- *Corresponding Author:
- E. A. Ghaemi
Department of Microbiology, Golestan University of Medical Science, Gorgan, Iran
E-mail: eghaemi@yahoo.com
| Date of Submission | 07 November 2015 |
| Date of Revision | 18 October 2016 |
| Date of Acceptance | 17 November 2016 |
| Indian J Pharm Sci 2016;78(6):763-768 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this study, the effect of sub-MIC concentrations of the ZnO nanoparticle on the expression of the alpha-hemolysin gene as a significant virulence factor of Staphylococcus aureus is assessed. The effect of sub-MIC concentration (312.5 μg/ml) of the ZnO nanoparticle on the hemolysis phenomenon was studied phenotypically on Blood agar media with and without ZnO nanoparticles and genetically by Real Time PCR method in media containing and without ZnO. The hemolysis phenomena of all S.aureus isolates in a blood agar with a sub-MIC concentration and 1/2, 1/4, and 1/8 MIC concentrations of ZnO nanoparticles was completely restrained. After performing Real Time PCR, the amount of hla gene expression in 12 h culture in media with 1/2 and 1/4 MIC concentration of ZnO nanoparticles was reduced, but gene expression in the 1/8 MIC concentration was did not decrease. Hemolysis by S. aureus was decreased in the presence sub-MIC concentration of ZnO nanoparticles. The use of ZnO nanoparticle in sub-MIC concentration as cover of artificial instruments such as catheter, intravascular catheters or shunts to control bacterial infection is suggested for further study.
Keywords
S. aureus, zinc oxide (ZnO) nanoparticle, α-haemolysin, gene expression, sub-minimum inhibitory
Staphylococcus aureus is Gram-positive, facultative anaerobic cocci, which is the most important species in the Staphylococcus genus from a medical perspective [1]. Methicillin-resistant S. aureus (MRSA) and vancomycin intermediate sensitive S. aureus (VISA) strains, which are mainly seen in hospitals, are one of the problems in the field of nosocomial infection treatments in the 21st century [2]. Search for drugs or factors with an ability to eliminate this bacterium in healthy carriers or patients can immensely reduce the risk of nosocomial infections and prevent deaths and additional expenses [3]. Increase in the use of artificial tools and instruments and intravascular catheters and tubes are important factors responsible for the spread of this bacterium and other pathogenic bacteria in hospitals [4]. Reducing the risk of infection transmission by adding antimicrobials, especially nanoparticles, to artificial instruments can decrease nosocomial infection [5]. One of the difficulties of working with antimicrobials at high dosage is that as a result of prolonged usage or high density they can cause toxicity in host [6].
Alpha-toxin is the most important cytotoxic factor produced by S. aureus and the first identified bacterial exotoxin from pore-forming toxins categories. Alphatoxin is a toxin with 293 amino acids, which is secreted in the form of water-soluble monomers from bacterial cells and is produced by 95% of S. aureus strains. Alpha-toxin has a heptagon structure [7]. The encoding gene of α-toxin is the hla gene, which is the genetic loci responsible for α-toxin activity, including 1620 bp [8]. In addition to leucocytes and red blood cells, α-toxin can cause lysis on platelet population. In human, mouse and rabbit erythrocytes, it causes haemolysis and in some cases leads to dermonecrotic problems and hypodermic bleeding [9]. Alpha-toxin is also important in pneumonia, sepsis, septic arthritis, brain abscesses and S. aureus corneal infections [10]. In addition to the ability to create pores, this toxin induces the release of cytokines and chemokine's such as interleukin 6 and interleukin 1 and tumour necrosis factor-α (TNF-α) [11].
The expression of α-toxin monomers is controlled by several overall regulatory systems, one of which is the accessory gene regulator (agr) locus, which is activated when quorum sensing (bacteria population) happens and performs the primary control in producing hla with the help of RNA III. This happens in the late-log and stationary phases of bacteria growth [12]. Inorganic antimicrobials, such as zinc oxide (ZnO), magnesium oxide (MnO), and cupric oxide (CuO) nanoparticles, have been known as safe antimicrobials for human and animal use [13]. ZnO nanoparticles affect bacteria in various ways; connecting to the SH groups (thiol) of bacterial enzymes and proteins, affecting electron transmission and cellular respiration, generating H2O2, active oxygen and reactive oxygen species (ROS), affecting the DNA and electrochemically destroying cell wall and membrane [14]. In this study, the effects of sub-minimum inhibitory concentration of ZnO nanoparticles on α-haemolysin gene expression in S. aureus are determined.
