- *Corresponding Author:
- Yang Gao
Department of Stomatology, Jiaozhou Central Hospital Of Qingdao, Shandong 266000, China
E-mail: zhangjiaruiwzn@163.com
| This article was originally published in a special issue, “Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “8-17” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of this study was to select the optimal ratio of a compound Chinese medicine mouthwash containing Magnolia officinalis, Galla chinensis and Geranium strictipes and to evaluate the effectiveness of caries prevention through animal experiments, providing a scientific basis for the clinical application of compound Chinese medicine mouthwash. Using a uniform design method to screen the optimal compatibility of three drugs, rat caries model was established using 30 Sprague-Dawley rats were selected and randomly divided into a traditional Chinese medicine experimental group, positive control group and blank control group, each with 10 rats. Streptococcus mutans was inoculated and administered in the cariogenic feed diet 2000 and 5 % sucrose solution. Rat mouths were rinsed with compound Chinese medicine mouthwash (Chinese medicine experimental group), chlorhexidine mouthwash (positive control group) and distilled water (blank control group). After continuous treatment for 4 w, the rats were euthanized; the effectiveness and safety of the compound Chinese medicine mouthwash in preventing caries were comprehensively evaluated through colony counting, the Keyes scoring method, changes in rat weight, the organ coefficient, and hematoxylin and eosin staining of the buccal mucosa. The minimum inhibitory concentrations of Magnolia officinalis, Galla chinensis and Geranium strictipes against Streptococcus mutans are 12, 24 and 30 mg/ml, respectively. The optimal formula included 18 mg/ml of Magnolia officinalis, 48 mg/ml of Galla chinensis and Geranium strictipes of 30 mg/ml. After counting the colonies in rats, it was found that the positive control group and the traditional Chinese medicine experimental group were significantly lower than the blank control group in three indicators, total colony count, Streptococcus mutans colony count and Streptococcus mutans colony level. There was no significant difference between the positive control group and the traditional Chinese medicine experimental group. Keyes caries score for the Chinese medicine experimental group was significantly lower than that for the blank control group in all four levels of caries. After changes in rat body weight, organ coefficients, and hematoxylin and eosin staining of rat cheek mucosa, it was found that there was no significant difference between the Chinese medicine experimental group and the blank control group. The compound Chinese medicine mouthwash has a significant inhibitory effect on the growth of Streptococcus mutans and dental caries in rats and it has a certain degree of biosafety as a local oral medication.
Keywords
Streptococcus mutans, levothyroxin, thyroid-stimulating hormone, thyroxine, thyroglobulin antibody, thyroid peroxidase antibody
Caries is a disease in which dental hard tissue undergo chronic and progressive damage under the influence of various factors, mainly bacteria[1]. World Health Organization (WHO) ranks dental caries as the 3rd most harmful disease to human health after cardiovascular disease and cancer. Caries is also one of the complications of orthodontic treatment and its main manifestation during the orthodontic process is enamel demineralization[2]. Caries is caused by the acid production of cariogenic bacteria in a suitable environment, leading to demineralization of hard tissues in teeth[3]. Among them, Streptococcus mutans (S. mutans) is one of the main cariogenic bacteria[4]. Early enamel demineralization has a significant impact on the microesthetics of orthodontic treatment[5]. After enamel demineralization, opaque spots or plaques appear on the tooth surface, which not only affect the patient's appearance and endangers dental health. In severe cases, orthodontic treatment may even need to be discontinued[6]. Although the invisible orthodontic technique without brackets optimizes the difficulty of oral hygiene compared to fixed orthodontic techniques, the large coverage area and long wearing time of invisible orthodontic appliances can also cause enamel demineralization in poor oral hygiene control, especially in the adolescent population[7].
Natural medicines have a long history of application, low toxicity, side effects which are easy to obtain and is cost-effective; they even have bactericidal and anti-inflammatory effects. Therefore, they are widely used in basic research and clinical practice[10]. Traditional Chinese medicine preparations are widely used in oral cavity and a large number of studies have shown that traditional Chinese medicine has good effects in inhibiting the growth of cariogenic bacteria[11]. However, currently, most of the reports on this type of traditional Chinese medicine focus on the effects of a single herb and there is limited research on traditional Chinese medicine formulas. Traditional Chinese medicine formulas reflect the characteristics of traditional Chinese medicine symptom differentiation and treatment, fully leveraging the synergistic effects of drugs and the multitarget effects of multiple effective ingredients[12]. With increasing attention to the safety of mouthwash, the advantages of traditional Chinese medicine mouthwash are gradually becoming apparent. However, there is a lack of systematic research on traditional Chinese medicine mouthwashes from basic components to application, leading to gaps in scientific and objective knowledge of their therapeutic effects. There are few reports on their toxicity and adverse reactions, failing to provide an experimental basis for clinical application.
