- *Corresponding Author:
- B. Chatterjee
Shobhaben Pratapbhai Patel School of Pharmacy and Technology Management (SPPSTM), SVKM’S NMIMS (Deemed to be University), Mumbai 400056, India
E-mail: bdpharmaju@gmail.com
| Date of Received | 31 July 2021 |
| Date of Revision | 10 June 2022 |
| Date of Acceptance | 21 March 2023 |
| Indian J Pharm Sci 2023;85(2):388-402 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Sunflower oil, a commonly available vegetable oil, is used as a lipid component in nanoemulsion. When a nanoemulsion is loaded into an aqueous gel platform for transdermal delivery, skin permeation of a drug depends on the release of nanosize droplets from the gel. Semisolid characteristics, such as viscosity, spreadability, rheological properties, can greatly affect the drug release and permeation. We have formulated a nanoemulsion using sunflower oil as lipid carrier and raloxifene as a model drug by cold emulsification method. The most suitable nanoemulsion was composed of lipid:mixture of surfactant-cosurfactant (Tween 20-Transcutol® P, 3:1 w/w) in a ratio of 12.5:87.5 %. This formulation was loaded into carbopol 940 based gel in various ratios. Mechanical evaluation of the most suitable nanoemulgel (nanoemulsion: carbopol, 1:1 weight ratio) resulted in 94.0±4.62 g of hardness and 4.99±0.057 cm of spreadability. Shear-thinning and pseudoplastic behavior were evidenced in rheological evaluation. Ex vivo skin permeation study on rat skin showed a significant increase in the cumulative amount of drug permeation and steady-state permeation flux compared to the conventional gel. Increasing carbopol concentration showed increased viscosity and reduction in ex vivo drug permeation. The permeation enhancing effect of sunflower oil and Tween 20, nanosize droplets and enhanced retention of the dosage form over skin cumulatively increase the ex vivo drug permeation. Alternately, the potential of raloxifene transdermal delivery by sunflower oil-based nanoemulsion can be established.
Keywords
Nanoemulsion, nanoemulgel, sunflower oil, Tween 20, ex vivo permeation
One of the significant advantages of the transdermal route is the avoidance of first-pass metabolism[1]. Drug delivery through the skin has gained considerable interest as a reduction in size to nanoscale provides deeper skin penetration[2,3]. Nanoemulsion has been reported as a useful alternative formulation to increase the permeability and bioavailability of lipophilic drugs by enhancing their absorption through the skin[4]. Nanoemulsions have a higher solubilization capacity than simple micellar solutions. Their thermodynamic stability offers advantages over unstable dispersions, such as emulsions and suspensions, because they can be manufactured with little energy input (heat or mixing) and longer shelf life[5]. However, the topical delivery of nanoemulsion in liquid may not be so effective due to its low viscosity leading to poor skin retention. If the cream is prepared as an oil base, it may last longer on the skin, but that will eventually cause less user acceptance due to greasiness. The oily base cream also retards drug release[6]. Therefore, modifying the nanoemulsion physical state by incorporating gelling agents could be a better alternative to the transdermal delivery system. Nanoemulgel formulation is a combined preparation in which nanoemulsions are incorporated into a gel matrix for therapeutic and application-related improvement. The high surface area due to nanosize provides bioadhesivity and formation of a film, enhancing drug penetration to the blood via transdermal route owing to occlusive and hydrating properties[7]. Carbopol based aqueous gel system is a common platform for applying drugs over the skin for topical or systemic purposes. Nanoemulsion loaded into carbopol gel, often called as nanoemulgel could be a good vehicle for hydrophobic drugs and provides better patient compliance than liquid formulation. Several researchers have reported improvement of bioavailability of different poorly water soluble drugs, such as piroxicam[8], glibenclamide[9], carvedilol[10], etc. by transdermal delivery of nanoemulgel.