Materials and Methods
ZnO nanoparticles of the size 10-30 nm with a purity of above 99% were obtained from the US Research Nanomaterial Co. ZnO nanoparticle is a white powder, insoluble in water, with a melting point of 1975° special surface area (SSA) of 20-60 m2/g, and density of 5.606 g/cm3.
Preparation of ZnO nanoparticle suspensions
A homogenized solution of 3 mg pure ZnO nanoparticle powder with 10 ml of pure propylene glycol as solvent was obtained through sonication with Q-Sonica XL- 2000 ultrasonic homogenizer at 20000 HZ frequency and 6 respective dilutions including 5000, 2500, 1250, 625, 312.5 and 156 μg/ml were obtained from that solution by addition of propylene glycol as diluents.
Selection sub-MIC dose of ZnO nanoparticle
For 26 isolations of S. aureus which showed clear β-haemolysis on sheep blood agar, minimum inhibitory concentrations (MIC) was determined through the agar dilution method [15]. Bacteria were placed into 3 groups: The MIC in first (10 isolates), second (10 isolations) and the third (6 isolations) groups were 625, 1250 and 2500 μg/ml, respectively. A 312.5 μg/ml dose was used as sub-MIC for all isolates. Accordingly, 1/2, 1/4 and 1/8 MIC doses was actually used for the three groups.
Effect of sub-MIC concentration of ZnO nanoparticles on haemolysis in the phenotypic method
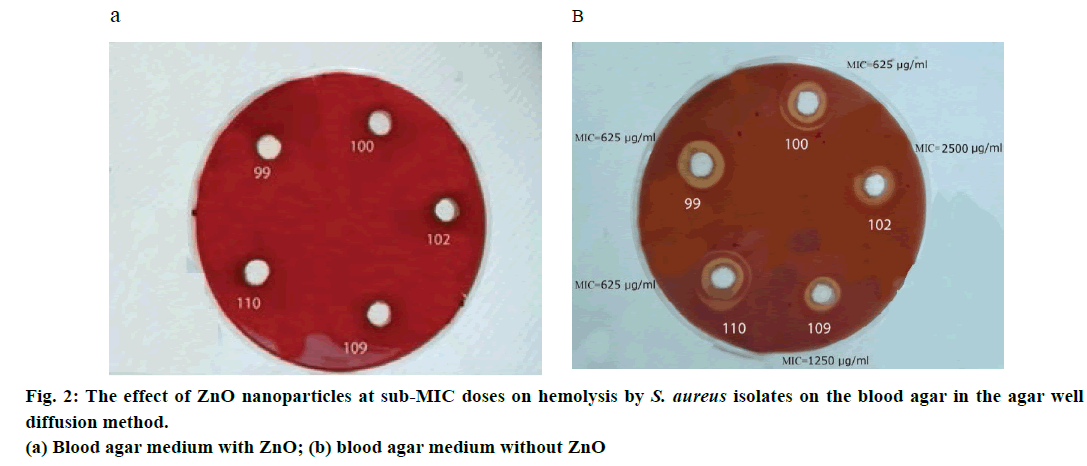
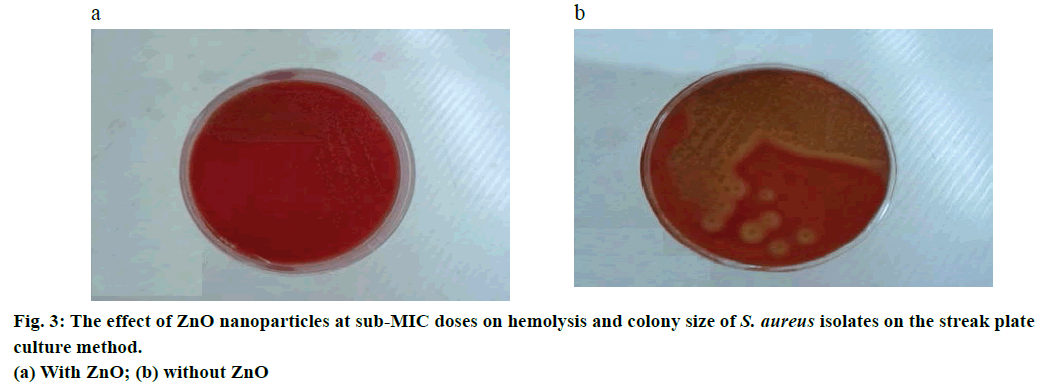
Two methods of agar-well diffusion and streak plate were used in the phenotypic method. Two series of blood agar with 5% sheep blood were prepared. The 312.5 μg/ml concentration (sub-MIC dose) of ZnO nanoparticles was used for one series, and the other series was prepared in a blood agar without nanoparticles. A 100 μl of 105 cfu/ml suspension of each S. aureus isolates was obtained and added to both of the above series. After 24 h of incubation at 37°, colony formation and also haemolysis on the surface of the two series of culture media were detected and recorded.