Studies have shown that Magnolia officinalis, Galla chinensis and Geranium strictipes have inhibitory effects on S. mutans[13-15]. Huang et al.[16], research has shown that Magnolia officinalis has a significant inhibitory effect on glucosyltransferase, which plays a crucial role in the development of dental caries. Zhang et al.[14], summarized the anti caries effect of Galla chinensis, suggesting that it can inhibit oral cariogenic bacteria, inhibit tooth demineralization and promote tooth mineralization. Xie et al.[17], suggests that Galla chinensis may inhibit the cariogenicity of the oral biofilm and it appears to be a promising source of new agents that may prevent dental caries. Peng et al.[15], confirmed through animal model experiments that Geranium strictipes has an inhibitory effect on dental caries. Yang et al.[18], also confirmed that Geranium strictipes can inhibit the formation of dental caries. Therefore, based on the theory of compound formulas in traditional Chinese medicine[19], it is reasonable to predict that the compound formulas of these three traditional Chinese medicines may have excellent anti-caries effects. However, there have been no research reports on the combination of these three drugs for the prevention and treatment of dental caries. This study compounded these three traditional Chinese medicines and determined their optimal proportions through a uniform design method to prepare a compound Chinese medicine mouthwash. Through the preparation and animal experiments of compound Chinese medicine mouthwash, the biological safety and caries inhibition effect of this mouthwash were studied, providing a scientific basis for further development of safe and effective compound Chinese medicine mouthwash.
Materials and Methods
Preparation of compound Chinese medicine mouthwash:
Materials: Magnolia officinalis, Galla chinensis and granules of Geranium strictipes were obtained from EFong Pharmaceuticals. Further, 2,3,5-Triphenyltetrazolium Chloride (TTC), Brain Heart Infusion (BHI) culture medium and Mitis salivarius (MS) agar base were used in this study.
Bacterial strain and culture conditions: The strain used in this experiment was S. mutans (American Type Culture Collection (ATCC), 25 175) and was obtained from the China General Microbiological Culture Collection Center (CGMCC), which was anaerobically cultured in BHI medium at 37° in an incubator containing 800 ml/l of Nitrogen (N2), 100 ml/l of Carbon dioxide (CO2) and 100 ml/l of Hydrogen (H2).
Determination of antibacterial effect of each single drug:
Using TTC as a viable indicator, the Minimum Inhibitory Concentration (MIC) of Magnolia officinalis, Galla chinensis and Geranium strictipes against S. mutans was determined using the Oxford cup method.
Experimental plan:
Using Magnolia officinalis, Galla chinensis and Geranium strictipes as three investigation factors, according to the U8 (85) uniform design table and the U8 (85) usage table[20]. The MIC of each drug was taken and the drug concentrations were divided into 8 levels, and fitted into 8 compound formulas with different drug combinations (Table 1).
| Different drug concentrations | Magnolia officinalis | Galla chinensis | Geranium strictipes |
|---|---|---|---|
| 1 | 12 | 36 | 60 |
| 2 | 14 | 52 | 50 |
| 3 | 16 | 32 | 40 |
| 4 | 18 | 48 | 30 |
| 5 | 20 | 28 | 65 |
| 6 | 22 | 44 | 55 |
| 7 | 24 | 24 | 45 |
| 8 | 26 | 40 | 35 |
Table 1: Uniform design experimental plan (mg/ml).
Antibacterial activity testing of various compound formulas:
Using Oxford cup method[21], the antibacterial activity of each compound was tested according to the same detection method as that of a single drug. Sterilized pure water was used as the solvent control group and blank Oxford cups without any reagents were used as the negative control group which was placed in a 37° incubator for anaerobic cultivation for 24 h. After cultivation, the diameter of the antibacterial rings in each group was measured using a Vernier caliper scale and the difference between the diameter of the antibacterial rings in the experimental group. The control group was used as an indicator of antibacterial activity and the experiment was repeated three times.
Detection of the biofilm inhibition rate of various compound formulas: Fresh S. mutans bacterial solution with Optical Density (OD)600≈0.2 96-well plates made of imported Polyvinyl Chloride (PVC) material was selected as the attachment of S. mutans biofilm. The prepared bacterial solution (120 μl/well) and various concentrations of compound drugs (80 μl/well) were added to the 96-well plates so that the final concentration of drugs in each well was the same as that of the original compound. In addition, a Blank Control (BC) group was prepared by adding 120 μl of bacterial solution and 80 μl of sterilized water to each well while an experimental background group was prepared by adding 200 μl of the corresponding original concentration of compound drugs to the well, and a blank background group (blank well) was set up separately. After adding samples, 96-well plate was placed in an anaerobic incubator for constant temperature cultivation for 24 h.
96-well plates were cultured and crystal violet staining method[22] was used to determine the inhibition rate of the drug on the formation of S. mutans biofilm[23]. The bacterial suspension from each well was discarded and the cells were washed three times with distilled water. Then, 30 μl of 1 g/l crystal violet solution was added to each well separately. After 15 min of dyeing, the dye solution was poured out from each well and washed repeatedly with distilled water. After drying at room temperature, a mixture of ethanol and acetone (4:1) was added to each well, and the OD600 value of each well was measured using Enzyme-Linked Immunosorbent Assay (ELISA) technique. The experiment was repeated three times and the biofilm inhibition rate of each compound was calculated according to the following formula
Biofilm inhibition rate=blankcontrol group-blankbackground group-(experimentalcontrol group-experimentalbackground group)/(blankcontrol group-blankbackground group)
The formula represents the OD600 values corresponding to each group.
