- *Corresponding Author:
- Y. Luan
Key Laboratory for Forest Resources Conservation and Utilization in the Southwest Mountains of China, Ministry of Education, Southwest Forestry University, Kunming 650224, China
E-mail: LuanTeam@163.com
| This article was originally published in a special issue,“New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “255-263” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Sulfamethoxazole suppress the secretion of exosomes from tumors, however its roles in modulation of proliferation, invasion and metastasis of adenocarcinoma cells, and tumor associated macrophages polarization remained unclear, therefore this study was carried on to elaborate the mechanisms. Exosomes of lung adenocarcinoma cell line A549 were extracted by ultracentrifugation; sulfamethoxazole was added and identified by Western blot method. The expression levels of surface markers of M1-type and M2-type macrophages were detected by quantitative polymerase chain reaction. A549 cells were cocultured with polarized M2-type macrophages, and the proliferation and migration ability of A549 cells was examined by cell counting kit-8 and scratch assays. Western blot was used to detect the expression of phosphoinositide-3-kinase, phospho-phosphoinositide-3-kinase, protein kinase B and phospho-protein kinase B. Animal experiments showed that lung adenocarcinoma-derived exosomes could promote the growth of tumor by the polarization of M2-type macrophages, and identification of lung adenocarcinomaderived exosomes by the addition of sulfamethoxazole and exosomes. Lung adenocarcinoma-derived exosomes were activated by phosphoinositide-3-kinase/protein kinase B signaling pathway to promote the polarization of M2-type macrophages to enhance the proliferation and migration of cancer cells. In addition, lung adenocarcinoma-derived exosomes can promote tumor growth in a subcutaneous mouse tumor model. Lung adenocarcinoma-derived exosomes promote the polarization of M2-type macrophages and promote the development of lung cancer through phosphatidylinositol-3-kinase/ protein kinase B signaling pathway.
Keywords
Sulfamethoxazole, lung adenocarcinoma, phosphoinositide-3-kinase, cell counting kit-8, Western blot
Lung Cancer (LC) is one of the most common cancers and the leading cause of cancer morbidity and mortality worldwide[1,2]. According to the World Health Organization classification of lung tumors in 2021, Non-Small Cell LC (NSCLC) accounts for about 85 % of all diagnosed LC[2]. Although the current diagnosis and treatment technology of NSCLC has been rapidly developed, the 5 y survival rate is only 15 % due to the majority of patients with locally advanced or extensive metastasis[3]. Therefore, it is particularly important to clarify the mechanism of LC progression and find new targets for NSCLC treatment. The Tumor Microenvironment (TME) is complex and diverse, compromising tumor-suppressive immune cells and tumor-promoting immune cells[4]. Tumor-associated macrophages are one of the immune cell populations of TME that promote tumor growth by inhibiting the tumor-suppressive immune cells, often leading to poor prognosis in patients with malignancy[5]. Macrophages can differentiate into different phenotypes according to microenvironments and there are mainly two phenotypes; classically (M1) and alternatively (M2) activated macrophages. M1-type macrophages exhibit pro-inflammatory, pro-immune and anti-tumor features, while M2-type macrophages orchestrate an immunosuppressive phenotype and can promote tissue remodeling and tumor progression through the secretion of anti-inflammatory cytokines[6]. Tumor-associated macrophages are considered to be M2-type macrophages, present in the TME and influence the metastasis of various cancers by interacting with cancer cells[7]. Studies have shown that the communication between tumor cells and macrophages plays a key role in mediating Tumor-Associated Macrophage (TAM) polarization. For example, Transforming Growth Factor-Beta (TGF-β) can promote the differentiation of inactivated macrophages into TAM-like immunosuppressive phenotype, which increases the expression levels of anti-inflammatory cytokines and decreases the expression levels of pro-inflammatory cytokines. Exosomes are small vesicles derived from cells and carry various biomolecules. Exosomes are key mediators of intercellular communication in TME. It has been reported that tumor-derived exosomes can promote tumor angiogenesis, metastasis and immune suppression by changing the phenotype and function of recipient cells[8]. Oral antibacterial drug Sulfamethoxazole (SFX) acts as a specific inhibitor of exosomes secretion in cancer cells, thereby effectively inhibiting the growth and metastasis of cancer without obvious toxicity. In the current study, we aimed to investigate how lung adenocarcinoma-derived exosomes induce macrophage polarization and the effect on tumor progression.