Effect of sub-MIC dose of ZnO nanoparticles on α-haemolysin gene expression
The effect of nanoparticles on α-haemolysin gene expression in blood broth environment and in both log growth phase at 4 h and stationary phase at 12 h was studied [16]. To achieve this, two series of Mueller- Hinton broth culture media containing 5% sheep blood were prepared; one series without nanoparticles, and the other with 312.5 μg/ml concentration ZnO nanoparticles as the sub-MIC dose. A 105 cfu/ml suspension was obtained from fresh 24 h culture of each isolate and 100 μl of it was added to both media with and without nanoparticles. All tubes were incubated at 37° in equal conditions. Some of the tubes were removed from incubators after 4 h (the log phase) and others after 12 h (the stationary phase) incubation and after being sealed, the tubes were kept in -70° until the extraction of RNA.
RNA extraction method
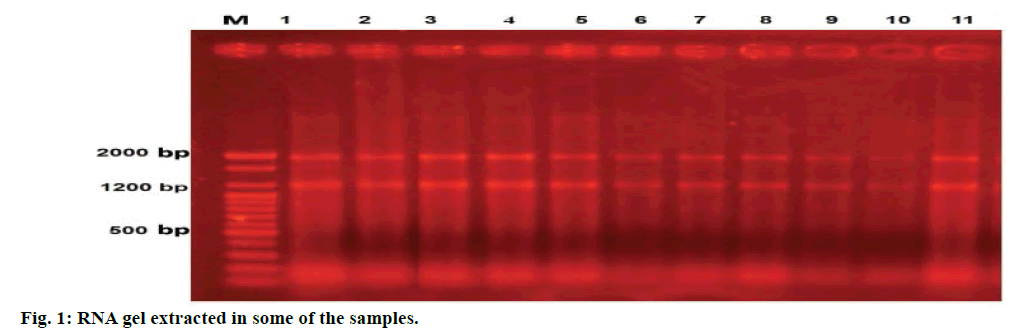
The phenol-chloroform method with RNX-Plus solution (CinnaGen Co.) was used to extract RNA. In view of the high amount of peptidoglycan in the S. aureus cell wall, 10 mg/ml lysozyme was used to disrupt the cellular wall in this study [12]. The density (ng/ μl) of the extracted RNA was studied using picodrop biophotometer at 260 nm (A260) wavelength. The quality and integrity of RNA were tested using agarose gel electrophoresis and ethidium bromide staining, so that two bands of 1.2 kb (16S) and 2 kb (23S) were seen (Figure 1). Fermantes DNase I, RNase-free kit was used to discard the possible existing DNA, Revert Aid First Standard cDNA (Thermo Scientific co.) was used to make cDNA.
Evaluation of α-homlysin (hla) gene expression
Specific primers of the hla gene and gyrB were selected from the study reported by Oogai et al., and were confirmed in the National Center for Biotechnology Information (NCBI) website using Blast pairing location (Table 1) [17]. To perform real time polymerase chain reaction (PCR), the QuantiFast SYBR Green RT-PCR kit obtained from Kiagen was used with ABI Prism 7300 Applied Biosystems Thermocycler. Expression levels of target genes in prepared cDNA samples from different isolates based on the gyrB gene were normalized as internal controls. The CT, ΔCT (ΔCT=CT, target -CT, reference) and ΔΔCT (ΔΔCT=ΔCtsample-ΔCtcalibrator) were analysed according the REST 2009 software. Finally, the change in the gene expression (fold change) was calculated through the 2-ΔΔCT formula [18].
