lot numbers: 20171012; 20171018; 20171019; 20170812; 20171020 and 20171215); LPS, Interleukin-1 beta (IL-1β), Interleukin-6 (IL-6), Tumor Necrosis Factor alpha ( TNF-α) kits were all purchased from Shanghai Jianglai Biotechnology Co., Ltd. (Cat. No: A21429; 1531673487; 1529727651 and 1533756928); Radioimmunoprecipitation Assay (RIPA) lysate (Beyotime Institute for Biotechnology, Lot No: P0008); TLR4 antibody (Abcam, Lot No: Ab72604); NF-κB antibody (Abcam, Lot: AB59346).
Main instrument:
Fully automatic biochemical analyser (Mindray, model BS-220); electrophoresis apparatus (Beijing Liuyi, model DYY-6C); high-speed freezing centrifuge (Thermo Fisher Scientific, model Fresco21); full wavelength microplate reader (BioTek, model epoch); microscope (Olympus, model BX51); gel imaging analyzers (BioTek, model ChemiDoc MP); fluorescence quantitative Polymerase Chain Reaction (PCR) instrument (BioTek, model ABI-7500).
Modeling and grouping:
Seventy-two SD male rats were randomly divided into blank control group, model group, silymarin group and soothing liver mixture group (low, medium and high dosage), with 12 rats in each group. In addition to the blank control group, all other groups were treated with rifampicin 100 mg/kg by gavage with isoniazid 100 mg/kg for modeling and 10 ml/kg by gavage once daily for 8 w.
Experimental administration:
During antituberculosis drugs administration, rats in each group were treated by drug gavage. According to the dose division between human and animal, the equivalent dosage of TCM for rats was 1.37 g/100 g rat weight, the rats in low medium and high dose soothing liver mixture groups were respectively gavage at 50, 100, 200 mg/kg doses, the silymarin group was gavage at 180 mg/kg doses and the blank control group and model group were gavage with equal volume of normal saline, which was continuously administered for 8 w. The dead rats were excluded and the modeling was judged whether to be successful according to the relationship between histopathological changes in the rat liver and changes in serum liver function indexes in subsequent studies. The success animal modeling criteria of DILI[5]: ALT and AST were increased 1-3 times and liver pathological sections showed various degrees of hepatocellular necrosis, portal inflammation and so on.
Observation indicators:
After the last administration, rats in each group were fasted without water for 16 h and the rats were anesthetized using 3.5 % chloral hydrate and sacrificed by cervical dislocation, blood was collected from eyeballs and liver tissues were collected.
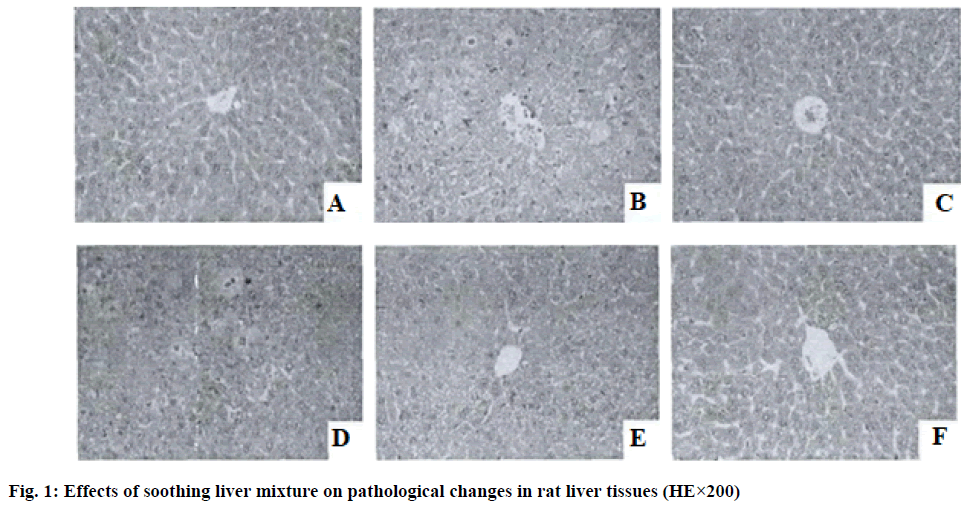
Pathological changes in rat liver tissue (Haemotoxylin and Eosin (HE) staining): The same section of the right lobe of rat liver was cut to 1 cm×1 cm×0.5 cm liver tissue, fixed in 4 % formalin solution, dehydrated, transparent and embedded in paraffin, prepared to sections with a thickness of 4 μm and the pathological changes in the liver tissues of rats in each group were observed by HE staining method under a 200 lens.