Cytotoxicity testing of various compounds: The cytotoxicity of each compound was detected using the Cell Counting Kit-8 (CCK-8) method[24]. 3rd generation gingival fibroblasts with good growth status were prepared after trypsin digestion and inoculated into a 96-well plate at a density of 6000 cells/well. After 24 h of cultivation in a 50 ml/l CO2 incubator at 37°, the original culture medium was discarded. An experimental group, experimental background group, control group and blank background group were set up. Dulbecco's Modified Eagle Medium (DMEM) culture medium with different combinations of drugs was added to each experimental group. The experimental background group was composed of each group's drug solution; negative control group was composed of DMEM culture media with Phosphate Buffered Saline (PBS), 10 μl CCK-8 solution and 100 μl DMEM medium. After 5 min, aspiration the solution from each well was discarded, rinsed and then 10 µl of CCK-8 solution and 100 μl of DMEM was added to each well under dark conditions. After incubation for 2 h, ELISA was performed to detect the OD value at a wavelength of 450 nanometers and the results were recorded. The experiment was repeated for three times and the cell viability was calculated using the following formula
Cell activity=experimental group-experimentalbackground group/control group-blankbackground group
Application of multi-index comprehensive evaluation method to screen out the optimal compound formula:
The rescaling method was applied to perform dimensionless treatment on the antibacterial effect. Using the multi-index comprehensive evaluation method[25], the above three experimental indicators were weighted by 1/3, and their Comprehensive Index (CI) Z (Z=1/3 cell activity+1/3 biological wave inhibition rate+1/3 cell activity) was calculated. The compound with the highest Z-value was considered the optimal compound.
Animal model preparation:
Experimental grouping: 30 male Sprague-Dawley (SD) aged 21 d were randomly divided into a traditional Chinese medicine Experimental Group (EG), Positive Control (PC) group and BC group, with 10 rats in each group. Rats were randomized, using a computer based random order generator and the weight of the rats was recorded daily. Optimal Chinese medicine mouthwash was used as an important experimental group solution, the compound chlorhexidine mouthwash was used as the PC group solution, and distilled water was used as the BC group solution.
Rats were kept in individually ventilated cages at a temperature of 24° with 50 % humidity, 60 air exchanges/hour in the cages and 12/12 h light/dark cycle with the lights on at 6:00 AM. The maximum caging density was 5 mice from the same litter and gender starting from weaning. Bedding and wood shavings were provided, and all the materials, including lids, feeders, bottles, bedding including water were sterilized before use.
The research team monitored animals twice daily. Health was monitored by weight, food, water intake, general assessment of animal activity, panting and fur condition, once a day. If serious health problems occurred in rats during the experiment, they were excluded.
Inhibition of endogenous bacteria:
Rats aged between (22-24) d were subjected to adaptive feeding and endogenous bacterial inhibition for a period of 3 d. They were fed uniformly mixed with ampicillin sodium (1000 g of regular feed/2 g) and drinking water containing penicillin sodium (800 000 units, 200 ml of physiological saline/bottle). Oral cotton swabs were collected from rats aged 25 d to identify the inhibition of endogenous bacteria in the oral cavity.
Inoculation of cariogenic bacteria:
Rats aged between (26-28) d were infected with cotton swabs soaked in saturated S. mutans suspension (1 ml/rat) in their oral cavity. On 29th d, oral cotton swabs were collected from rats to detect the colonization of S. mutans.
Drug manipulation:
During the period from 25 d to the end of the experiment, the BC group, PC group, and EG were all fed a diet of Keyes 2000 and 5 % sucrose aqueous solution. After 30-50 d, sterile cotton swabs were dipped in 1 ml of experimental solution from each group until saturation. The remaining solution was applied to the tooth surface, tongue and oral mucosa of the rat molars, and the remaining solution was injected into the rat's mouth for rinsing to ensure that the solution was properly administered. Experimental drug intervention was conducted twice a day, with each quadrant treated for 15 s. After treatment, feed and water were withheld for 2 h. The drug intervention experiment lasted for 3 w and the health status of the rats, including weight, was recorded daily.
Sample collection:
Saliva collection: On 50th d, 0.2 % 0.4 ml/100 g of pilocarpine nitrate was injected into the abdominal cavity of each rat. 100 μl/rat of stimulating saliva was collected and diluted 1000 times. 10 μl of this saliva was evenly applied to BHI medium to count the total bacteria in the rat's mouth. Another 10 μl was applied to Murashige and Skoog (MS) medium to orally administered S. mutans to rats, the number of Macrophage (M) colonies was counted and all culture media were incubated under anaerobic conditions of 80 % of N2, 20 % CO2 at 37° for 12 h for S. mutans colony counting.
S. mutans colony level=(S. mutans colony count/total colony count)×100 %
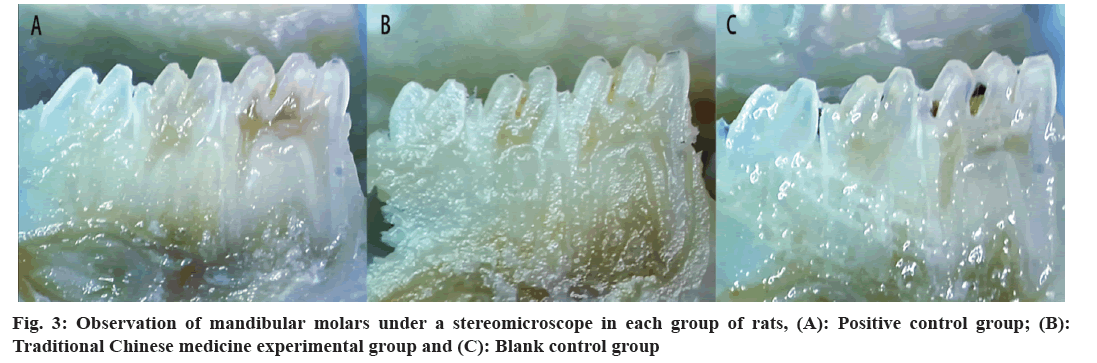
Collection of mandible samples: After 51 d, all the rats were euthanized under anesthesia with pentobarbital sodium. The mandibles of the rats were dissected and treated in a high-pressure steam pot for 5 min. The soft tissue on the jawbones was cleaned and rinsed thoroughly. The mandible samples of the rats were then placed in a 0.4 % ammonium urea solution (with 10 % paraformaldehyde solution added) and stained for 12 h. Using a 0.1 mm thick and 25 mm diameter ultrathin diamond chip, the dental arch was cut open along the mesial and distal directions of the mandible, and the caries of the rat mandibular molars were evaluated using the Keyes caries scoring method under a stereomicroscope. The range and depth of ammonium urate infiltration into rat teeth represent the extent and severity of caries. Statistical analysis was performed according to the Keyes caries scoring method[26].