Materials and Methods
Cell culture:
A549 lung adenocarcinoma (CL-0016, Pricella), cells were cultured in Dulbecco’s modified eagle medium (Grand Island Biological Company (GIBCO), United States of America (USA)) containing 10 % fetal bovine serum (GIBCO, USA) and 1 % penicillin-streptomycin and put in incubators at 37°, with 5 % Carbon dioxide (CO2) and 90 % relative humidity. THP-1 human monocyte cell line (CL-0233, Pricella) was cultured in THP-1 cell special medium (CM-0233, Pricella) and placed in 37°, 5 % CO2 incubator. THP-1 cells were induced to differentiate into macrophages by Phorbol Myristate Acetate (PMA) (100 ng/ml). The experiment was divided into three groups; control group, M0 macrophage and in M0+exosome group, macrophages after exosomes treatment of A549 cells (PMA (100 ng/ml) induced THP-1 cells for 48 h, gently washed twice with Phosphate Buffer Solution (PBS); 200 μg/ml exosomes treated this macrophage, cultured for 24 h); in M0+SFX group, macrophages treated with SFX (macrophages are treated with 100 μm SFX for 24 h) and in M0+Interleukin-4 (IL-4) group, M0 macrophages were induced by IL-4 (20 ng/ml) for 72 h.
Extraction and identification of exosomes:
A549 cell culture supernatants were collected after 48 h of culture with 10 % exosomes-free serum, centrifuged at 300 ×g at 4° for 10 min, at 3000 ×g at 4° for 25 min and at 10 000 ×g at 4° for 1 h. The supernatant was then collected, filtered with a 0.22 μm filter, centrifuged at 100 000 ×g 4° for 3 h and the supernatant was discarded, resuspended in 100 μl PBS and stored at -80°. Western blot was used to identify the protein markers CD9, CD63 and Tumor Susceptibility Gene-101 (TSG101) of exosomes to confirm the quality of exosomes. Exosomes quantification was performed using the Bicinchoninic Acid (BCA) protein detection kit.
Real-Time quantitative Polymerase Chain Reaction (RT-qPCR):
The messenger Ribonucleic Acid (mRNA) level of CD163 in M0 macrophages was detected by RT-qPCR. Total RNA was extracted from cell lines with TRIzol reagent (Takara). The total RNA was reverse transcribed into complementary Deoxyribonucleic Acid (cDNA) with gold standard reverse transcription kit, and RT-qPCR was performed on a Z480 PCR machine. Amplification condition predenatured 94° 30 s (1 cycle) , 94° 5 s, 60° 30 s (40 cycles), Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) as parameter, calculated by 2-ΔΔCt. The primers are shown in Table 1.
| Genes | Forward primers (5'-3') | Reverse primers (5'-3') |
|---|---|---|
| ARG1 | ACTTAAAGAACAAGAGTGTGATGTG | GGCAACCCAGGTAACCCTTA |
| IL-10 | GGCACCCAGTCTGAGAACAG | CCGGTAGTGAACCCGTTGAT |
| CD163 | TTTGTCAACTTGAGTCCCTTCAC | TCCCGCTACACTTGTTTTCAC |
| iNOS | TTCAGTATCACAACCTCAGCAAG | TGGACCTGCAAGTTAAAATCCC |
| GAPDH | ACGGATTTGGTCGTATTGGG | CGCTCCTGGAAGATGGTGAT |
Table 1: Primer Sequences
Cell Counting Kit-8 (CCK-8) for cell proliferation:
A549 cells were co-cultured with macrophages polarized to the M2 phenotype and plated in 96-well plates in 100 μl of suspension. Each sample was repeated three times. After culturing the cells in the incubator for 12, 24, 36 or 48 h, 10 μl of CCK-8 reagent (Tokyo, Dojindo, Japan) was added to each well and incubated for 1-4 h. The absorbance was measured at a wavelength at 450 nm.