| Name | Sesquence (5 to 3) | Product size (bp) |
|---|---|---|
| hla-F | 5-GGGGACCATATGATAGAGATT-3 5-TGTAGCGAAGTCGGTGAAA-3 |
154 |
| gyr-F | 5-CGC AGG CGA TTT TAC CAT TA-3 5-GCT TTC GCT AGA TCA AAG TCG-3 |
141 |
Table 1: Selected Primers For hla And gyrb Genes
Results and Discussion
In both of the phenotypic methods in question, the agarwell diffusion method (Figure 2) and streak plate culture method (Figure 3), haemolysis was stopped in 24 h culture with 1/2 and 1/4 sub-MIC doses of ZnO nanoparticles and no haemolysis was seen around the colony, while nanoparticle-free wells had visible beta haemolysis.
a-haemolysin gene expression in 26 haemolysisgenerating isolates of S. aureus was studied in three groups with 2500, 1250, 625 μg/ml MIC in a blood agar culture media with (312 μg/ml concentration) and without ZnO nanoparticles at log phase at 4 h and stationary phase at 12 h using real time PCR method (Table 2). In the log phase at 4 h, α-haemolysin gene expression increased from 1.89 to 4.85 times, in all three groups, but in the stationary phase at 12 h, α-haemolysin gene expression decreased to 0.36 and 0.57 in the first and second group, respectively, but it increased up to 1.7 in the third group. In both MRSA and methicillinsensitive S. aureus (MSSA) isolates the α-haemolysin gene expression in stationary phase (12 h) is decreased but this decrease is greater in MSSA (Table 3). While S. aureus α-haemolysin gene expression in stationary phase (12 h) has a significant decrease in isolates derived from patients and in isolates derived from carriers it shows a relative increase (Table 4).
| Group names (number of isolations) |
MIC μg/ml |
∆∆ Ct at 4th h (log phase) | 2-∆∆CT at 4th h (gene expression) |
∆∆Ct at 12th h (stationary phase) |
2 -∆∆CT At 12th h (gene expression) |
|---|---|---|---|---|---|
| First group (10) | 625 | -0.92 | 1.89 | 1.72 | 0.36 |
| Second group (10) | 1250 | -2 | 4 | 0.79 | 0.57 |
| Third group (6) | 2500 | -2.28 | 4.85 | -0.78 | 1.7 |
Table 2: α-hemolysin gene expression change in three groups of s. aureus isolates with a sub-mic dose of zno nanoparticle in both log and stationary phases
| Resistance type of strains (number of isolations) | ∆∆Ct at 4th h log phase | 2 -∆∆CT at 4th h (gene expression) | ∆∆CT at 12th h stationary phase | 2 -∆∆CT at 12th h (gene expression) |
|---|---|---|---|---|
| MSSA (19) | -1.84 | 3.58 | 1.01 | 0.49 |
| MRSA (7) | -1.59 | 3.01 | 0.1 | 0.93 |
Table 3: α-hemolysin gene expression at sub-mic doses of nanoparticles in mssa and mrsa isolates
| Derivation source (number of isolations) | ∆∆Ct at 4th h log phase | 2-∆∆CT at 4th h (gene expression) | ∆∆CT at 12th h (stationary phase) | 2-∆CT at 12th h (gene expression) |
|---|---|---|---|---|
| Patients (16) | -1.1 | 2.14 | 1.68 | 0.31 |
| Healthy earners (10) | -2.83 | 7.11 | -0.76 | 1.69 |
Table 4: α-hemolysin gene expression at sub-mic doses of nanoparticles in isolates derived from patients and healthy carriers
With the increase in resistance to antibiotics and side effects of synthetic drugs against bacterial infection in the recent years, the tendency towards usage of metal oxide nanoparticles has been growing. Preventing microbial infections is one of the main applications of these nanoparticles. Adding high dosage of nanoparticles to artificial instruments, especially intravascular catheters, urinary catheters and stitching and bandage equipment can prevent the establishment and growth of pathogenic bacteria. But the real danger is that increasing the usage of high doses of nanoparticles and repeating the usage of nanoparticle-treated material can lead to unknown risks in humans and the environment. Quorum sensing, which is based on cell signalling between bacteria [18], led us consider very low dosage, rather than high dosage that kill and discard bacteria of nanoparticles to decrease the expression of virulent genes.
