- *Corresponding Author:
- Shweta Jain
College of Veterinary Science and Animal Husbandry, Dau Shri Vasudev Chandrakar Kamdhenu Vishwavidyalaya, Durg, Chhattisgarh 491001, India
E-mail: drshwetavety@yahoomail.co.in
| Date of Received | 19 June 2025 |
| Date of Revision | 23 June 2025 |
| Date of Accepted | 15 November 2025 |
| Indian J Pharm Sci 2025;87(6):257-266 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
People and pharmaceuticals of world are attracting towards herbs and alternates for prophylactic and therapeutic uses based on their folklore utilization. Hygrophila spinosa plant was extensively used in traditional systems of medicine for treating various ailments like jaundice, rheumatism, anemia, inflammation, pain, urinary infections, oedema and gout. Alternative medicine including cow urine distillate (ark) maintains well-being and in recent decades researches on cow urine distillate are gaining interest unveiling its therapeutic potential scientifically. It is claimed as novel pharmaceutical composition particularly for its bio enhancer activities of commonly used antibiotics, anti-fungal and anti-cancer drugs. This study was performed with the aim to evaluate 28 d repeated dose oral safety profile of Hygrophila spinosa extract and Kosli cow urine distillate using hemato-biochemical parameters, antioxidant status and histopathological parameters in Albino rats. The findings of study showed no significant difference in average body weight gain, hemato-biochemical parameters, oxidative status and histopathological examination of control and various treatment group rats. Hygrophila spinosa, cow urine distillate and their combination are safe for treatment of this duration.
Keywords
Subacute toxicity, Hygrophila spinosa, cow urine distillate, combination
The paradigm shift of mankind to herbal and alternative medicine has explored new frontier in global healthcare. The pharmaceuticals are attracting towards herbs and alternates based on their folklore utilization for making fortune with these. The plant kingdom has rich and diverse secondary metabolites which serve as a chemical scaffold for the development of new therapeutic compounds and adjuvant therapy[1]. The phytopharmaceuticals are easily available, safe, economic and are suitable for prophylactic as well as therapeutic uses.
Hygrophila spinosa (H. spinosa) plant is widely distributed and used as a folk medicine in tropical Africa, India, China, Nepal and Malaysia[2]. In Ayurveda, medicinal use of its seeds, roots and panchang (i.e., roots, flowers, stem, fruits and leaves ash burnt together has been described. All parts of the plant are extensively used in traditional systems of medicine for treating various ailments like jaundice, rheumatism, anemia, inflammation, pain, urinary infections, oedema and gout[3]. The aerial parts and the roots of H. spinosa used as demulcent, aphrodisiac, diuretic, urinary and liver tonic. The root contains an alkaloid named hygrosterol acts as antioxidants. The pharmacological properties of H. spinosa such as hepatoprotective, antibacterial, antitumor and antidiabetic were investigated by various researchers.
Cow urine has been used extensively in Indian system of medicinal preparations since time immemorial as cited in ancient holy texts like Charaka Samhita, Sushruta Samhita, Vridhabhagabhatt, Atharva Veda, Bhavaprakash, Rajni Ghuntu, Amritasagar, etc.,[4]. Alternative medicine including Cow Urine Distillate (CUD) (ark) maintains well-being and in recent decades researches on CUD are gaining interest unveiling its therapeutic potential scientifically. In Susruta and in Charak use of cow urine have been mentioned for various ailments such as weight loss, jaundice, reversal of certain cardiac and renal diseases, indigestion, stomach ache, diarrhea, edema, anemia, hemorrhoids and skin diseases. A number of studies conducted over the past few years have reported that gomutra is capable of curing blood pressure, blockage in arteries, arthritis, diabetes, jaundice, cancer, thyroid, asthma, psoriasis, gynaecological problems and as antiseptic etc.,[5]. Mechanism for antibacterial activity of CUD is due to its hydrogen bonding interaction with the amino acids Val71 and Asp73 of bacterial proteins as indicated by in silico docking studies of active compounds of CUD[6]. Council for Scientific Industrial Research (CSIR) India had obtained US patent (No.689690/6410059) for CUD, which claimed a novel pharmaceutical composition[7] particularly for its bio enhancer activities of commonly used antibiotics, anti-fungal and anti-cancer drugs. Bio enhancer is the agent that enhances the potency or efficacy of a substance with which it is administered. It often improves the solubility or adsorption of medicine, or inhibits the action of drug-metabolizing enzymes[8] and enhances the permeability of the cytoplasmic membrane for drug entry[9]. Bio enhancing property of cow urine as well as its distillate was extensively researched. But, toxicological evaluation of Kosli CUD and its combination with H. spinosa has not yet been investigated.
Therefore, 28 d repeated dose subacute toxicity study is designed to confirm the safety of H. spinosa Extract (HSE) and Kosli CUD and their combination using hemato-biochemical parameters, antioxidant status and histopathological parameters.