Scratch wound assay:
A549 cells were co-cultured with macrophages polarized to the M2 phenotype, and then A549 cells were prepared into cell suspensions, which were evenly seeded on a six-well plate. After 24 h, the cells in each group were treated according to the respective requirements and incubated in incubator with 5 % CO2 at 37°. After the cell density reaches 90 %, used a 100 μl sterile pipette to conduct a scratch experiment, with the scratch tip as perpendicular to the cell as possible to ensure that the scratch widths in each group are basically the same. Cells were washed twice with PBS and then cultured for 24 h in serum free medium at 37°, 5 % CO2. Photographs were taken at 0 and 24 h after the cell was scratched and the cell migration rate was calculated based on the change in the scratch distance.
Western blot analysis:
Total protein was extracted by radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology). Protein concentrations were determined by the BCA protein detection kit (Beyotime Institute of Biotechnology). It was separated by 8 %-12 % sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane. After incubation, Tris Buffered Saline Tween-20 (TBST) with 5 % skim milk for 1 h at room temperature 4°, then it was incubated overnight with the corresponding primary antibody. Primary antibodies used were CD9, CD63, TSG101, Phosphatidylinositol-3-Kinase (PI3K), phospho-PI3K (p-PI3K), Protein Kinase B (AKT), and phospho-AKT (p-AKT). After incubation with horseradish peroxidase anti-rabbit secondary antibody for another 2 h, band gray value analysis was performed using ImageJ software (National Institutes of Health (NIH)) with GAPDH as internal reference for comparative analysis.
Animal models and tumor Hematoxylin and Eosin (HE) stain:
Adult male Bagg and Albino (BALB/C) nude mice, (6-8) w, (Shanghai Laboratory Animal Research Center, China, Shanghai) were fed in a 25°±1° environment with a relative humidity of 50 %-60 % and worked for 12 h alternately day and night with enough food and water. The design and the process of this animal experiment were approved by the animal ethics committee and in accordance with the NIH guidelines. Nude mice were randomly