The main organs namely, heart, liver, spleen, lungs and kidneys, were collected on 51st d, dissected and weighed. The organ coefficients of each major organ in each rat were calculated.
Organ coefficient=organ weight/body weight×100 %
Oral mucosa collection: The buccal mucosa of the same part of each rat was cut to a size of approximately 2 mm×4 mm. The cells were fixed in 4 % paraformaldehyde solution for 24 h. After Hematoxylin and Eosin (H&E) staining, the integrity of the oral mucosal tissue structure, the degree of cell arrangement and the presence of adverse reactions such as proliferative changes, inflammatory reactions and necrosis were observed under a microscope.
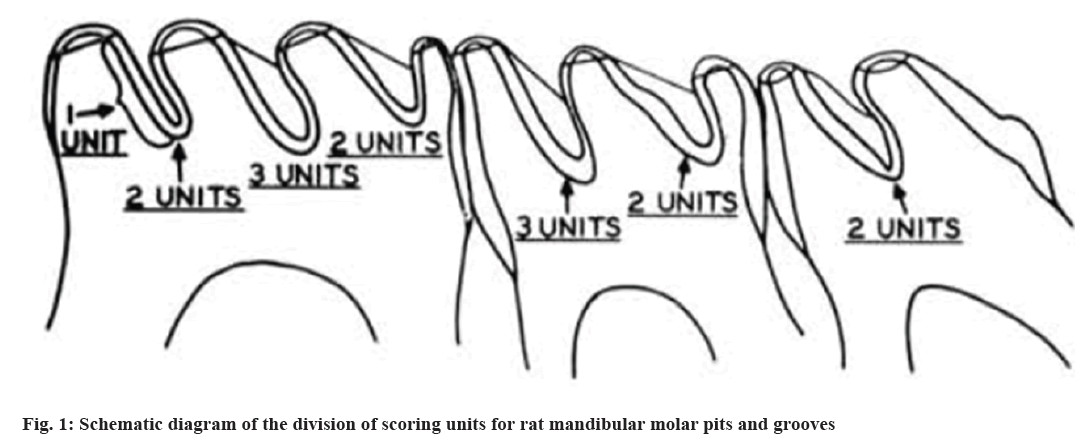
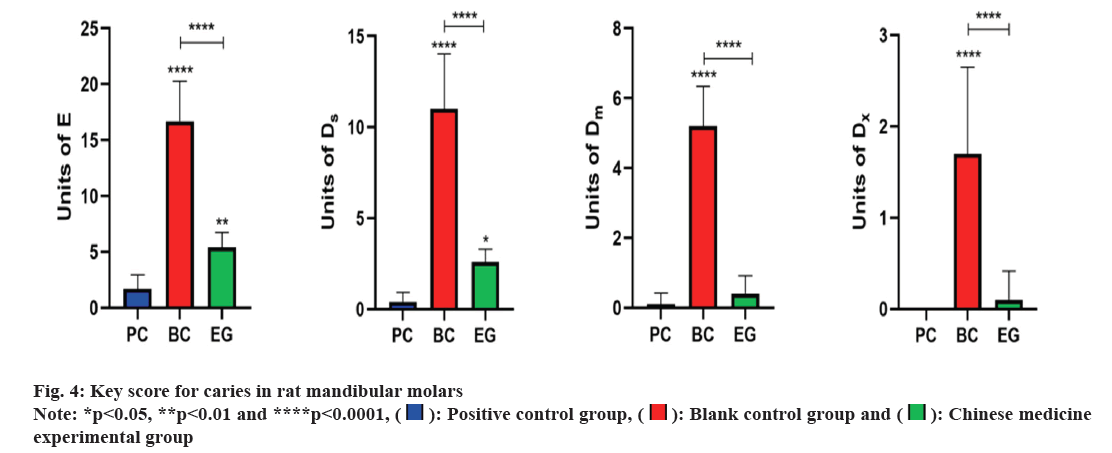
Keyes scoring method for mandibular molar pit and fissure caries in rats: According to the Keyes caries scoring system[26], pit and fissure caries are classified into four levels namely, pure Enamel (E) caries, which refers to caries lesions confined to the enamel layer; mild Dentin (Ds) caries, refer to caries that affect <1/4 of the enamel and outer layer of dentin; moderate Dentin (Dm) caries, refers to caries that affect 1/4 to 3/4 of the thickness of the dentin and widespread Dentin (Dx) caries, refers to the depth of caries that exceeds 3/4 of the thickness of the dentin and even affects the entire dentin layer. As shown in fig. 1, the scoring units for each mandibular molar groove are 7 for the 1st molar, 5 for the 2nd molar and 2 for the 3rd molar. By observing the range of caries, according to the Keyes scoring criteria in rat molars with the naked eye, four levels of caries were scored and summed; the severity of molar caries was evaluated overall using the four levels of caries.