Sunflower oil, rich in linoleic acid (66 %), also contains oleic acid (21.3 %), palmitic acid (6.4 %), arachidonic acid (4 %), stearic acid (1.3 %) and behenic acid (0.8 %)[11]. The use of sunflower oil in sunscreen lotion[11] and as lipid component in nanoemulsions for various food elements have been reported earlier[12,13]. However, it has not been studied as lipid component for transdermal nanoemulsion or nanoemulgel. In their research, Babanejad et al.[14] have developed a polyol by sunflower oil-propargyl alcohol reaction for oral delivery of raloxifene hydrochloride[14].
Raloxifene is a second-generation selective estrogen receptor modulator used to treat osteoporosis and invasive breast cancer in postmenopausal women. Current oral therapy’s effectiveness with raloxifene is limited because of poor bioavailability (only 2 %) due to its low solubility and higher first-pass metabolism[15]. Raloxifene is generally supplied at a daily dose of 60 mg as an oral tablet. Administration of such a high dose increases the risk of developing a blood clot in the patient’s body. Therefore, the transdermal route of drug delivery for raloxifene could be a viable option. In our previous research, we have observed improved bioavailability of raloxifene by transdermal transfersomes[14]. Our group has also formulated raloxifene ethosomes containing transdermal delivery system for better skin permeation[16]. Raloxifene was also formulated as self-nano emulsifying oral drug delivery system[17], transdermal Nano-Lipid Carrier (NLC) [18], and nanoparticle with permeation enhancer[19].
NLC based raloxifene transdermal system suffers from stability issues and complicated fabrication techniques. The use of chemical permeation enhancer for repeated use could be toxic to the skin. Sunflower oil-based nanoemulsion can be better for transdermal permeation. Raloxifene transdermal gel formulation using sunflower oil as lipid carrier has not been reported before. In the present research, we have formulated a sunflower oil-based nanoemulsion, converted it to gel for enhanced skin permeation of the drug. The semisolid-state characterization and their effect on the drug permeation have been emphasized.
Materials and Methods
Reagents and materials:
The active pharmaceutical ingredient raloxifene (Batch No: 3479BX) was purchased from Binzhou Neophar Pharmaceutical Co. LTD, China. Sunflower oil (Lam Soon Edible Oils Sdn Bhd, Malaysia), palm oil (PPB Group Berhad, Malaysia) and olive oil (Deoleo SA, Malaysia) were purchased from different commercial sources. The surfactants, such as sorbitan monolaurate (Span 20), polyoxyethylene 20 sorbitan monolaurate (Tween 20), and Labrafil® M1944 CS were obtained from Merck, Germany. The cosurfactant, Transcutol® P, chemically diethylene glycol monoethyl ether (Gattefosse, France) was a gift from Elite Organic Sdn Bhd, Malaysia. Lauroglycol FCC was purchased from Gattefosse, France. Gelling agent carbopol 940 was purchased from Acros Organics (USA). Acetonitrile, methanol, ethanol, dihydrogen phosphate, potassium hydroxide and triethanolamine were purchased from Merck, Germany.
Comparison between oils:
Raloxifene solubility in different oils (sunflower oil, lauroglycol, palm oil, olive oil) was determined by adding an excess amount of the drug in 2 ml of oil in 5 ml capacity stoppered vials. The samples were placed in an incubator shaker (New Brunswick, USA)for 24 h at 150 rpm at room temperature to reach equilibrium. The samples were then filtered, and the aliquot absorbance was measured after the required dilution by Ultraviolet (UV)-visible spectrophotometer (Shimadzu, Japan) at a wavelength of 279 nm. Raloxifene solubility in different oils was calculated using the standard curve generated with six different standard concentrations of raloxifene (0.5, 1, 2, 5, 10, 15 µg/ml). The blank solution was prepared by dissolving 100 µl of the corresponding oils in 10 ml methanol.
Selection of surfactant and cosurfactant:
The surfactant was selected based on the emulsification ability of the chosen oils in water. Three types of surfactants (Tween 20, Span 20 and Labrafil®) were screened in this research. Different concentrations of each surfactant in water were prepared by continuous stirring with a magnetic stirrer. Then, 2.5 ml of the surfactant solution was placed into a centrifugal tube, and 10 µl of oil was added and mixed using a vortex mixer. The gradual addition of 10 µl of oil was continued until the sample became turbid. The process was repeated three times.