Haemolysis in S. aureus is controlled by α, β, δ and γ haemolysin genes. Among these four types of haemolysin, α-haemolysin is the most important, since, in addition to its haemolytic effect on red blood cells, it leads to the death of platelets and white blood cells. It also contributes to cutaneous disorders, because it is dermonecrotic [19]. The results of our study showed that 1/2 up to 1/8 MIC densities of ZnO nanoparticles can inhibit the haemolysis in all tested isolates of S. aureus in 24 h cultures, meaning that although bacterial growth continues, at least one pathogenic property is discarded. It means that despite the decrease in nanoparticle dose and reducing its side effect occurrences, the expression of virulence factors of bacteria can be prevented. Therefore, one can hope that by adding low doses of these nanoparticles to catheters, bandages, sterile gauzes and so on the pathogenicity of the bacteria can be prevented.
One of the significant findings of this study is that with sub-MIC doses of ZnO nanoparticles, although bacterial growth did not stop and the bacteria survived, colony size is conspicuously smaller than when they grow in a nanoparticle-free culture media [7]. Change in colony size can ensue the bacteria’s change into a strain of small colony variant (SCV) [20], which needed more research for confirmation.
When investigating α-haemolysin gene expression as one of the most important factors leading to haemolysis in the presence of low concentration of ZnO nanoparticles, we found that only after the bacteria reached the stationary phase, the gene expression showed a significant decrease at transcription level; while in the log phase not only had the gene expression decreased, but also increased. On the other hand, our study showed that reduction in hla gene expression in stationary phase occurred on 1/2 up to 1/4 MIC doses of ZnO nanoparticles but not in 1/8th MIC dose of this nanoparticle. These results showed that ZnO nanoparticles at sub-MIC doses could reduce haemolysis gene expression, but when the dose of the nanoparticles was less than 1/4 of MIC, this phenomenon could not be seen. Our results showed that α-haemolysin gene expression in the stationary phase reduced to almost 4.8 times than that in the log phase, which explained the cessation of haemolysis in a 24 h culture.
While sub-MIC doses of ZnO nanoparticles in a 24 h culture had well restrained haemolysis, α-haemolysin gene expression had only relative reduction and various reasons attribute for this phenomenon. Our opinion is that (1) the hla haemolysin gene may be transcribed but not translated, (2) the amount and density of the α-haemolysin produced in the presence of ZnO nanoparticles may not be enough to cause lysis in red blood cells or even if there has been a partial lysis, it is not visible by macroscopic view and (3) there is the possibility that sub-MIC doses of ZnO nanoparticles have restrained the expression of other encoding haemolysin genes in addition to α-haemolysin, such as β, γ and δ haemolysins, leucocidin, which needs further study.
One of the significant findings of our study was that in MSSA strains, the reduction of α-haemolysin gene expression in the presence sub-MIC dose ZnO nanoparticles, was almost twice less than that of MRSA strains, which might be due to the higher resistance of MRSA strains to these nanoparticles. Ansari et al. [21] had determined the MIC of ZnO nanoparticles on MRSA at 2000 μg/ml in their study, which was similar to our findings. Our study showed that sub-MIC doses of ZnO nanoparticles significantly reduced the α-haemolysin gene expression in patients compared to isolates derived from healthy carriers. Although the reason for this is not known to us, the importance of using these nanoparticles in low concentration to cure patients became obvious.
ZnO nanoparticles in sub-MIC doses up to 1/8 MIC in a 24 h blood agar completely prevents haemolysis, but only the doses of 1/2 up to 1/4 MIC could significantly reduce transcription from α-haemolysin gene in a 12 h culture. Sub-MIC doses of ZnO nanoparticles could be used with artificial instruments, especially intravascular and urinary catheters, stitching and bandage equipment, to stop contamination and growth of pathogenic bacteria, but this requires further investigation.
Acknowledgements
Authors would also like to thank Ms. Fatemeh Shakeri, Ms. Sedigheh Livani, Ms. naemeh Javid and Mr. Masoud Bazoori for their invaluable assistance in running the project.