Materials and Methods
Preparation of HSE and CUD:
Leaves of H. spinosa from paddy fields of district Rajnandgaon, (C.G.) were collected, shade dried, powdered and stored in air tight container. Extraction was done in Soxhlet apparatus for 18- 24 h using 30 % distilled water and 70 % methanol as solvent. The extract thus obtained was allowed to evaporate, transferred to air tight container for storage.
Natural voiding, morning, mid-stream urine from Kosli cows of 2-3 y age was collected in a sterile container and distillate was prepared using a glass distillation apparatus. The preparation was stored at 4°C for further study.
Experimental animals:
Hundred adult healthy Albino rats of either sex weighing 150 to 200 g from Laboratory Animal House, College of Veterinary Science and A. H., Anjora, Durg were used for the study. Rats were allocated in ten groups of ten animals (five male and five females in each group). The animals were housed in polypropylene cages and maintained under standard laboratory conditions (27°C±2°C temperature and 12/12 h light/dark cycle) throughout the experiment. All the animals were observed regularly during entire experimental period. The study was carried out with prior approval by the Institutional Animal Ethical Committee (IAEC), College of Veterinary Science and Animal Husbandry, Durg (C.G.), India (No. 445/GO/ ReBi/ S/01/CPCSEA) and followed the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The experiments were carried out in the Department of Veterinary Pharmacology and Toxicology, College of Veterinary Science and Animal Husbandry, Anjora, Durg and Laboratory Animal House, College of Veterinary Science and Animal Husbandry, Anjora, Durg, Chhattisgarh (India).
Experimental protocol:
The study was done according to OECD, 2008 for a period of 28 d[10]. The design of experiment is depicted in Table 1. Rats were monitored daily for general health, mortality, behavioral abnormality, signs and symptoms of toxicity during entire period of experiment. During the experimental period, body weight changes of rats were recorded at weekly intervals (i.e. on d 0, 7, 14, 21 and 28).
| Group | No. of animal/ group | Treatment | |
|---|---|---|---|
| Male | Female | ||
| I | 5 | 5 | Control: Normal saline |
| II | 5 | 5 | CUD 1.25 ml/kg/day |
| III | 5 | 5 | HSE 100 mg/kg /day |
| IV | 5 | 5 | CUD 1.25 ml/kg/day + HSE 100 mg/kg/day |
| V | 5 | 5 | CUD 2.5 ml/kg/day |
| VI | 5 | 5 | HSE 200 mg/kg/day |
| VII | 5 | 5 | CUD 2.5 ml/kg/day + HSE 200 mg/kg/day |
| VIII | 5 | 5 | CUD 5 ml/kg/day |
| IX | 5 | 5 | HSE 400 mg/kg/day |
| X | 5 | 5 | CUD 5 ml/kg/day + HSE 400 mg/kg/day |
Table 1: Experimental Design for Subacute Oral Toxicity of HSE, CUD and Their Combination in Albino Rats
Collection of samples and analysis:
On d 29 of experiment, blood samples were collected from all the animals by puncturing retro-orbital plexus in sterilized vials containing Ethylenediaminetetraacetic Acid (EDTA) (10 IU/ml of blood) for hematological investigation and in centrifuge tubes without anticoagulant for biochemical evaluation. Serum was harvested for biochemical analysis. For estimation of antioxidant parameters blood was collected in Eppendorf?s containing Acid citrate dextrose @ 1.5 ml/10 ml blood.
Tissue samples of rats were collected after humane sacrifice by cervical dislocation under light anaesthesia using Diethyl ether. Sacrificed animals were subjected to detailed post mortem examination. Liver was collected rapidly and placed in petri dish on ice bath. Extraneous tissues were trimmed off, some part was immediately deep frozen for analysing lipid per oxidation. Vital organs like liver, lung, heart, spleen and kidney were collected in 10 % formaline solution for histopathological examination. Data obtained was analysed statistically by using IBM Statistical Package for Social Sciences (SPSS) version 25 software and presented as a mean±standard error of the mean (M±SEM) at (p?0.05) level of significance.