divided into two groups (n=5 in each group). Control group has M0 macrophages; exosomes group has A549 cells after exosomes-stimulated macrophages. The cell solution was injected subcutaneously into the posterior flank of the nude mice, and the tumor volume and weight were measured and recorded every 5 d. At the end of the experiment, the mice were euthanized and the tumor tissues were obtained for subsequent experiments and then the tumor nude were sectioned at 5 μM and stained with HE stain to evaluate the volume of tumor.
Statistical methods:
All statistical analysis and graphs were performed by GraphPad Prism 9.0 software and ImageJ software, and all values are shown as Standard Deviation (SD)±mean. Comparison between the two groups was performed using the t-test and the Chi-square (χ2) test for independent samples, and p<0.05 was considered statistically significant.
Results and Discussion
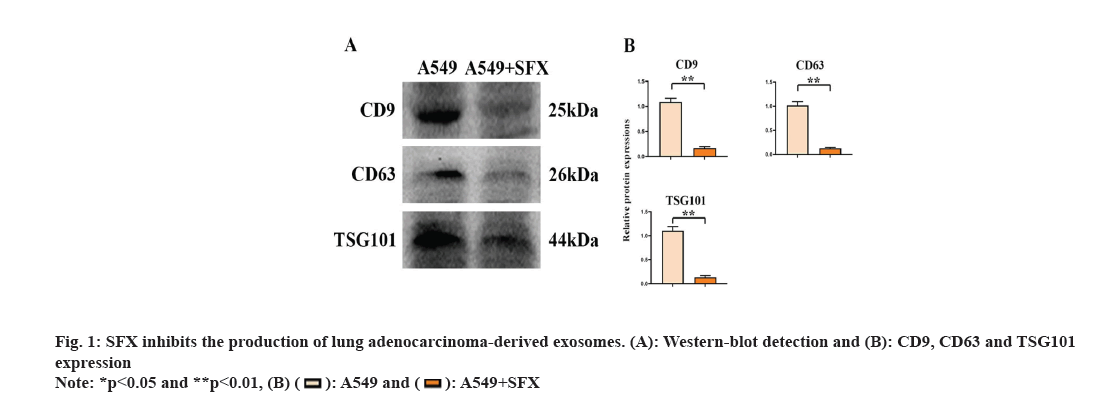
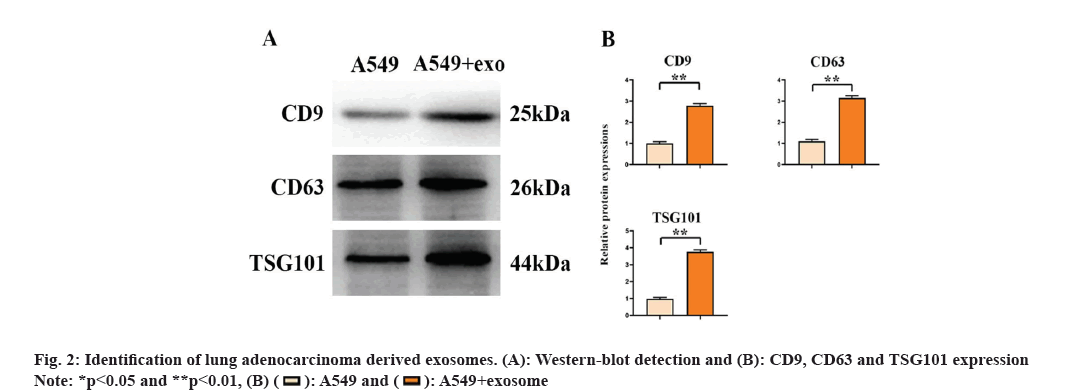
Lung adenocarcinoma-derived exosomes can promote the growth of M2 macrophages. With SFX treatment, we found significantly reduced expression of CD9, CD63 and TSG101 (fig. 1). Through the detection of the surface markers of exosomes derived from A549 cells, we found that CD9, CD63 and TSG101 expression was significantly elevated in the A549+exo group relative to the A549 group. This indicates that exosomes were successfully extracted from lung adenocarcinoma as shown in fig. 2.
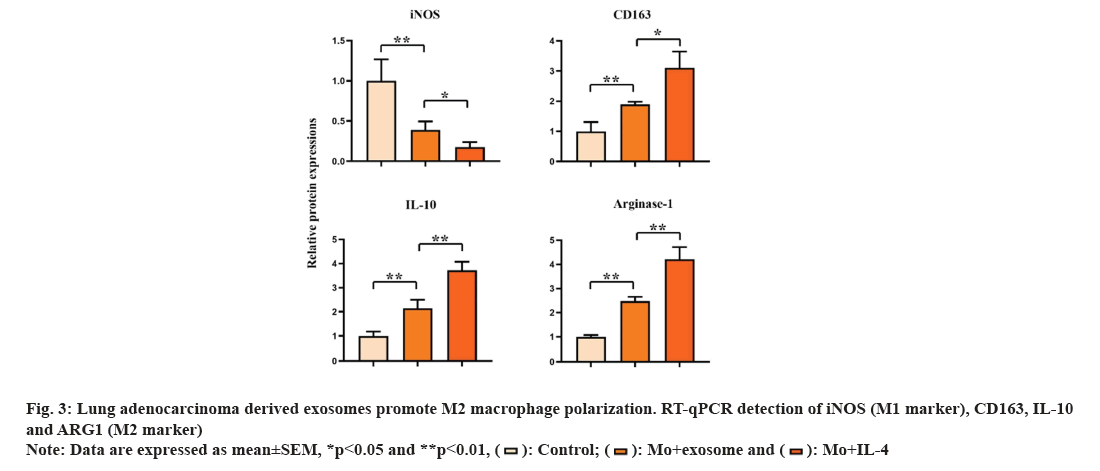
M1 and M2 phenotypes of macrophages play different roles in tumor. We detected the expression of M1 macrophage marker (inducible Nitric Oxide Synthase (iNOS)) and M2 macrophage marker (CD163, IL-10, Arginase 1 (ARG1)) by Reverse Transcription-Polymerase Chain Reaction (RT-PCR). The result showed that the expression of iNOS in control group was significantly higher than that in M0+exosome group and M0+IL-4 group (p<0.05). The expression of CD163, IL-10 and ARG1 in M0+exosome group and M0+IL-4 group was significantly higher than that in control group (p<0.05). These results suggested that lung adenocarcinoma-derived exosomes promote the polarization of M0 macrophages toward M2 as shown in fig. 3.