Biochemical indexes detection in rats: For LPS content measurement, 3-4 ml blood samples were collected from rats, centrifuged at 3000 r/min for 15 min and yellow plasma was collected into Eppendorf (EP) tubes and stored at -40° in a freezer for further use. The LPS content of rat plasma was detected by using the LPS kit and each operation was performed strictly according to the kit's instructions; liver function index detection: The serum levels of ALT, AST and ALP of rats were detected by an automatic biochemical analyzer; oxidative stress indicators and inflammatory factors detection: 5 ml of blood samples were collected from rats, centrifuged at 3000 r/min for 15 min and the blood supernatant was collected into EP tube and stored at -70° in a freezer for further use. SOD was measured by the xanthine oxidase method, MDA by the thiobarbituric acid method, while the remaining indexes were measured by the Enzyme- Linked Immunosorbent Assay (ELISA) and each procedure was performed strictly according to the kit instructions.
Rat liver tissue TLR4 and NF-κB microRNA (miRNA) expression detection: 50 mg liver tissue was obtained from rats, Trizol solution was added to extract total RNA and complimentary DNA was synthesized and amplified for the purpose according to the reverse transcription kit instructions. Reaction system was 0.4 μl upstream primer and downstream primer each, 2 μl of template, 7.2 μl of double distilled water and 10 μl of master mix. Reaction conditions was 95° 5 min→95° 15 s→60° 60 s with 40 cycles. Primer sequences are detailed in Table 1. The Real Time Reverse Transcription-Polymerase Chain Reaction (RTPCR) system was used to perform lysis curve analysis and the miRNA expression levels were calculated by the 2-ΔΔCT method.
| Gene | Primer sequences | Product length (bp) |
|---|---|---|
| TLR4 | Forward:5’-ATGGCATGGCTTACACCACC-3’ | 237 |
| Reverse:5’-GAGGCCAATTTTGTCTCCACA-3’ | ||
| NF-kB | Forward:5’-GGTCACCCATGGCACCATAA-3’ | 231 |
| Reverse:5’-AGCTGCAGAGCCTTCTCAAG-3’ | ||
| actin | Forward:5’-CCCATCTATGAGGGTTACGC-3’ | 116 |
| Reverse:5’-TTTAATGTCACGCACGATTT-3’ |
Note: TLR4: Toll-Like Receptor-4 and NF-kB: Nuclear Factor Kappa B
Table 1: TLR4/NF-KB Primer Sequences
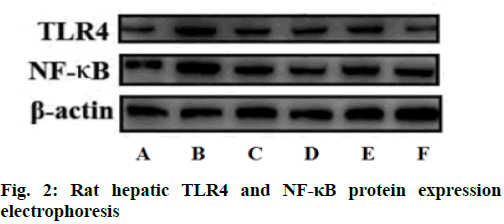
Rat liver tissue TLR4 and NF-κB expression assay (Western blotting): 50 mg liver tissue was collected and ground in liquid nitrogen and 100 μl pre-cooled RIPA lysate was added, standing on ice for 30 min. Protein quantification was performed by Bicinchoninic Acid (BCA) protein concentration and 30-50 μg were loaded on the Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), after which running gel, transmembrane, blocking and other operations were completed. Protein antibody was added to incubate overnight, Tris-Buffered Saline with 0.1% Tween® 20 detergent (TBST) washed the membrane and primary antibody was added, put in 4° freezer incubation overnight. The next day rinsed with Phosphate Buffered Saline (PBS), secondary antibodies were added, incubated at 37° for 1 h and Enhanced Chemiluminescence (ECL) was developed, after which relevant protein expression levels were assessed by grey scale analysis.
Statistical methods:
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) 22.0. The measurement data were expressed by (x̄±s), normally distributed and homogeneous in variance and the independent samples t-test was used for comparison between groups, while the paired t-test was used for comparison within groups and p<0.05 was considered statistically significant.