Results and Discussion
Average Body weight: The effect of CUD, HSE and their combination on Average body weight after a 28 d repeated oral administration in Albino rats is presented in Table 2. There was no significant difference in body wt. of rats among different groups on d 0, d 7, d 14, d 21 and d 28.
| Group | I | II | III | IV | VI | V | VII | VIII | IX | X | Level of significance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Normal saline | HSE:100 mg/kg /day | CUD: 1.25 ml/kg/day | HSE 100 mg/kg /day+ CUD: 1.25 ml/kg/day | HSE: 200mg/kg /day | CUD: 2.5 ml/kg/day | HSE 200mg/kg /day+CUD: 2.5 ml/kg/day | HSE: 400 mg/kg /day | CUD: 5 ml/kg/day | HSE 400mg/kg /day+ UD: 5 ml/kg/day | |
| Average Body Weight (g)/ Rat 0 d |
177.2±6.389 | 178.2±6.974 | 168.±4.976 | 182.0±6.090 | 176.9±6.105 | 175.0±5.194 | 180.9±6.345 | 182.8±4.767 | 182±6.002 | 185±4.756 | NS |
| 7 d | 192.8±7.097 | 184.5±10.668 | 177.5±6.710 | 194.8±5.929 | 189.3±6.750 | 193.0±5.170 | 191.6±9.271 | 190.8±6.530 | 193±5.312 | 191.5±6.085 | NS |
| 14 d | 198.5±7.751 | 190.6±10.404 | 184.1±7.430 | 191.1±6.626 | 196.7±7.003 | 203.0±5.532 | 201.9±8.421 | 196.5±6.830 | 189.2±6.673 | 193.1±7.023 | NS |
| 21 d | 200.3±8.210 | 195.3±9.788 | 192.1±8.580 | 197.4±6.281 | 205.1±7.293 | 208.0±6.971 | 205.3±7.993 | 202.1±7.548 | 182.5±8.745 | 196.1±8.056 | NS |
| 28 d | 209.5±8.759 | 203.3±9.408 | 196.4±7.830 | 204.2±7.548 | 204.7±6.962 | 220.7±9.007 | 213.4±7.645 | 203.7±7.283 | 194.8±7.157 | 199.4±8.887 | NS |
Note: The values are Mean±Standard error of mean of n=10 animals. Different superscripts show significant difference (p≤0.05) * in a row. NS: Non significant
Table 2: Effect of 28 D Repeated Dose Sub Acute Oral Toxicity of HSE, CUD and Their Combination on Average Body Weight of Albino Rats
Hemato-Biochemical parameters and antioxidant status: The effect of CUD, HSE and their combination on hemato-biochemical parameters and anti-oxidant status after a 28 d repeated oral administration in Albino rats are presented in Table 3-Table 5 and fig. 1, respectively.
| Group | I | II | III | IV | V | VI | VII | VIII | IX | X | LS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Normal saline | HSE: 100 mg/kg /day | CUD: 1.25 ml/kg/day | HSE 100mg/kg /day+CUD: 1.25 ml/kg/day | HSE: 200mg/kg /day | CUD: 2.5 ml/kg/day | HSE 200mg/kg /day+CUD: 2.5 ml/kg/day | HSE: 400mg/kg /day | CUD: 5 ml/kg/day | HSE 400mg/kg /day+CUD: 5 ml/kg/day | |
| Hb (g/dl) | 13.493±0.137 b | 14.436±0.280a | 13.541±0.162b | 14.616±0.341a | 14.715±0.238a | 13.635±0.201b | 14.820±0.18a | 15.027±0.164a | 13.377±0.266b | 15.092±0.190a | * |
| PCV (%) | 38.17±1.376 b | 41.67±0.422a | 38.00±1.291b | 41.67±1.022a | 39.67±0.882ab | 38.1±1.046 b | 40.83±0.792ab | 40.50±0.563ab | 38.67±0.882ab | 41.50±0.764a | * |
| TEC (millions/ ?l of blood) | 7.977± 0.264b | 9.013±0.182a | 8.058±0.183b | 9.032±0.185a | 8.800±0.171a | 8.085± 0.166 b | 9.083±0.247a | 8.949±0.136a | 8.158±0.124b | 9.198±0.163a | * |
| TLC (thousands/?l of blood) | 5.628±0.240 | 5.767±0.310 | 5.967±0.178 | 5.97±0.181 | 5.883±0.146 | 5.852± 0.178 | 5.790±0.151 | 5.750±0.307 | 5.590±0.112 | 5.742±0.320 | NS |
| MCV (fl) | 48.237±2.804 | 46.337±1.173 | 47.249±1.840 | 46.165±0.971 | 45.143±1.190 | 47.413± 2.116 | 45.201±1.932 | 45.310±0.923 | 47.464±1.389 | 45.226±1.412 | NS |