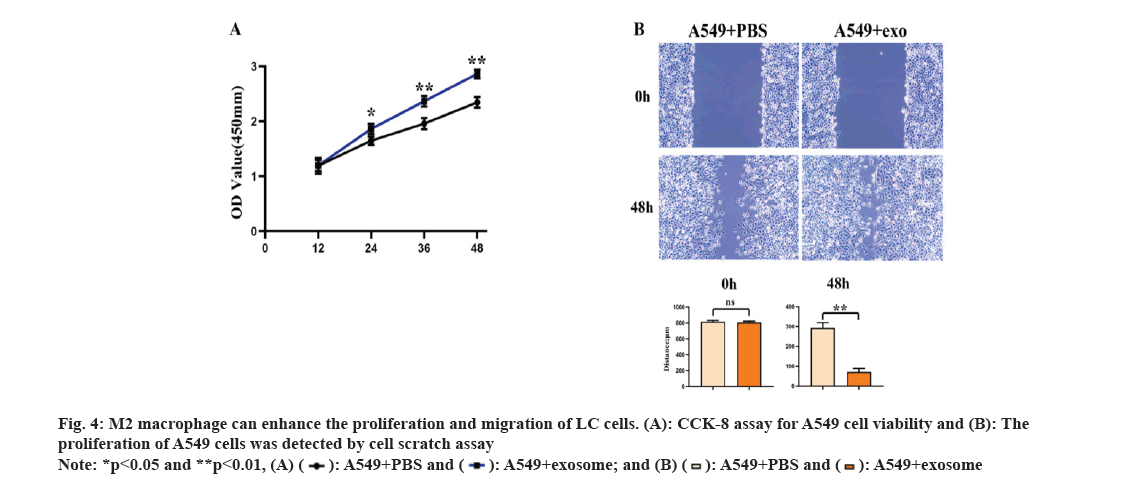
In order to study the effects of secretions derived from lung adenocarcinoma cells on the polarization of M2 type macrophages and their biological functions in lung adenocarcinoma cells, we co-cultured the polarized M2 type macrophages with A549 cells and then tested the proliferation and migration abilities of A549 cells. The results of CCK-8 test and the scratch wound assay showed that (fig. 3A and fig. 3B) the proliferation and migration abilities of A549+exosome group were significantly higher than those of A549+PBS group. These results suggested that exosomes induced M2 macrophages derived from lung adenocarcinoma promote the proliferation and invasion of LC cells as shown in fig. 4.
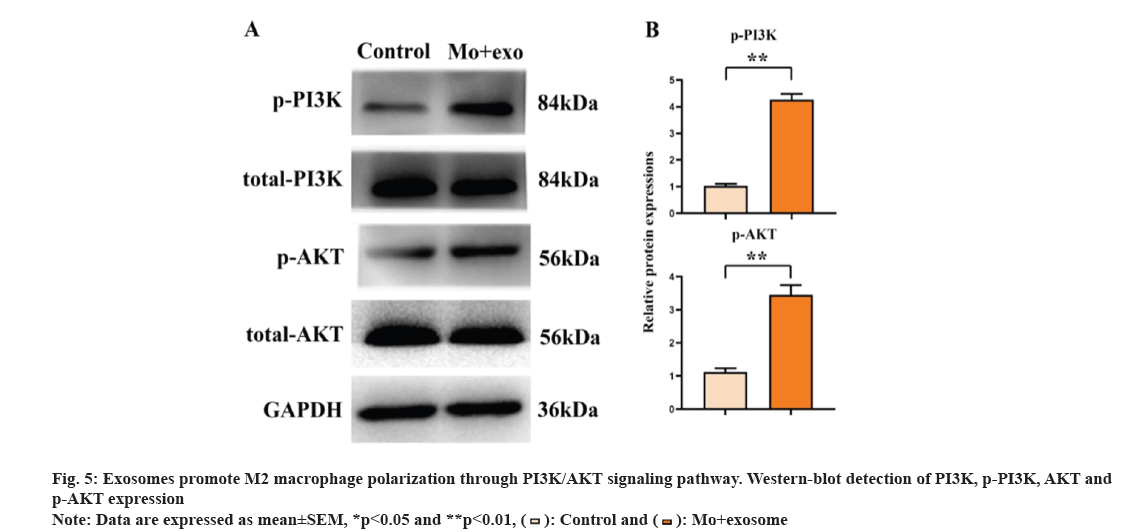
The PI3K/AKT pathway can regulate a variety of cellular functions, including the polarization of macrophages. Therefore, it was speculated that exosomes can promote the polarization of macrophages through PI3K/AKT signaling pathway. The result of Western blot (fig. 5) showed that the expression level of p-PI3K and p-AKT was higher in the exosomes group compared with the control group. These results suggested that lung adenocarcinoma-derived exosomes promote the activation of PI3K/AKT signaling pathway.