Results and Discussion
In the blank control group rats, the hepatocytes of the liver tissue were arranged in an orderly manner, the structure of the hepatic lobules was intact, the hepatocytes dispersed outward from the central vein showed a radial arrangement and no hepatocyte necrosis and inflammatory cell infiltration were observed in the pericentral and confluent zones of the central vein; in the model group, the structure of the liver tissue was significantly damaged, with variably shaped nodules appearing around the hepatic lobules, disorganized hepatocyte cords and marked inflammatory cell infiltration around the central vein vs. the confluent zone; the liver tissue structure of the rats in the silymarin group was almost normal and scattered inflammatory cell infiltration was seen around the hepatic lobules and the pericentral and confluent zones of the central vein and the degree of liver injury was significantly reduced in the silymarin group compared with the model group; soothing liver mixture can effectively improve the liver tissue lesions of rats, as the drug dose increases, the liver tissue damage of rats continuously alleviates, the high dose soothing liver mixture group rats have intact hepatocyte cords and hepatic lobule structure, the inflammatory cell infiltration of the pericentral and confluent zones of central vein is significantly alleviated and the efficacy is similar to that of silymarin group as shown in fig. 1.
The levels of ALT, AST and ALP in the serum of rats in all other groups were significantly increased compared with the blank control group (p<0.05); compared with the model group, the serum ALT, AST and ALP levels of rats in the silymarin group and the soothing liver mixture group at each dose were significantly decreased (p<0.05); compared with the low and middle dose soothing liver mixture groups, the serum ALT, AST and ALP levels of rats in the high dose soothing liver mixture group were significantly decreased (p<0.05); there was no significant difference between the high dose soothing liver mixture group and silymarin group in all the indexes of liver function in serum samples (p>0.05) as shown in Table 2.
| Group | Number of animals (n) | Dose (mg/kg) | ALT | AST | ALP |
|---|---|---|---|---|---|
| Blank control group | 8 | - | 32.14±1.15 | 53.46±5.04 | 47.25±5.34 |
| Model group | 8 | - | 63.85±6.03a | 84.57±6.69a | 151.46±18.79a |
| Silymarin group | 8 | 180 | 40.19±2.70ab | 58.02±4.04ab | 96.52±17.76ab |
| Low dose soothing liver mixture group | 8 | 50 | 57.26±3.87abc | 78.94±6.53abc | 128.31±16.59abc |
| Medium dose soothing liver mixture group | 8 | 100 | 43.74±2.28abc | 63.54±5.16abc | 103.47±12.74abc |
| High dose soothing liver mixture group | 8 | 200 | 37.62±1.57ab | 52.39±3.88ab | 88.27±10.26ab |
Note: Compared with the blank control group, ap<0.05; compared with the model group, bp<0.05 and compared with the high dose soothing liver mixture group, cp<0.05. ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase and ALP: Alkaline Phosphatase
Table 2: Effects of Soothing Liver Mixture on Serum Liver Function Indexes in Rats (IU/l)
When compared with the blank control group, the levels of SOD and GSH-Px in the serum of rats in all other groups were significantly decreased (p<0.05) and the levels of MDA were significantly increased (p<0.05); compared with the model group, the serum SOD and GSH-Px levels of rats in the silymarin group and the soothing liver mixture group at each dose were significantly increased (p<0.05), while the MDA levels were significantly decreased (p<0.05); compared with the low dose soothing liver mixture group, the serum levels of SOD and GSH-Px were significantly increased (p<0.05), while the levels of MDA were decreased (p<0.05) in the high dose soothing liver mixture group; there were no significant differences in all oxidative stress indexes in the serum between rats in the high dose soothing liver mixture group and those in silymarin group or the medium dose soothing liver mixture group (p>0.05) as shown in Table 3.