| MCH (pg/dl) | 16.998±0.516 | 16.064±0.548 | 16.837±0.342 | 16.228±0.570 | 16.766±0.516 | 16.901± 0.442 | 16.357±0.333 | 16.814±0.328 | 16.434±0.543 | 16.419±0.197 | NS |
| MCHC (g/dl) | 35.588±1.323 | 34.632±0.358 | 35.841±1.290 | 35.171±1.124 | 37.241±1.349 | 35.870± 1.170 | 36.378±0.981 | 37.132±0.553 | 34.720±1.263 | 36.452±1.010 | NS |
| Neutrophils (%) | 23.500±2.012 | 22.333±1.202 | 24.667±1.520 | 23.000±1.366 | 25.000±1.033 | 24.667± 1.856 | 23.667±2.418 | 23.167±1.327 | 23.500±1.586 | 23.167±1.721 | NS |
| Eosinophils (%) | 0.667±0.333 | 0.833±0.167 | 0.833± 0.307 | 0.667±0.333 | 0.833±0.477 | 1.000 ± 0.365 | 1.000±0.365 | 0.833±0.477 | 0.833±0.401 | 0.667±0.333 | NS |
| Basophils (%) | 0.167±0.167 | 0.000±0.000 | 0.000±0.000 | 0.000±0.000 | 0.000±0.000 | 0.000 ± 0.000 | 0.167±0.167 | 0.000±0.000 | 0.167±0.167 | 0.333±0.211 | NS |
| Monocytes (%) | 1.333±0.333 | 2.000±0.632 | 1.333±0.422 | 1.667±0.422 | 1.333±0.422 | 1.833 ± 0.401 | 1.667±0.615 | 1.833±0.307 | 1.500±0.342 | 1.833±0.654 | NS |
| Lymphocytes (%) | 69.167±1.327 | 70.500±1.500 | 69.667±1.174 | 69.833±2.400 | 70.000±1.897 | 70.667± 2.290 | 71.500±2.012 | 70.667±1.961 | 69.833±1.922 | 68.833±3.146 | NS |
Note: The values are Mean±Standard error of mean of n=10 animals. Different superscripts show significant difference *p≤0.05 in a row. NS: Non significant and LS: Level of significance
Table 3: Effect of 28 D Repeated Dose Sub Acute Oral Toxicity of HSE, CUD and Their Combination on Hematological Parameters of Albino Rats
| Group | I | II | III | IV | V | VI | VII | VIII | IX | X | LS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Normal saline | HSE : 100 mg/kg/day | CUD: 1.25 ml/kg/day | HSE 100 mg/kg /day+CUD: 1.25 ml/kg/day | HSE: 200 mg/kg /day | CUD: 2.5 ml/kg/day | HSE 200mg/kg /day+CUD: 2.5 ml/kg/day | HSE: 400 mg/kg /day | CUD: 5 ml/kg/day | HSE 400mg/kg /day+CUD: 5 ml/kg/day | |
| AST (IU/l) | 138.667±1.820 | 136.500±2.156 | 137.500±1.996 | 138.333±3.490 | 137.833±2.330 | 136.667±2.155 | 135.333± 2.603 | 139.000±3.777 | 134.000±2.887 | 138.833±4.254 | NS |
| ALT(IU/l) | 77.167±2.903 | 76.167±2.822 | 73.167±3.280 | 77.000±3.225 | 77.500±1.607 | 79.167±2.482 | 79.333±3.084 | 76.667±3.913 | 77.333±2.333 | 75.000±2.805 | NS |
| ALP(IU/l) | 109.115±8.506 | 103.177±3.496 | 104.000±5.475 | 105.632±9.444 | 105.013±5.147 | 106.737±3.323 | 102.043±4.488 | 106.148±8.100 | 107.875±6.659 | 108.445±3.477 | NS |
| BUN (mg/dl) | 13.333±2.404 | 13.167±1.905 | 14.167±1.990 | 13.667±0.803 | 13.000±3.055 | 13.000±0.856 | 12.000±1.000 | 12.667±1.706 | 12.833±0.946 | 13.167±2.414 | NS |
| Creatinine (mg/dl) | 0.448±0.044 | 0.437±0.026 | 0.487±0.052 | 0.420± 0.026 | 0.495±0.065 | 0.463±0.055 | 0.480±0.052 | 0.475±0.053 | 0.513±0.055 | 0.503±0.047 | NS |
| Cholesterol (mg %) | 29.792±1.870 | 29.748±1.145 | 27.322±0.904 | 30.340±1.441 | 29.610±1.414 | 28.168±0.601 | 29.813±1.110 | 31.052±1.188 | 30.672±0.764 | 30.772±0.623 | NS |
| Total protein (g/dl) | 6.463±0.331 | 6.562±0.308 | 6.887±0.242 | 6.898±0.285 | 6.888±0.270 | 7.508±0.466 | 7.070±0.394 | 6.832±0.337 | 6.780±0.380 | 7.133±0.494 | NS |
| Bilirubin (mg/dl) | 0.808±0.050 | 0.783±0.045 | 0.767±0.046 | 0.790±0.034 | 0.718±0.039 | 0.797±0.035 | 0.763±0.037 | 0.765±0.027 | 0.807±0.058 | 0.792±0.046 | NS |
| Albumin (g/dl) | 3.627±0.113 | 3.673±0.154 | 3.722±0.039 | 3.817±0.060 | 3.707±0.080 | 3.940±0.078 | 3.932±0.147 | 3.807±0.103 | 3.780±0.104 | 3.627±0.094 | NS |