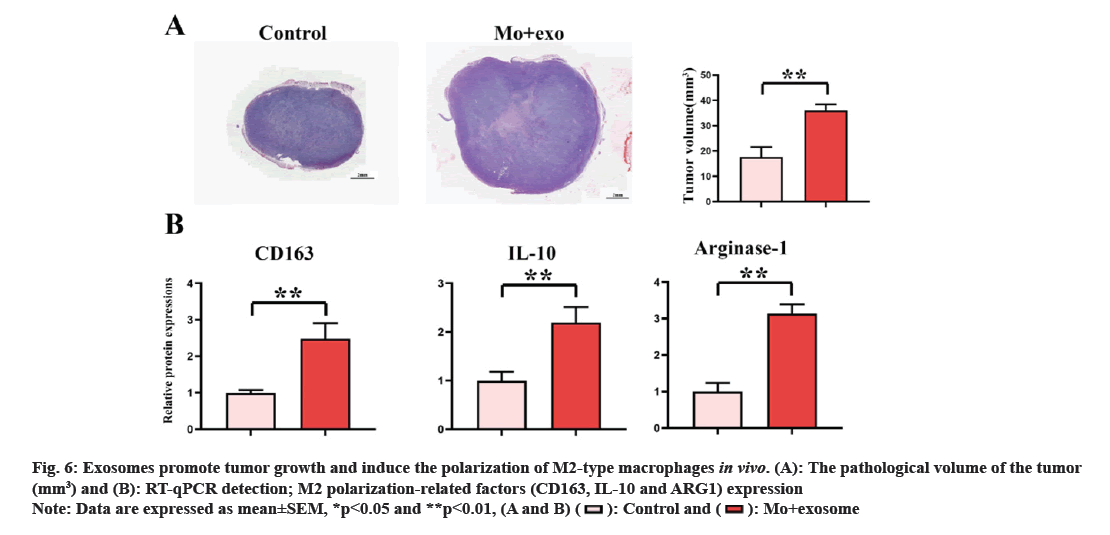
The effect of lung adenocarcinoma derived exosomes on M2 macrophage polarization and tumor growth was verified by establishing the subcutaneous tumor mouse model. The results of animal experiments showed (fig. 6A) that the tumor volume of the exosomes group was significantly larger than that of the control group. RT-qPCR results showed (fig. 6B) that the expression levels of CD163, IL-10 and ARG1 in M0+exosome group were significantly higher than those in control group (p<0.05). The results of animal experiments showed that lung adenocarcinoma derived exosomes can accelerate the growth of tumor through macrophage M2 polarization.
The role of TME in tumor is the focus of research in recent years. TME refers to the environment around the tumor[9], which consists of immune cells, fibroblasts, tumor cells, surrounding blood vessels and extracellular matrix, and plays a crucial role in the development of cancer. Because immune cells are an important component of the TME and matter much, interactions between the host defense of the immune system and tumor cells have been intensively studied over the past decade. TAM is a major component of TME immune cells and is considered to play a central role in the progression of tumor and to be a therapeutic target. Studies have shown that tumor metastasis may be influenced by non-tumor cells in the TME[10]. In addition, it has been demonstrated that TAM performs different functions at different stages of tumor progression, including promoting tumor killing, angiogenesis, cancer cell migration and invasion, inhibiting anti-tumor immune cells and mediating chemo-resistance. Numerous studies have shown that tumor-promoting factors in the TME influence macrophage polarization[11]. Macrophages are mainly polarized to M1 or M2 phenotype and fulfill different functions. In the TME[12], the M1-type macrophages mainly function as a tumor-suppressor and M2-type macrophages mainly play a tumor-promoting role. M2-like TAM account for approximately 70 % of NSCLC[13]; M2-like TAM can inhibit the cell Cluster of Differentiation (CD) 4 and CD8 T and can induce an immunosuppressive microenvironment that promotes the development of tumors by the over-expression of immune regulatory molecules, ARG1, IL-10, Programmed Death-Ligand 1 (PD-L1) and Cytotoxic T Lymphocyte Antigen 4 (CTLA-4). The study showed that the polarization of M2-type macrophages can promote the growth of tumors[14].