| Group | Number of animals (n) | Dose (mg/kg) | SOD (U/ml) | MDA (nmol/ml) | GSH-Px (U/ml) |
|---|---|---|---|---|---|
| Blank control group | 8 | - | 236.49±20.68 | 3.27±0.31 | 288.46±31.37 |
| Model group | 8 | - | 161.49±18.37a | 5.61±0.63a | 208.43±23.81a |
| Silymarin group | 8 | 180 | 198.42±20.49ab | 4.57±0.44ab | 254.59±26.34ab |
| Low dose soothing liver mixture group | 8 | 50 | 182.47±19.92abc | 4.98±0.54abc | 233.46±20.57abc |
| Medium dose soothing liver mixture group | 8 | 100 | 199.72±21.48ab | 4.64±0.21ab | 246.54±23.61ab |
| High dose soothing liver mixture group | 8 | 200 | 216.73±22.09ab | 4.35±0.46ab | 266.47±25.68ab |
Note: Compared with the blank control group, ap<0.05; compared with the model group, bp<0.05 and compared with the high dose soothing liver mixture group, cp<0.05. SOD: Superoxide Dismutase; MDA: Malondialdehyde and GSH-Px: Glutathione Peroxidase
Table 3: Effects of Soothing Liver Mixture on Serum Oxidative Stress Indicators in Rats (x̄±s)
When compared with the blank control group, serum IL-1β, IL-6 and TNF-α levels were all significantly increased (p<0.05); compared with the model group, serum IL-1β, IL-6 and TNF-α levels of silymarin group and soothing liver mixture group were all significantly reduced (p<0.05); serum IL-1β, IL-6 and TNF-α of rats in silymarin group and high dose soothing liver mixture group had no significant difference (p>0.05) as shown in Table 4.
| Group | Number of animals (n) | Dose (mg/kg) | IL-1β (pg/mg) | IL-6 (pg/mg) | TNF-α (ng/mg) |
|---|---|---|---|---|---|
| Blank control group | 8 | - | 4.11±0.28 | 8.72±0.94 | 5.27±0.84 |
| Model group | 8 | - | 8.49±1.16a | 15.65±2.04a | 15.68±2.14a |
| Silymarin group | 8 | 180 | 6.48±0.85ab | 13.38±1.84ab | 10.23±1.28ab |
| Low dose soothing liver mixture group | 8 | 50 | 7.32±0.94abc | 14.73±1.92ac | 13.05±1.47abc |
| Medium dose soothing liver mixture group | 8 | 100 | 6.57±0.76abc | 13.86±1.88ac | 10.72±1.65ab |
| High dose soothing liver mixture group | 8 | 200 | 5.81±0.57ab | 11.87±1.49ab | 9.19±1.38ab |
Note: IL-1β: Interleukin-1 beta; IL-6: Interleukin-6 and TNF-α: Tumor Necrosis Factor alpha
Table 4: Effects Of Soothing Liver Mixture on Serum Levels of Inflammatory Factors in Rats (x̄±s)
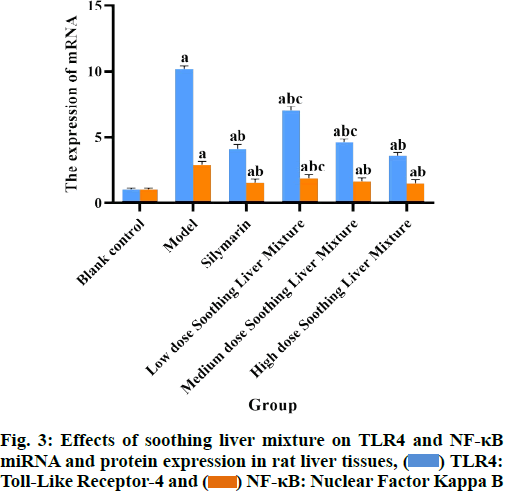
Compared with the blank control group, the levels of LPS and the levels of TLR4 and NF-κB miRNA and protein expression in the plasma of rats in all other groups were significantly increased (p<0.05); compared with the model group, the levels of LPS and TLR4 and NF-κB miRNA and protein expression in the plasma of rats in the silymarin group and the soothing liver mixture group at each dose were significantly reduced (p<0.05); compared with low dose soothing liver mixture group, the plasma LPS content and TLR4 and NF-κB miRNA and protein expression in high dose soothing liver mixture group were significantly reduced (p<0.05); LPS levels and TLR4 and NF-κB miRNA and protein expression in plasma of rats in silymarin group and high dose soothing liver mixture group had no significant differences (p>0.05) as shown in Table 5 and fig. 2-fig. 4.