| A: G ratio | 1.346±0.127 | 1.297±0.084 | 1.225±0.120 | 1.279±0.095 | 1.232±0.140 | 1.219±0.204 | 1.387±0.204 | 1.310±0.121 | 1.380±0.218 | 1.215±0.234 | NS |
| Calcium (mg/dl) | 9.385±0.530 | 9.477±0.469 | 9.638±0.873 | 9.747±0.588 | 9.487±0.703 | 9.493±0.296 | 9.932±1.131 | 9.893±0.203 | 9.800±0.645 | 9.920±0.751 | NS |
Note: The values are Mean±Standard error of mean of n=10 animals. Different superscripts show significant difference (P<0.05)* in a row. NS: Nonsignificant and LS: Level of significance
Table 4: Effect of 28 D Repeated Dose Subacute Oral Toxicity of HSE, CUD and Their Combination on Biochemical Parameters of Albino Rats
| Group | I | II | III | IV | V | VI | VII | VIII | IX | X | LS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Normal saline | HSE : 100mg/kg /day | CUD: 1.25 ml/kg/day | HSE 100 mg/kg /day+CUD:1.25 ml/kg/day | HSE: 200 mg/kg /day | CUD:2.5 ml/kg/day | HSE 200mg/kg /day+CUD: 2.5 ml/kg/day | HSE: 400 mg/kg /day | CUD:5 ml/kg/day | HSE 400 mg/kg/day+CUD: 5 ml/kg/day | ||
| Serum Antioxidant parameter |
LPO (mmol/mg Hb) | 0.799±0.159 | 0.783±0.080 | 0.782±0.122 | 0.506±0.038 | 0.616±0.191 | 0.573±0.065 | 0.620±0.089 | 0.510±0.049 | 0.633±0.177 | 0.732±0.108 | NS |
| GSH (mmol/mg Hb) | 0.167±0.005 | 0.163±0.005 | 0.176±0.007 | 0.160±0.005 | 0.169±0.003 | 0.176±0.007 | 0.161±0.005 | 0.162±0.005 | 0.173±0.004 | 0.169±0.005 | NS | |
| GPx (Units/ ml) | 44.915±13.932 | 65.506±7.163 | 53.155±16.844 | 59.015±9.740 | 51.437±16.158 | 49.563±13.132 | 60.228±9.180 | 35.274±5.160 | 53.927±16.303 | 61.344±17.435 | NS | |
| LPO in liver tissue (nmole/g ) | 59.231±6.344 | 65.897±7.887 | 65.641±5.838 | 61.368±3.857 | 58.547±4.931 | 65.299±11.884 | 60.000±5.264 | 69.402±9.268 | 55.385±4.955 | 56.667±8.452 | NS | |
Note: The values are Mean±Standard error of mean of n=10 animals. Different superscripts show significant difference (*p≤ 0.05) in a row. NS: Non significant and LS: Level of significance
Table 5: Effect of 28 D Repeated Dose Subacute Oral Toxicity of HSE, CUD and Their Combination on Antioxidant Status of Albino Rats
Hematological parameters viz., Hemoglobin, PCV, TEC, TLC, DLC, MCV, MCH and MCHC were recorded[11]. No significant difference in TLC, MCH, MCV, MCHC and DLC % amongst all treatment groups. Group III, IV, VI, VII, IX and X showed higher level of Hemoglobin and TEC as compared to control group. Higher PCV % in groups III, IV and X was found to that of group I animals.
Analysis of serum for biochemical marker enzymes (Aspartate Aminotransferase (AST), Alanine Transaminase (ALT), Alkaline Phosphatase (ALP)), total protein, albumin, A:G ratio, bilirubin, cholesterol, Blood Urea Nitrogen (BUN), creatinine and calcium using semi-auto analyser (Elitech Micro lab 300) revealed no significant difference in control and treatment groups.
For antioxidant profiling, Red Blood Cells (RBC) suspension was used to estimate reduced glutathione[12] using Dithio-bos-2-nitro benzoic acid reagent. Hemolysate was used to estimate lipid peroxidation[13] and glutathione peroxidase[14]. Antioxidant profile showed no significant difference in Lipid peroxidation in serum and liver tissues in terms of production of Malondialdehyde (MDA) level using Thiobarbituric Acid (TBA) method, glutathione level and glutathione peroxidase activity.