Statistical methods:
Statistical analysis was performed for the measurement of the data using Data Processing System (DPS) version 9.01 statistical software. A binomial stepwise regression was conducted with the CI-Z as the dependent variable “y” and the three factors of Magnolia officinalis, Galla chinensis and Geranium strictipes as independent variables “x”. The inspection level was Alpha (α) was found to be 0.05. Further, statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 23.0 and GraphPad Prism 8.0 version statistical software was used to generate the statistical graphs for the measured data. Shapiro-Wilk test was used to test the normality of the data. Antibacterial effect, biofilm inhibition rate, cell activity, oral bacterial count, S. mutans level, Keyes score for mandibular molar caries, weight change and organ coefficient in rats all showed normal distribution, expressed in the form of (x̄±s). The statistical method used Analysis of Variance (ANOVA) to analyze whether there were statistically significant differences in oral bacterial counts, levels of S. mutans, Keyes score of mandibular molar caries change in rat weight and organ coefficients among the groups.
Results and Discussion
MIC of each single drug against S. mutans was studied. Using the Oxford cup method, the antibacterial activity of each compound was tested according to the same detection method as that of a single drug. MIC of Magnolia officinalis, Galla chinensis and Geranium strictipes against S. mutans are 12, 24 and 30 mg/ml, respectively.
Data processing and analysis of various compound formulas and optimal compound formula results were analysed. Using Magnolia officinalis, Galla chinensis, and Geranium strictipes as three evaluation factors, 8 compound combinations were designed based on the Uniform design U8 (85) and usage table. A binomial stepwise regression analysis was conducted using the comprehensive indicator Z as the dependent variable “y” where antibacterial effect, biofilm inhibition rate and cell activity were considered to be X1, X2 and X3 respectively and were termed as independent variables. The regression equation, y=0.38345+0.01539X2-0.00019X12-0.00017X22-0.00013X32+0.00018X1X3 was obtained and the significance test showed p=0.03<0.05 (R=0.9937, Standard error (S)=0.0251 and F=31.6848), indicating high reliability and statistical significance.
After optimizing the obtained regression equation, it was determined that when the CI-Z was maximized, the combination of various factors was the optimal compound which included 18 mg/ml of Magnolia officinalis, 48 mg/ml of Galla chinensis and 30 mg/ml of Geranium strictipes (Table 2).
| Group | Antibacterial effect | Biofilm inhibition rate | Cell activity | Comprehensive index Z |
|---|---|---|---|---|
| 1 | 0.53±0.14 | 0.26±0.10 | 0.61±0.32 | 0.37±0.06 |
| 2 | 0.85±0.11 | 0.45±0.05 | 0.29±0.11 | 0.48±0.04 |
| 3 | 0.74±0.13 | 0.59±0.05 | 0.79±0.23 | 0.55±0.06 |
| 4 | 0.88±0.04 | 0.83±0.08 | 0.43±0.37 | 0.66±0.07 |
| 5 | 0.09±0.07 | 0.67±0.03 | 0.16±0.08 | 0.28±0.04 |
| 6 | 0.55±0.05 | 0.69±0.06 | 0.42±0.18 | 0.48±0.04 |
| 7 | 0.39±0.04 | 0.75±0.09 | 0.76±0.22 | 0.49±0.09 |
| 8 | 0.71±0.09 | 0.76±0.09 | 0.56±0.35 | 0.59±0.08 |
Table 2: Results of the antibacterial effect, biofilm inhibition rate, cell activity and comprehensive index z of each compound.
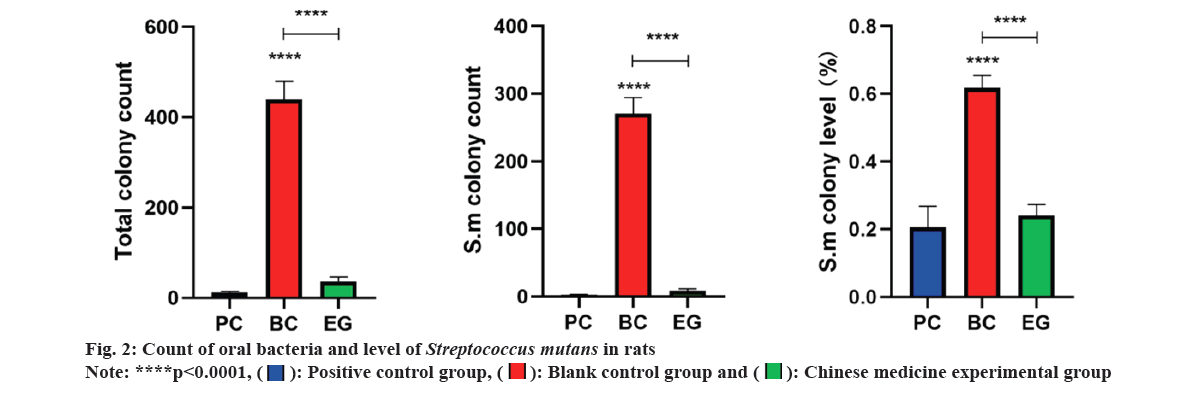
After statistical analysis, the results of oral bacterial count and S. mutans level in rats were studied (fig. 2). After pairwise comparison of each group, it was found that the total bacterial count index in BC group (438.80±40.46) was significantly higher than that in EG (36.10±10.05) and PC group (11.70±2.67). There was no significant difference between PC group and EG group. S. mutans colony count index in BC group (270.70±23.40) was significantly higher than in EG (8.70±2.75) and the PC group (2.40±0.84). There was no significant difference between PC group and EG group. In terms of S. mutans colony level indicators, BC group (0.62±0.04) was significantly higher than EG group (0.24±0.03) and PC group (0.21±0.06). There was no significant difference between PC and EG groups.