| Group | Number of animals (n) | Dose (mg/kg) | LPS (EU/ml) | miRNA expression levels | Protein expression levels | ||
|---|---|---|---|---|---|---|---|
| TLR4 | NF-κB | TLR4 | NF-κB | ||||
| Blank control group | 8 | - | 0.22±0.20 | 1.00±0.10 | 1.00±0.00 | 1.00±0.10 | 1.00±0.00 |
| Model group | 8 | - | 1.61±0.78a | 10.18±0.24a | 2.92±0.26a | 3.13±0.28a | 2.64±0.25a |
| Silymarin group | 8 | 180 | 0.82±0.57ab | 4.12±0.33ab | 1.53±0.27ab | 1.75±0.18ab | 1.35±0.18ab |
| Low dose soothing liver mixture group | 8 | 50 | 1.03±0.55abc | 7.01±0.34abc | 1.87±0.26abc | 2.32±0.24abc | 1.54±0.19abc |
| Medium dose soothing liver mixture group | 8 | 100 | 0.74±0.49ab | 4.62±0.25abc | 1.64±0.25ab | 1.81±0.27abc | 1.38±0.14ab |
| High dose soothing liver mixture group | 8 | 200 | 0.48±0.29ab | 3.58±0.29ab | 1.48±0.29ab | 1.26±0.20ab | 1.27±0.19ab |
Note: Compared with the blank control group, ap<0.05; compared with the model group, bp<0.05 and compared with the high dose soothing liver mixture group, cp<0.05. LPS: Lipopolysaccharide; TLR4: Toll-Like Receptor-4 and NF-κB: Nuclear Factor Kappa B
Table 5: Effects of Soothing Liver Mixture on TLR4 and NF-kB Mirna and Protein Expression in Rat Liver Tissues (x̄±s)
LPS is the main component of endotoxin, which is able to produce oxygen free radicals to cause lipid peroxidation to damage hepatocytes[6], but also promote the synthesis and release of inflammatory mediators, chemotactic neutrophils and other various pathways to cause microcirculation disorders in liver tissue, resulting in hepatocyte hypoxia ischemia and causing mitochondrial damage, followed by induction of hepatocyte apoptosis and necrosis, ultimately leading to liver failure[7]. LPS has been shown to play an important role in liver injury and its mechanism is complex and involves several signal pathways, the most critical of which is TLR4/NF-κB signal pathway[8]. TLR4 plays an important role in immune response and inflammatory reaction, which can recognize xenoantigens and act through signaling to produce proinflammatory factors, being a gateway protein for inflammatory signal transmission[9]. LPS binds to TLR4 and signals intracellularly and activates IkappaB Kinase (IKK) to induce IκB undergoes ubiquitination for degradation with phosphorylation and activates NF-κB signal pathway that promotes IL-1β, IL-6 and TNF-α and other inflammatory factors to be released in large amounts[10]. Inflammatory factors can be divided into anti-inflammatory cytokines and pro-inflammatory cytokines, among which IL-1β, IL-6 and TNF-α all belong to proinflammatory cytokines and can form a cascade amplifying effect through feedback regulation, inflammatory factors can again activate NF-κB signal pathway, so as to form an inflammatory waterfall effect and aggravate liver injury[11]. It can be seen that blocking oxidative stress damage and inhibiting TLR4/NF-κB signal pathway is of great significance in arresting the progression of liver damage.
The current study found that the mechanism by which rifampicin causes DILI may lie in the blockade of intrahepatic cholestasis caused by hepatic bile metabolism[12]; the mechanism by which isoniazid causes DILI may lie in the fact that its metabolites can cause oxidative stress reaction in the body and cause oxidative type liver injury[13]. The disease meets the criteria of the dialectical treatment of TCM and in TCM, DILI is actually dampness and heat in course of time, liver Yin loss, liver disease affecting spleen such that the spleen is deficient and deficiency, long time then deficiency of both Qi and Yin. Dampness and internal heat inclusion depletes Qi and blood fluid such that stasis poison endogenous growth and poor flow of Qi and blood, and stasis poison cannot be discharged so that the condition is aggravated continuously[14]. In this study, soothing liver mixture was used to treat liver injury induced by antituberculosis drugs, in the formula, R. bupleuri is acrid and bitter in taste and cold in nature, functioning as soothing the liver and invigorating splenic yang, and reconciling superficies and interior; S. sarmentosum can clear heat and detoxify, drain dampness and degrade jaundice, conduct unobstructed flow of Qi, and clear dampness and heat effectively; Bran P. lactiflora Pallas may suppress liver Yang and nourish blood and collect Yin; Rhizoma A. macrocephalae fried with bran can dry