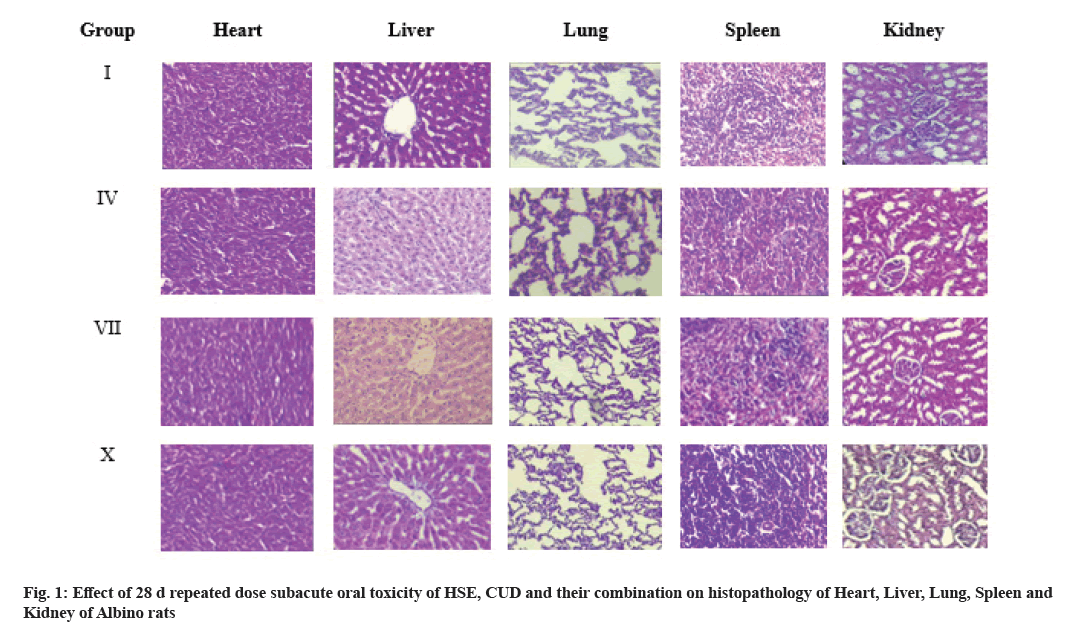
Necropsy of vital organs (lung, heart, liver, kidney and spleen) revealed no gross pathological changes. Histopathological examination of these organs in various treatment groups showed normal architecture with no abnormality.
In this subacute toxicity study, various groups did not produce any sign of toxicity and death at various doses of treatments. A gradual increase in average body weight gain was observed in different treatment groups as in case of control group animals indicating safety of treatments. Maximum average weight gain (45.7 g) was found in group V rats against control group rats (32.3 g).
Analysis of hematological parameters has higher prediction for the toxicity of medicinal plants. Among hematological parameters, higher level of Haemoglobin and TEC in Group III, IV, VI, VII, IX and X and higher PCV % in Group III, IV and X were within the normal range.
Liver and kidney are the major organs implicated in xenobiotic action. While liver is the site of drug metabolism, excretion of drug takes place in the kidney[15]. Change in activity of serum enzymes present in one or more organs reflects damage to the related organs and is proportional to the extent of damage. Serum AST is a mitochondrial enzyme released from heart, liver and skeletal muscle. Increased serum AST activity is observed with both reversible and irreversible injury to hepatocytes and can be seen following hepatocellular injury and cholestasis. ALT plays a role in amino acid catabolism and inter-organ nitrogen transport. It is a cytosolic enzyme primarily present in the liver. Serum levels of AST and ALT are increased on damage to the tissue producing them. Thus, serum estimation of ALT which is fairly specific for liver tissue is of greater value in liver cell injury, whereas AST level may rise in acute necrosis or ischaemia of other organs such as the myocardium, besides liver injury. Among many tissues, cells from liver, bone, kidney, intestinal mucosa, and placenta have the greatest ALP activity. Liver ALP contributes over half the serum activity[16]. Essential markers for evaluation of kidney functions are BUN, and serum creatinine level (depicting glomerular filtration, tubular reabsorption, tubular secretion). Observation of serum enzyme (ALT, AST and ALP) activities, BUN, creatinine level, cholesterol level, Total protein, Albumin, A: G ratio and calcium levels showed no significant difference. Thus, the extract, CUD or their combination produced no toxic effects on the metabolism or excretion of the products of metabolism. Additionally, no deviation in the antioxidant profile indicated safety of HSE, CUD and their combination in this study.
Results of histopathological examination revealed normal liver parenchyma with intact hepatic cord and central vein with normal radiating hepatocytes, without necrotic, degenerative or fatty changes. The histoarchitecture of kidney revealed normal architecture of tubules, glomerulus and Bowman?s capsule with no degenerative changes. Similarly, histopathology of spleen tissue showed no abnormalities and that of heart showed conserved cardiac muscles, connective tissue and myosin filament. Normal cellular structure of lung tissues was conserved with normal bronchioles. The overall result reveals HSE, CUD and their combination in the given doses are safe with no potential of toxicity. The present study is in corroboration of toxicological studies by many researchers.