Ethanol and Transcutol® P were screened for suitability as a cosurfactant for this preparation. A mixture of surfactant:co-surfactant at a weight ratio of 1:1 was prepared (Smix). Then, the Smix was mixed with the selected oil in different proportions. Then, 10 μl of water was added to the oil phase and vortex mixed. The gradual addition of water was continued until the sample became turbid. The phase separation of each sample was continuously observed. The study was repeated three times.
Preparation of nanoemulsion:
The oil in water nanoemulsion of raloxifene was prepared by a cold emulsification method which involves mixing oil and aqueous phase. The oil phase was a mixture of raloxifene in oil at a concentration of 25 mg/ml. A 15 % v/v surfactant solution was prepared by dissolving the required amount of surfactant in water. The surfactantcosurfactant mixture, termed Smix was prepared by adding cosurfactant to the surfactant solution. Then, the aqueous phase was gradually mixed with the oil phase using a vortex. The mixture was then subjected to sonication (Qsonica, USA) for 30 sec to prepare the nanoemulsion. Different trial batches of nanoemulsion were prepared with varying oil and Smix ratios to find the most stable formulation. The composition of various batches is presented in Table 1.
| Formulation code | Surfactant (tween 20): Co-surfactant (Transcutol P®) (Smix, weight ratio) | Oil : Smix (weight ratio) | Observation for phase separation |
|---|---|---|---|
| NE 1 | 01:01 | 01:09 | Yes |
| NE 2 | 01:01 | 01:08 | Yes |
| NE 3 | 01:01 | 01:07 | Yes |
| NE 4 | 01:01 | 01:06 | Yes |
| NE 5 | 01:01 | 01:03 | Yes |
| NE 6 | 01:01 | 01:01 | Yes |
| NE 7 | 01:01 | 02:01 | Yes |
| NE 8 | 01:01 | 02:08 | yes |
| NE 9 | 01:01 | 03:02 | Yes |
| NE 10 | 01:01 | 03:01 | Yes |
| NE 11 | 01:01 | 05:02 | Yes |
| NE 12 | 01:01 | 09:01 | Yes |
| NE 13 | 02:01 | 01:07 | No |
| NE 14 | 02:01 | 02:07 | No |
| NE 15 | 03:01 | 01:07 | No |
| NE 16 | 03:01 | 02:07 | No |
| NE 17 | 03:01 | 03:07 | Yes |
Table 1: Association of miR-132 and SOX4 Expression with Clinicopathological Parameters in Colon Cancer Tissues (Number, Positive Rate %)
Characterization of nanoemulsion:
Thermodynamic stability: Nanoemulsion was deliberately exposed to different stress conditions to examine physical stability. The samples were centrifuged (Hettich, Germany) at 3000 rpm for 10 min followed by visual detection of phase separation, if any. Visual observation for phase separation was also done after challenging the nanoemulsions to three repeated freeze-thaw cycles, and different storage temperatures of 45° and about 4° for 3 d. No sign of phase separation was considered acceptance criteria for the preliminary selection of nanoemulsion.
Percentage transmittance: Percentage transmittance of nanoemulsion formulations was determined by UV-visible spectrophotometer (Shimadzu, Japan) at 650 nm, following the methods described by Ali et al.[20]. The absorbance of nanoemulsion sample, after 1:100 times dilution in water, was measured using water as blank, and the data were converted to transmittance using the following equation. All the samples were studied in triplicates (n=3). Percentage transmittance determines the clarity of the solution.
Percentage Transmittance=100×10-A
Where -A is the absorbance of the sample.
Refractive Index: A refractometer measured the refractive index of nanoemulsion formulation at 25°. Around 2-3 drops of nanoemulsion sample was taken on the prism and subjected for measurement of refractive index by an Abbe refractometer (Boreco, Germany). Special care was taken to ensure that the sample was evenly distributed on the entire surface of the prism to avoid any dry spots or bubbles. Each sample was studied in triplicates.