dampness and alleviate water retention, replenish Qi and strengthen spleen and co-treat with S. sarmentosum for the syndrome of spleen deficiency stagnation; fructus aurantii fried with bran can cure stagnation and swelling, regulating vital energy and relax philtrum; S. miltiorrhiza induces menstruation to relieve menalgia, invigorates blood flow and removes blood stasis; pericarpium citri reticulatae removes dampness to reduce phlegm, treat spleen and stomach diseases; and liquorice reconciles these herbs. The whole formula functions as eliminating dampness and heat, removing stasis, clearing the hollow viscera, relieving diarrhea turbidity, clearing away heat and toxic materials and soothing the liver and invigorating splenic yang. Modern pharmacology has shown that saikosaponin-d can play an anti-hepatic fibrosis role by inhibiting TLR4/NF-κB signal pathway[15]; the compound extract of S. sarmentosum can inhibit the degree of lipid peroxidation in hepatocytes and improve the ability of hepatocytes to scavenge oxygen free radicals[16]; total glucosides of paeony can inhibit the progression of inflammation in rats with nonalcoholic fatty liver disease by down-regulating High Mobility Group-B1 (HMGB1)/TLR4 signal expression[17]; Atractylenolides I and III from Rhizoma A. macrocephalae are able to inhibit activity of IL-1β, IL-6 and TNF-α and other inflammatory factors[18]; Hesperidin and its derivatives in fructus aurantii have certain anti-inflammatory activity, while they exhibit the effect of inhibiting hepatocyte apoptosis[19]; water soluble phenolic acids and fatsoluble diterpenoids, the main active components of S. miltiorrhiza, have been shown to have effects such as improving microcirculation and anti-inflammation[20], which fit well with the mechanism of DILI disease.
In this study, we show that liver tissue architecture is significantly damaged in rats with liver injury induced by antituberculosis drugs and the body has expanded oxidative stress and inflammatory reaction, as well as increased TLR4 and NF-κB miRNA and protein expression levels were significantly unregulated. Soothing liver mixture can alleviate liver injury in rats, reduce body oxidative stress and inflammatory reaction, down-regulate TLR4, NF-κB miRNA and protein expression levels. These results suggest that soothing liver mixture may inhibit LPS/TLR4/NF-κB signal pathway, which reduces oxidative stress injury in liver tissue with the release of inflammatory factors, thereby protecting hepatocytes and improving liver function in rats.
Taken together, soothing liver mixture may play a protective role against tuberculosis drug-induced liver injury by inhibiting LPS/TLR4/NF-κB signal pathway. Although the findings suggest that the mechanism of action of soothing liver mixture may be linked to LPS/ TLR4/NF-κB signal pathway, this study failed to target this pathway for in-depth study and future experiments should be conducted through cell models for more meticulous exploration.
Conflict of interests:
The authors declared no conflict of interest.
References
- Yang X, Tu R, Yang J. Research advances in drug-induced liver injury. J Clin Hepatol 2020;36(3):509-13.
- Yang M, Li Z, Dou D. Current analysis of an epidemiological study of drug-induced liver injury in mainland China. J Integr Tradit Chin West Med Liver Dis 2020:30(1);13-6.
- Guo R, Zhang L. A review of pathogenesis of drug induced liver injury. J Integr Tradit Chin West Med Liver Dis 2020;30(1):27-30.
- Zhang L, Lu Y. Mechanisms of drug-induced liver injury in first-line antituberculosis drugs. Clin Med J 2020;18:(7):21-5.
- Luo X, Du Q, Deng Y. Model establishment of rifampicin induced drug liver injury in rats and evaluation of nephrotoxicity. Chin J Clin Pharmacol 2020;36(11):1488-91.
- Ji M, Lou Z, Tan M. Interventionist study of Aconitum carmichaeli Radix extracts on portal vein endotoxin and small intestinal histomorphology in rats with alcoholic liver injury. Zheji Med J 2018;40(6):551-72.
- Ding R, Zhao D, Hu Z. Unfractionated heparin alleviates liver injury in endotoxemic mice by inhibiting inflammatory response and apoptosis. Adv Anat Sci 2017;23(5);502-5.
- Niu Y, Liu J, Liu X. Action mechanism of detoxification purgation and eliminating stasis formula based on TLR4/NF-κB pathway on intervention of endotoxin induced liver injury. Li Shizhen Med Materia Med Res 2020;31(5);1029-33.