Sub-acute toxicity study of the extract of seeds (100, 200, 400 mg/kg) of Asteracantha longifolia Nees (Linn) in rats did not show any significant changes in body weight, food and water intake, haematobiochemical, and histopathological parameters[17]. Investigation with the leaves of H. auriculata (250 and 500 mg/kg) against cisplatin, gentamycin, and paracetamol drug-induced nephrotoxicity in albino Wister rats and reported its excellent therapeutic potential for nephrotoxicity and as antioxidants[18]. Subacute toxicity study (28 d) of hydroalcoholic extract of H. spinosa at dose of 300 and 600 mg/ kg revealed safety of treatment by revealing no significant difference in body weight, activity of marker enzymes (Glutamic Pyruvic Transaminase (GPT), Glutamic Oxaloacetic Transaminase (GOT), ALP) in serum and liver tissue; level of plasma and tissue lipid profiles (cholesterol, phospholipids and triglyceride) and no histopathological changes in control and treatment group animals[19].
Antioxidant assays supported the antioxidant property of H. auriculata. The maximum 2,2-Diphenyl-1- Picrylhydrazyl (DPPH) radical scavenging activity, maximum superoxide radical scavenging activity, maximum of Molybdenum (Mo6+) reduction and Ferric ion (Fe3+) reduction by methanol leaves extract of H. auriculata were 72.82±0.19 %, 81.72±0.28 %, 79.68±0.41 % and 47.88±0.47 %, respectively at 120 ?g/ml concentration[20].
Increased body weight of Wistar rats on 28th d by treatment of CUD 5 ml/kg and 10 ml/kg body weight (358.33±7.49 g and 331.67±10.77 g, respectively) as in control group (360.00±11.55 g) was reported[21]. There was no significant difference in the value of cholesterol in these groups (79.38±7.61 and 77.75±7.68 mg/dl) from normal control group (65.70±3.09 mg/dl). The creatinine level in normal and CUD group (5 ml/kg and 10 ml/kg body weight) were 0.39±0.01, 0.32±0.04 and 0.24±0.04 mg/dl respectively.
Results of sub-acute toxicity study of urine distillate of Deoni, Malanad Gidda and Kasargod cow breeds in rats are in corroboration with this study[22]. No significant (p>0.05) difference was reported in AST, ALT, serum creatinine and BUN levels as compared to control group. No gross pathological changes and normal histological features of the organs were found.
Research on combination therapy of CUD and medicinal plants extract (Tagetes erecta, Ocimum sanctum, Syzgium aromaticum and Acalypha indica plants) against five pathogenic gram-negative bacteria-Salmonella typhimurium, Klebsiella pneumoniae, Escherichia coli, Enterobacter aerogenes and Asiatic cholera reported improvement in antibacterial effect of plant extracts by CUD[23]. The combination therapy had more profound effect than the individual plant extract against all the bacterial strains at the specified level.
Evaluation of the anti-inflammatory and antioxidant activity of wheat grass powder (100, 200 and 400 mg/ kg doses) fortified with CUD in Wistar rats showed significant inhibition of rat paw edema and inhibition was maximum at 400 mg/kg (40.06 %) at the 5 h. Antioxidant activity using ferric thiocyanate method showed significant reduction (61.09 %) in peroxide production. The study revealed that fortification with CUD enhanced the antioxidant and anti-inflammatory activities of wheat grass powder[24].
Findings on antioxidant activity of four plant extracts viz., Acalyphaindica, Gymnema sylvestre, Tamarindus indica and Murraya koenigii, in combination with CUD using the DPPH free radical scavenging assay indicated the antioxidant activity of Azadirachta indica was significantly higher with indigenous cow ark (100 ?g/ ml) in comparison to other plant extracts and reference Ascorbic acid[25]. Evaluation of antioxidant profile of CUD with Allium sativum by DPPH and radical scavenging assay[26] revealed IC50 for CUD, plant extract and the combination were 5, 3.5 and 5.9 m/g/?g/g, respectively as compared to Ascorbic acid (3.2 m/g/?g/g).
Recently, much attention has been focused on using natural products as an alternative therapy for the treatment of different ailments, but herbal medicine requires long term therapy. Oral subacute toxicity study assesses adverse effects or possible health hazards which may likely to occur by repeated administration of test substance over a relatively limited period of time. Repeated dose 28 d oral toxicity study is done in rodents preferably the rats. The findings of this study suggests that CUD, HSE and their combination can be used for given duration without any adverse effect.
Conflict of interests:
The authors declared no conflict of interests.
References
- Süntar I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem Rev 2020;19(5):1199-209.
- Almeida MR, Almeida SM. Hygrophila schulli (Hamilt.) species. India Biodiversity Portal 2020.
- Proom P, Anbu Jeba SJ, Viyayakuma S, Varatharajan R, Jayaraj P. Hepato-protective effect of Hygrophila auriculata (Schumach.) Heine. Proc Seminar Medicinal Aromatic Plants (MAPS 2008;2009.