Keyes scoring results of caries in rat mandibular molars were analysed. The mandibular molars of rats in each group were observed under a stereomicroscope (fig. 3). In BC group, significant pit and fissure caries were observed in the mandibular molars of rats. No smooth surface caries were observed in the mandibular molars of rats in each group. The pit and fissure caries of each group of rats were measured using the Keyes scoring method (fig. 4). After pairwise comparison of each group, it was found that E level of caries, BC group (16.70±3.59) was significantly higher than the EG (5.40±1.35) and PC group (1.70±1.25); this group was significantly higher than EG group (p<0.01). In Ds level of pit and fissure caries, BC group (11.00±3.02) was significantly higher than the EG (2.60±0.70) and PC group (0.40±0.52); PC group was significantly higher than EG group (p<0.05). Similarly, Dm level of pit and fissure caries, BC group (5.20±1.14) denoted significantly higher score than EG (0.40±0.52) and PC groups (0.10±0.32). There was no significant difference between the PC and EG groups. Further, Dx level of pit and fissure caries, showed high score in BC group (1.70±0.95) than EG (0.10±0.32) and PC groups (0.00±0.00). No significant difference was found between PC and EG groups.
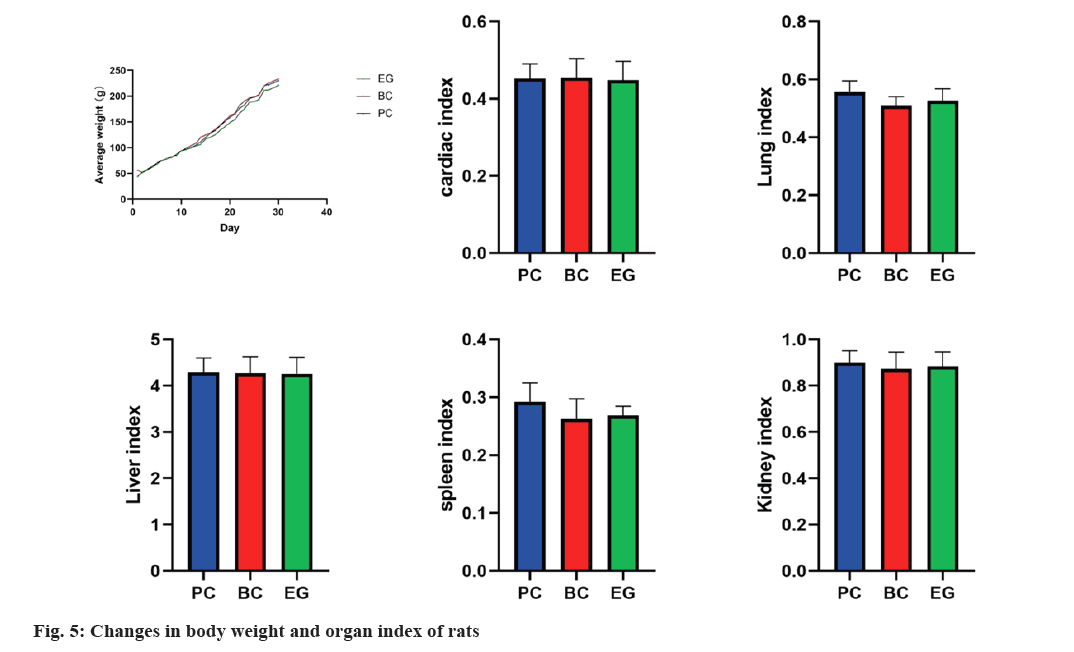
Weight changes and organ index results of rats were assessed. Statistical analysis was conducted on the body weight and organ index of each group of rats (fig. 5). After pairwise comparison, there was no significant difference between the groups.
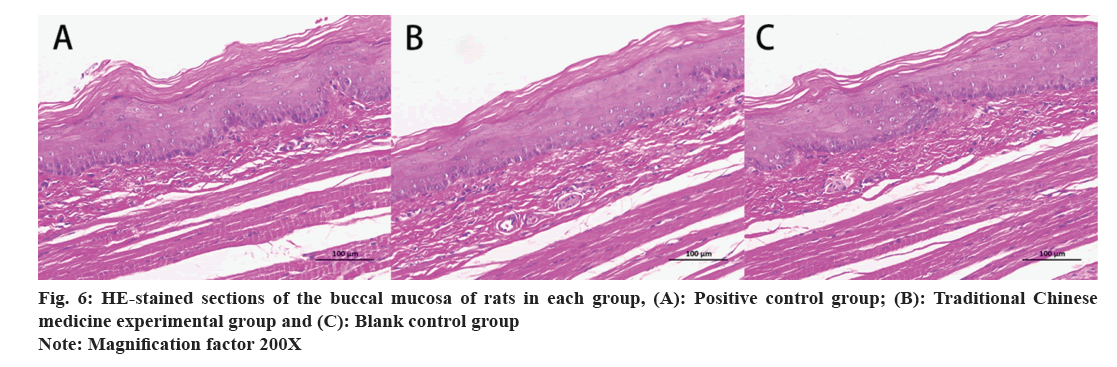
H&E staining results of oral and buccal mucosa of rats were evaluated. Oral mucosa of rats was collected, stained with H&E, and microscopically observed to determine the integrity of the oral mucosal tissue structure, the degree of neat cell arrangement, and the presence of adverse reactions such as proliferative changes, inflammatory reactions, and necrosis. As shown in fig. 6, the oral mucosal tissue structures of the PC group, EG and BC group were all intact, with neatly arranged cells, and no adverse symptoms, such as proliferative changes, inflammatory reactions, or necrosis, were observed with no significant differences.