- Zhang L, Ma X, Wang J. Kidney-failure diet influence against TLR4/MyD88/NF-κB signal pathways and inflammatory status in CRF rats. Chin J Gerontol 2021;41(2):345-50.
- Wang Y, Gao Y, Li F. Effects of Yi Qi Huo Xue Hua Yu Decoction combined with hepatic arterial chemoembolization on TLR4 receptors and downstream inflammatory factors in patients after hepatic resection. Chin J Integr Tradit West Med Liver Dis 2021;31(3):234-7.
- Hou D, Liu Z, Chui S. Tanshinone II-A modulates TLR4/IκBα/NFκB signal pathway to inhibit LPS induced cellular inflammation. Chin Pharmacol Bull 2021;37(2);210-4.
- Wang C, Cheng F, Huang B. Protective effect and mechanism of aqueous extract of persimmon leaf against liver injury induced by combination of isoniazid and rifampicin in mice. J Guangxi Med Univ 2020:37(1);37-43.
- Peng XY, Luo XH, Yang Q, Cheng ML, Han B, Xie RJ. Interventional effect of bicyclol on isoniazid-induced liver injury in rats and the expression of glucose-regulated protein 78, and growth arrest and DNA-damage-inducible gene 153. Zhonghua Gan Zang Bing Za Zhi 2019;27(2):133-9.
- Wang X, An J, Wang Z. Protective effects of TFP on antituberculosis drugs-induced liver injury in mice. Chin J Clin Pharmacol 2021;37(6):713-7.
- Zhai Y, Zhao Y, Xiang R. Study on action mechanism of the anti-liver cancer effect of Xiao Chaihu Tang based on weighted network pharmacology. Chin J Med Chem 2020;30(11);658-68.
- Qian L, Zhang W, Lv L. Effects of compound extracts of Sedum sarmentosum on D-galactosamine-induced acute liver injury in mice. Hebei J Tradit Chin Med 2018;40(10):1540-5.
- Zhang C, Yang L, Li J. Exploration of the action mechanism of Paeonia lactiflora Pallas-Radix bupleuri medicine on the treatment of hepatocellular carcinoma using network pharmacology. Chin Arch Tradit Chin Med 2020;38(2):175-9.
- Wang H, Kang X, Zhu Y. Exploring the underlying molecular mechanism of Rhizoma Atractylodis macrocephalae in the treatment of ulcerative colitis based on network pharmacology and molecular docking techniques. J Zhejiang Chin Med Univ 2020;44(9):916-23.
- Li C, Yang Y, Leng D. Study progress of chemical composition and pharmacological effects of fructus aurantii. J Liaoning Univ Tradit Chin Med 2019:21(2);158-61.
- Cheng P, Huang S, Yang Y. Research on anti-inflammatory effect and molecular mechanism of Salvia miltiorrhiza-Sophora flavescens extract by network pharmacology. Chin Pharmacol Bulletin 2021:37(2);270-6.
 ) TLR4:
Toll-Like Receptor-4 and (
) TLR4:
Toll-Like Receptor-4 and ( ) NF-κB: Nuclear Factor Kappa B
) NF-κB: Nuclear Factor Kappa B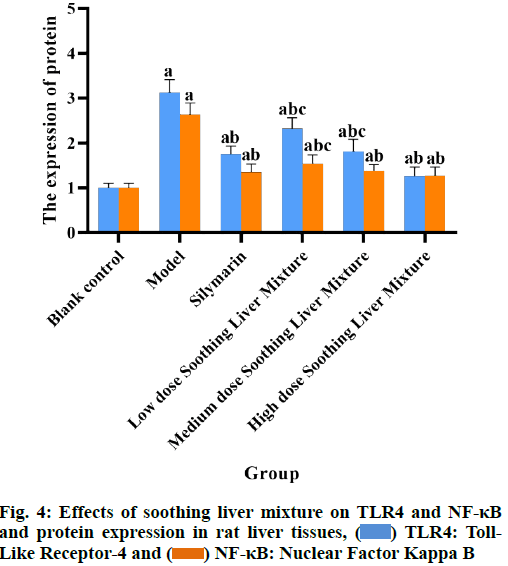
 ) TLR4: Toll-
Like Receptor-4 and (
) TLR4: Toll-
Like Receptor-4 and ( ) NF-κB: Nuclear Factor Kappa B
) NF-κB: Nuclear Factor Kappa B