Exosomes are closely related to cancer, and exosomes derived from cancer cells can regulate the phenotype of immune cells in TME[15]. Exosomes are small vesicles with a lipid bilayer membrane structure, and the diameter is 30~150 nm. Exosomes can be secreted into the blood, urine and cerebrospinal fluid by various cells, including tumor cells[16]. Exosomes can transfer biologically active substances such as nucleic acids, proteins and lipids between cells. In cancer, exosomes can induce angiogenesis, cell migration and proliferation, inflammatory responses, immunosuppression, escape from immune surveillance and metastasis[17]. For example, Chen et al.[18] found that exosomes derived from epithelial ovarian cancer cells can convert macrophages into TAM, which can promote tumor proliferation and migration; exosomes can promote liver metastasis of colorectal cancer by promoting M2 macrophage polarization to enhance epithelial-mesenchymal transition and secrete vascular endothelial growth factor[19]. Studies have found that SFX acts as an inhibitor of Extracellular Vesicles (EVs) secreted by cancer cells by interfering with Endothelin Receptor A (ETA). SFX is an food and drug administration-approved oral antibiotic that has shown significant antitumor and anti-metastatic effects in mouse models of cancer xenografting, participating in the colocalization degradation of polyvesicle endosomes and lysosomes, and inhibiting exosomes production. We isolated exosomes by ultracentrifugation, CD9, CD63 and TSG101 were significantly reduced after SFX stimulation, indicating that SFX inhibited exosomes production and the exosomes stimulated M0 macrophages to increase the levels of M2 markers CD163, IL-10, ARG1 and decrease the level of M1 marker iNOS. The results showed that exosomes derived from lung adenocarcinoma cells could polarize M0 macrophages into M2 macrophages. In addition, the results of CCK-8 and scratch wound assay further indicated that co-culture of lung adenocarcinoma derived exosomes with A549 cells can enhance the ability of proliferation and migration of cells.
AKT was discovered 25 y ago and PI3K is its upstream regulator. The PI3K/AKT pathway is an intracellular signal transduction pathway that responses to extracellular signals and regulates cell survival, proliferation, metabolism, growth and angiogenesis[20]. Studies have shown that the PI3K/AKT pathway can regulate macrophage proliferation, polarization, migration, metabolism and inflammation[21]. For example, proprotein convertase subtilisin/kexin type 9 promotes the invasion and metastasis of colon cancer cells by regulating the epithelial-mesenchymal transition of tumor cells and activating the PI3K/AKT signaling pathway and macrophage polarization[22]; tumor-derived exosomes induce the polarization of M2-type macrophages and promote the progression of glioma through tumor suppressor factors[23]. These results suggest that the PI3K/AKT signaling pathway mediates the polarization of M2-type macrophage in lung adenocarcinoma-derived exosomes. PI3K/AKT has been proved to be a key regulator of macrophage regulation in tumor progression.
LC is one of the most common cancers and substantial progress has been made in immunotherapy as a new therapeutic strategy, but few clinical trials have been result full. The main challenge of immunotherapy is TME. TAM-mediated immunosuppressive TME can contribute to the suppression of adaptive anti-tumor immune responses. In the study, it is found that lung adenocarcinoma-derived exosomes can contribute to M2-type macrophage polarization by activating the PI3K/AKT signaling pathway, thereby promoting tumor growth. Therefore, this study may provide new therapeutic clues for LC patients.
Funding:
This work was supported by the Reserve Talents Project for Young and Middle-aged Academic and Technical Leaders of Department of Science and Technology of Yunnan Province (202105AC160047) and the National Natural Science Foundation of China (32160223), and the General Program of Science and Technology Department of Yunnan Province (2018FG001-039).