In orthodontics, caries is a persistent and widespread problem caused by the demineralization[27]. The current enamel remineralization therapy has limited impact on post orthodontic vitiligo lesions; so preventing caries is particularly important for orthodontic treatment[28]. Research has shown that multiple bacteria are closely related to dental caries. As S. mutans is the most closely related oral streptococcus to dental caries, so inhibiting the growth of S. mutans plays an important role in the prevention of dental caries[29]. This study is mainly divided into two parts; primarily, the preparation and screening of compound Chinese medicine mouth wash and its optimal compounds were carried out. On this basis, animal experiments were conducted with reference to the classic rat caries model to further study its biological safety, inhibitory effect on S. mutans, and preventive effect on caries, providing a scientific basis for subsequent clinical applications.
The in vitro antibacterial effect and plaque biofilm inhibition rate of this study showed that the compound Chinese medicine mouthwash had a significant inhibitory effect on the growth of S. mutans and plaque biofilm formation, which is consistent with the research results of Ren et al.[13-15]. Animal experiments were conducted using rat oral colony count and S. mutans colony level as indicators. The results showed that the total oral colony count and colony count were lower than that of the BC group and there was statistical significance. This confirms that the compound Chinese medicine mouthwash has inhibitory effects on the growth of S. mutans and the formation of plaque biofilms. The results of this study showed that there was a statistically significant difference in the Keyes score of rat mandibular molar caries between the EG and BC group where Keyes score of mandibular molar caries in the EG group was significantly lower than the BC group. This confirms that the compound Chinese medicine mouthwash has an inhibitory effect on dental caries. The main mechanism of drug in preventing dental caries is its direct inhibitory effect on the growth, acid production and sugar production of cariogenic bacteria[30]; inhibition of the adhesion of cariogenic bacteria and affecting the morphology, structure and activity of dental plaque biofilm[31]; inhibition of tooth demineralization and promoting remineralization and improving the acid resistance of teeth[32]. Traditional Chinese medicines, due to their complex compositions often have a synergistic effect of multiple anti-caries mechanisms[33]. The synergistic mechanism of the three traditional Chinese medicines in this study is not yet clear and further research should be conducted in the future.
Results of this study showed that there was no significant difference in weight change or organ index between the EG and BC group of rats. The oral mucosal tissue structure of the EG was intact, with neatly arranged cells and no adverse symptoms such as proliferative changes, inflammatory reactions, or necrosis. There was no significant difference compared with BC group. This indicates that the compound Chinese medicine mouthwash has no harmful effects on the health of rats and it has a certain degree of biosafety as a local oral medication.
The limitation of this study is that it confirms the inhibitory effect and biocompatibility of compound Chinese medicine mouthwash on S. mutans and dental caries. However, the taste of compound Chinese medicine mouthwash is bitter and artificial administration in animal experiments can avoid the problem of poor compliance. If further studies are to be conducted in the future, it is necessary to consider improving the taste of the compound Chinese medicine mouthwash.
Conflicts of interest:
The authors declared no conflict of interests.
References
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369(9555):51-9.
[Crossref] [Google Scholar] [PubMed]
- Richter AE, Arruda AO, Peters MC, Sohn W. Incidence of caries lesions among patients treated with comprehensive orthodontics. Am J Orthod Dentofac Orthop 2011;139(5):657-64.
[Crossref] [Google Scholar] [PubMed]
- Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Primers 2017;3:1-16.
[Crossref] [Google Scholar] [PubMed]
- Ajdic D, McShan WM, McLaughlin RE, Savic G, Chang J, Carson MB, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci USA 2002;99(22):14434-9.
[Crossref] [Google Scholar] [PubMed]
- Sudjalim TR, Woods MG, Manton DJ. Prevention of white spot lesions in orthodontic practice: A contemporary review. Aust Dent J 2006;51(4):284-9.
[Crossref] [Google Scholar] [PubMed]
- Travess H, Roberts-Harry D, Sandy J. Orthodontics part 6: Risks in orthodontic treatment. Br Dent J 2004;196(2):71-7.
[Crossref] [Google Scholar] [PubMed]
- Song ZX, Fang SS, Guo T, Wen Y, Liu Q, Jin ZL. Microbiome and metabolome associated with white spot lesions in patients treated with clear aligners. Front Cell Infect Microbiol 2023;13:1-12.
[Crossref] [Google Scholar] [PubMed]
- Marinho VCC, Chong LY, Worthington HV, Walsh T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2016;7(7):1-12.
[Crossref] [Google Scholar] [PubMed]
- Santos A. Evidence-based control of plaque and gingivitis. J Clin Periodontol 2003;30:13-16.
[Crossref] [Google Scholar] [PubMed]
- Ren YY, Zhang XR, Li TN, Zeng YJ, Wang J, Huang QW. Galla chinensis, a traditional Chinese medicine: Comprehensive review of botany, traditional uses, chemical composition, pharmacology and toxicology. J Ethnopharmacol 2021;278:1-22.