Financial support and sponsorship
This project was made possible with the financial support from Laboratory Sciences Research Center and Deputy of Research and Technology of Golestan University of Medical Sciences; to the authors wish to express their gratitude.
References
- Valaperta R, Tejada MR, Frigerio M, Moroni A, Ciulla E, Cioffi S, et al. Staphylococcus aureus nosocomial infections: the role of a rapid and low-cost characterization for the establishment of a surveillance system. N Microbiol 2010;33:223-32.
- Sakoulas G, Moellering RC, Eliopoulos GM. Adaptation of methicillin resistant Staphylococcus aureus in the face of vancomycin therapy. Clin Infect Dis 2006;42:40-50.
- Bien J, Sokolova O, Bozko P. Characterization of virulence factors of Staphylococcus aureus: Novel function of known virulence factors that are implicated in activation of airway epithelial proinflammatory response. J Pathog 2011;2011:601905.
- Gordon T, Perlstein B, Houbara O, Felner I, Banin E, Margel S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids and Surfaces A: Physicochem Eng Aspects 2011;374:1-8.
- Kluytmans J, Van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus epidemiology, underlying mechanisms and associated risks. Clin Microbiol Rev 1997;10:505-20.
- Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage 2011;1:111-14.
- Bhakids S, Tranum-Jensen J. Α toxin of Staphylococcus aureus. Microbial Rev 1991;55:733-51.
- Sharma-Kunikel BK, Wu Y, Tabor DE, Mok H, Sellman BR, Jenkins A, et al. Characterization of α-toxin hla gene variants, α-toxin expression levels and levels of antibody to α-toxin in hemodialysis and postsurgical patients with Staphylococcus aureus bacteremia. J Clin Microbiol 2015;53:227-36.
- Berube BJ, Sampedro GR, Otto M, Bubeck WJ. The psmα locus regulates production of Staphylococcus aureus α-toxin during infection. Infect Immun 2014;82:3350 - 8.
- Kwaka YK, Vikström E, Magnussonb KE, Vécsey-Semjén c B, Colque-Navarro a P. The Staphylococcus aureus, a toxin perturbs the barrier function in Caco-2 epithelial cell monolayers by altering junctional integrity. Infect Immun 2012;80:1670-80.
- Berube BJ, Vardenbura BJ. Staphylococcus aureus α-toxin: Nearly a century of intrigue. Toxins 2013;5:1140-66.
- Koenig RL, Ray JL, Maleki SJ, Smeltzer MS, Hurlburt BK. Staphylococcus aureus agr a binding to the RNA III-agr regulatory region. J Bacteriol 2004;186:7549-55.
- https://www.ncbi.nlm.nih.gov/pubmed/6369479.
- Yousef JM, Danial EN. In vitro antibacterial activity and minimum inhibitory concentration of zinc oxide and nanoparticle zinc oxide against pathogenic strains. J Health Sci 2012;2:38-42.
- Jones N, Ray B, Ranjit KJ, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad Spectrum of microorganisms. FEMS Microbial Lett 2008;279:71-6.
- Koohsari H, Ghaemi EA, Mozaffari AN, Moradi A. Association of agr gene expression with Staphylococcus aureus virulence genes in BHI broth. Med Lab J 2016;10:1-6.
- Oogai Y, Matsuo M, Hashimoto M, Kato F, Sugai M, Komatsuzawa H. Expression of virulence factors by Staphylococcus aureus growth in serum. Appl Environ Microbial 2011;77:8097-105.
- Arya M, Shegill IS, Williamson M, Gommersall L, Arya N, Patel HR. Basic principles of real-time quantitative PCR. Expert Rev Mol Diagn 2005;5:209-19.
- Song L, Hobaugh MR, Shustak C, Bayey H, Gouaux JE. Structure of staphylococcal α-haemolysin a heptameric transmembrane pore. Science 1996;274:1859-66.
- Xie Y, He Y, Irwin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol 2011;77:2325-31.
- Ansari MA, Khan HM, Khan AA, Sultan A, Azem A. Characterization of clinical strains of MSSA‚ MRSA and MRSE isolated from skin and soft tissue infections and the antibacterial activity of ZnO nanoparticles. World J Microbiol Biotechnol 2012;28:1605-13.