Acknowledgments:
This work was supported by the Engineering Research Center for Inheritance and Innovation of Traditional Chinese Medicine, The First Affiliated Hospital of Yunnan University of Traditional Chinese Medicine, Kunming 650034, China.
Conflict of interests:
The authors declared no conflict of interests.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [PubMed]
- Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumors: Impact of advances since 2015. J Thorac Oncol 2022;17(3):362-87.
[Crossref] [Google Scholar] [PubMed]
- Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Wu YL, et al. Lung cancer: Current therapies and new targeted treatments. Lancet 2017;389(10066):299-311.
[Crossref] [Google Scholar] [PubMed]
- Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res 2019;79(18):4557-66.
[Crossref] [Google Scholar] [PubMed]
- Toh B, Toh B, Abastado JP, Abastado JP. Myeloid cells: Prime drivers of tumor progression. Oncoimmunology 2012;1(8):1360-7.
[Crossref] [Google Scholar] [PubMed]
- Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest 2012;122(3):787-95.
[Crossref] [Google Scholar] [PubMed]
- Dehne N, Mora J, Namgaladze D, Weigert A, Brüne B. Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr Opin Pharmacol 2017;35:12-9.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: Trafficking, sorting and function. Genomics Proteomics Bioinformatics 2015;13(1):17-24.
[Crossref] [Google Scholar] [PubMed]
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24(5):541-50.
[Crossref] [Google Scholar] [PubMed]
- Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 2006;25:315-22.
[Crossref] [Google Scholar] [PubMed]
- Kumar V, Cheng P, Condamine T, Mony S, Languino LR, McCaffrey JC, et al. CD45 phosphatase inhibits STAT3 transcription factor activity in myeloid cells and promotes tumor-associated macrophage differentiation. Immunity 2016;44(2):303-15.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Li D, Cang H, Guo B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med 2019;8(10):4709-21.
[Crossref] [Google Scholar] [PubMed]
- Sun Z, Yang P, Singh T, Dhindsa J, Larsen JE, Fong KM, et al. Refining prognosis in non-small-cell lung cancer. N Engl J Med 2007;356(2):189-90.
[Crossref] [Google Scholar] [PubMed]
- Wang R, Zhang J, Chen S, Lu M, Luo X, Yao S, et al. Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer 2011;74(2):188-96.
[Crossref] [Google Scholar] [PubMed]
- Zeng Y, Fu BM. Resistance mechanisms of anti-angiogenic therapy and exosomes-mediated revascularization in cancer. Front Cell Dev Biol 2020;8:610661.
[Crossref] [Google Scholar] [PubMed]
- He G, Peng X, Wei S, Yang S, Li X, Huang M, et al. Exosomes in the hypoxic TME: From release, uptake and biofunctions to clinical applications. Mol Cancer 2022;21(1):1-22.
[Crossref] [Google Scholar] [PubMed]
- Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020;182(4):1044-61.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett 2018;435:80-91.
[Crossref] [Google Scholar] [PubMed]
- Wang D, Wang X, Si M, Yang J, Sun S, Wu H, et al. Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages. Cancer Lett 2020;474:36-52.
[Crossref] [Google Scholar] [PubMed]
- Zhu M, Li D, Wu Y, Huang X, Wu M. TREM-2 promotes macrophage-mediated eradication of Pseudomonas aeruginosa via a PI3K/Akt pathway. Scand J Immunol 2014;79(3):187-96.
[Crossref] [Google Scholar] [PubMed]
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 2005;9(1):59-71.
[Crossref] [Google Scholar] [PubMed]
- Wang L, Li S, Luo H, Lu Q, Yu S. PCSK9 promotes the progression and metastasis of colon cancer cells through regulation of EMT and PI3K/AKT signaling in tumor cells and phenotypic polarization of macrophages. J Exp Clin Cancer Res 2022;41(1):303.
[Crossref] [Google Scholar] [PubMed]
- Li M, Xu H, Qi Y, Pan Z, Li B, Gao Z, et al. Tumor-derived exosomes deliver the tumor suppressor miR-3591-3p to induce M2 macrophage polarization and promote glioma progression. Oncogene 2022;41(41):4618-32.
[Crossref] [Google Scholar] [PubMed]