[Crossref] [Google Scholar] [PubMed]
- Wong RWK, Hagg U, Samaranayake L, Yuen MKZ, Seneviratne CJ, Kao R. Antimicrobial activity of Chinese medicine herbs against common bacteria in oral biofilm: A pilot study. Int J Oral Maxillofac Surg 2010;39(6):599-605.
[Crossref] [Google Scholar] [PubMed]
- Leonti M, Casu L. Traditional medicines and globalization: Current and future perspectives in ethnopharmacology. Front Pharmacol 2013;4:1-13.
[Crossref] [Google Scholar] [PubMed]
- Ren SR, Yang YM, Xia MY, Deng YL, Zuo YL, Lei L, et al. A Chinese herb preparation, honokiol, inhibits Streptococcus mutans biofilm formation. Arch Oral Biol 2023;147:1-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang TT, Chu JP, Zhou XD. Anti-carious effects of Galla chinensis: A systematic review. Phytother Res 2015;29(12):1837-42.
[Crossref] [Google Scholar] [PubMed]
- Peng AZ, Yang XL, Lu Y, Lan H. Anti caries effects of extracts from Geranium strictipes R. Kunth. Lat Am J Pharm 2023;42(3):504-8.
- Huang BB, Fan MW, Wang SL, Han DX, Chen Z, Bian Z. The inhibitory effect of magnolol from Magnolia officinalis on glucosyltransferase. Arch Oral Biol 2006;51(10):899-905.
[Crossref] [Google Scholar] [PubMed]
- Xie Q, Li J, Zhou X. Anticaries effect of compounds extracted from Galla chinensis in a multispecies biofilm model. Oral Microbiol Immunol 2008;23(6):459-65.
[Crossref] [Google Scholar] [PubMed]
- Yang XL, Li QY, Yang Q, Lan H. Biomineralization of tooth enamel with toothpastes containing Geranium strictipes extracts. Lat Am J Pharm 2023;42(3):643-9.
- Luan X, Zhang LJ, Li XQ, Rahman K, Zhang H, Chen HZ, et al. Compound-based Chinese medicine formula: From discovery to compatibility mechanism. J Ethnopharmacol 2020;254:1-11.
[Crossref] [Google Scholar] [PubMed]
- Fang KT, Lin DKJ, Winker P, Zhang Y. Uniform design: Theory and application. Technometrics 2000;42(3):237-48.
- Foster J, Woodruff H. Microbiological aspects of penicillin: I. methods of assay. J Bacteriol 1943;46(2):187-202.
[Crossref] [Google Scholar] [PubMed]
- Negri M, Gonçalves V, Silva S, Henriques M, Azeredo J, Oliveira R. Crystal violet staining to quantify Candida adhesion to epithelial cells. Br J Biomed Sci 2010;67(3):120-5.
[Crossref] [Google Scholar] [PubMed]
- Ionescu AC, Brambilla E, Travan A, Marsich E, Donati I, Gobbi P, et al. Silver-polysaccharide antimicrobial nanocomposite coating for methacrylic surfaces reduces Streptococcus mutans biofilm formation in vitro. J Dent 2015;43(12):1483-90.
[Crossref] [Google Scholar] [PubMed]
- James N, Kini S, Pai S, Shenoy N, Kabekkodu SP. Comparative evaluation of corneal storage medias used as tooth avulsion medias in maintaining the viability of periodontal ligament cells using the cell counting kit-8 assay. Clin Cosmet Investig Dent 2022;14:87-94.
[Crossref] [Google Scholar] [PubMed]
- Rezaei J. Best-worst multi-criteria decision-making method. Omega Int J Manage Sci 2015;53:49-57.
- Keyes P. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res 1958;37(6):1088-99.
[Crossref] [Google Scholar] [PubMed]
- Lovrov S, Hertrich K, Hirschfelder U. Enamel demineralization during fixed orthodontic treatment-incidence and correlation to various oral-hygiene parameters. J Orofac Orthop 2007;68(5):353-63.
[Crossref] [Google Scholar] [PubMed]
- Hua F, Yang HY, He H. Current enamel remineralization therapies have limited effects on postorthodontic white spot lesions. J Evid-Based Dent Pract 2018;18(4):339-42.
[Crossref] [Google Scholar] [PubMed]
- Bhaumik D, Salzman E, Davis E, Blostein F, Li G, Neiswanger K, et al. Plaque microbiome in caries-active and caries-free teeth by dentition. JDR Clin Transl Res 2022;9(1):61-71.
[Crossref] [Google Scholar] [PubMed]
- Takahashi-Abbe S, Abbe K, Takahashi N, Tamazawa Y, Yamada T. Inhibitory effect of sorbitol on sugar metabolism of Streptococcus mutans in vitro and on acid production in dental plaque in vivo. Oral Microbiol Immunol 2001;16(2):94-9.
[Crossref] [Google Scholar] [PubMed]
- Kouidhi B, Al Qurashi YMA, Chaieb K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microb Pathog 2015;80:39-49.
[Crossref] [Google Scholar] [PubMed]
- Lynch RJM, Navada R, Walia R. Low-levels of fluoride in plaque and saliva and their effects on the demineralisation and remineralisation of enamel; role of fluoride toothpastes. Int Dent J 2004;54(5):304-9.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Zhang Z, Li S, Ye X, Li X, He K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014;92:133-47.
[Crossref] [Google Scholar] [PubMed]
 .
.
 experimental group.
experimental group.