- Pathak ML, Kumar A. Cow praising and importance of Panchyagavya as medicine. Sachitra Ayurveda 2003;5(1):56-9.
- Kishore SV, Rao LR, Ramesh B, Aditya AK. Indian cow urine distillation and therapeutic uses. Mintage J Pharma Med Sci 2015;4(1):1-5.
- Pant L, Thapa S, Dahal B, Khadka R, Biradar MS. In silico and in vitro studies of antibacterial activity of Cow Urine Distillate (CUD). Evid Based Complement Alternat Med 2024:1-9.
[Crossref] [Google Scholar] [PubMed]
- Khanuja SP, Kumar S, Shasany AK, Arya JS, Darokar MP, Singh M, et al. Pharmaceutical composition containing cow urine distillate and an antibiotic. United States patent US 6,410,059. 2002.
- Javed S, Ahsan W, Kohli K. The concept of bioenhancers in bioavailability enhancement of drugs: A patent review. J Sci Lett 2016;1:143-65. [Crossref]
[Google Scholar] [PubMed]
- Peterson B, Weyers M, Steenekamp JH, Steyn JD, Gouws C, Hamman JH. Drug bioavailability enhancing agents of natural origin (bioenhancers) that modulate drug membrane permeation and pre-systemic metabolism. Pharmaceutics 2019;11(1):33.
[Crossref] [Google Scholar] [PubMed]
- OECD guidelines for the testing of chemicals. Repeated dose 28-day oral toxicity study in rodents. OECD, Paris 2008:1-13.
- Jain NC. Haematological techniques in Schalms veterinary haematology, 4th Ed. Lea and Febinger, Philadelphia 1986:20-86.
- Prins HK, Loos JA. Biochemical methods in red cell genetics. Academic Press, New York 1969:127-9.
- Placer ZA, Cushman L, Johnon B. (1966). Estimation of products of lipid peroxidation (malondialdehyde) in biochemical system. Anal Biochem 1966;16(2):359-64.
[Crossref] [Google Scholar] [PubMed]
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967;70(1):158-69.
[Google Scholar] [PubMed]
- Unuofin JO, Otunola GA, Afolayan AJ. Evaluation of acute and subacute toxicity of whole-plant aqueous extract of Vernonia mespilifolia less. in Wistar rats. J Integr Med 2018;16(5):335-41.
[Crossref] [Google Scholar] [PubMed]
- Clampitt RB, Hart RJ. The tissue activities of some diagnostic enzymes in ten mammalian species. J Comp Pathol 1978;88:607-21.
[Crossref] [Google Scholar] [PubMed]
- Manivannan E, Kothai R, Arul B. Acute and subacute (28-day) toxicity assessment of ethanolic extract of seeds of Asteracantha longifolia Nees (Linn). Inter J Pharmaceut Res 2017;9(3):1-10.
- Riyazunnisa D, Reddy KS, Satyanarayana SV. Ethanolic extract from Hygrophila auriculata (schumach.) heine leaves exhibited a promising protective effect against drug-induced nephrotoxicity in rodents. J Pharm Negative 2023;14(2):614-21.
- Pattanayak SP, Sunita P. Antitumor potency and toxicology of an Indian ayurvedic plant, Hygrophila spinosa. Pharmacologyonline 2008;2(1):361-71.
- Anusha P, Immanuel SR. Antioxidant and antibacterial activities of leaves extract of Hygrophila auriculata (schumach.) Heine. J Pharmacogn Phytochem 2019;8(2):1784-9.
- Mahida NR, Mandali GC, Raval SK, Joshi BP. Protective effect of cow urine distillate in streptozotocin induced type 1 diabetes in rats. Indian J Vet Sci Biotechnol 2017;12(3) 127-31.
- Wadeyar S. Hypoglycaemic activity and safety studies of urine distillate of some indigenous cow breeds in rats. Karnataka Veterinary, Animal and Fisheries Sciences University, Bidar 2013.
- Pahal V, Kaur A, Dadhich KS. Effect of combination therapy using cow (Bos indicus) urine distillate and some Indian medicinal plants against selective pathogenic gram-negative bacteria. Inter J Pharmaceutical Sci and Res 2017;8(5):2134-42.
- Killari KN, Prasad K, Talluri MR, Bokam YK, Nadiminti SR, Kommavari CS. Antiinflammatory activity of wheat grass fortified with cow urine distillate. Indian J Pharm Sci 2019;81(3):521-6.
- Vinotha M, Thavasuraj S, Chinniah S, Nithya V. Antimicrobial, antibiofilm and antioxidant effects of medicinal plants extract with indigenous cow ark against human pathogens. Inter J Advanced Sci Technol 2020;29(3):569-83.
- Nithya V. Indigenous cow ark with Allium sativum-a key to therapeutic and an effective antioxidant to hepatocellular carcinoma. Int J Aqua Sci 2021;12(2):1631-44.