Viscosity and pH: Viscosity was measured using a Brookfield viscometer (Brookfield Engineering Laboratories, USA) by a C41 spindle at room temperature. Approximately 1 ml of each nanoemulsion was placed inside the plate and neatly closed. The measurement started at the speed of 0.6 rpm of the rotating spindle of the viscometer. The speed was increased consistently, and the measurement was recorded when the torque reached 10 %. The speed was progressively increased at a steady rate until the torque reached 100 %, with a 20 s interval between each sequential speed. The nanoemulsions pH was measured using a pH meter (Mettler-Toledo, China), duly calibrated before each measurement. All the samples for viscosity and pH were analysed in triplicates (n=3).
Determination of zeta potential: Zeta potential of the diluted nanoemulsion formulations (30 µl in 2 ml of purified water) was measured using a Zetasizer (Malvern, UK). This parameter was measured to determine the surface charge of oil droplets to determine the stability of the developed nanoemulsion formulation.
Droplet size and span (polydispersity index): The mean droplet size of the dispersed oil in nanoemulsion was examined by the Zetasizer (Malvern, UK) using diluted nanoemulsion (30 µl in 2 ml of purified water) as a sample. Span or polydispersity index was determined to evaluate the size distribution pattern of the nanoemulsion droplets.
Morphology and shape: The morphology and structure of the optimized nanoemulsion formulation were determined by Transmission Electron Microscopy (TEM) (Libra® 120, Carl Zeiss Microscopy LLC, Thornwood, NY, German). The nanoemulsion was diluted with distilled water (1:10, v/v). A drop (50 µl) of the nanoemulsion formulation was placed on carbon-coated copper grid (#300 mesh size and 3.05 mm in diameter). The grid was kept at room temperature for 30 min to dry and was subjected to TEM at an accelerating voltage of 120 kV, at 20-80 fold enlargement. The TEM images are recorded and analyzed visually.
Preparation of nanoemulgel: From the results of evaluation studies, the most suitable raloxifene nanoemulsion was selected and converted from liquid emulsion to semisolid nanoemulgel. Carbopol 940 was used as the gelling agent in different ratios to prepare different sets of nanoemulgel formulations. Two gm of carbopol 940 was added in 100 ml of water, stirred and kept for 24 h to hydrate and become a highly viscous solution. The nanoemulsion was then slowly added to the viscous solution of carbopol under magnetic stirring. The preparation pH was maintained between 7-8 by using triethanolamine to obtain the nanoemulgel. Different nanoemulgel formulations were prepared with varying nanoemulsion and carbopol ratio, described in section 3.4.
Characterization of semisolid gel:
Droplet size, Polydispersity Index (PDI) and pH: Droplet size and PDI of the nanoemulgel was measured by zeta sizer as per the method described in section 2.5.5. pH was also measured by pH meter. Stability of the nanoemulgel: The physical stability of the developed nanoemulgel formulations was determined. The integrity of the nanoemulgel after storing in ambient temperature (30 d), inside the freezer (30 d), at elevated temperature (35°, 7 d), and after challenging to three freeze-thaw cycles was evaluated. The formulations were checked visually for any change in consistency, color, or phase separation after exposure in the conditions mentioned above.
Rheology: Rheology of the developed gel was studied by a rheometer (Haake MARS, Thermo, Germany), connected with a universal temperature controller set to 25±0.5°. A PP35 Ti spindle was used, a set of parallel plates with a diameter of 35 mm. All samples were analyzed in triplicate. Up and down Controlled Rotational rate (CR) ramp was performed to determine the thixotropy and to study the flow behavior of nanoemulgel formulation. The range of shear rates was from 0.01 to 100 s-1 with a frequency of 1 Hz. The Linear Viscoelastic Region (LVR) measurement was carried out via an oscillation stress sweep test with a range of 0.01 to 100 Pa at a frequency of 1 Hz. The yield stress of nanoemulgel was determined through the rotational CR ramp at 0.1 to 100 Pa of shear stress with a frequency of 1 Hz. The data was collected by Haake RheoWin 3.61.000 software and analyzed to determine the difference between the rheological behavior of different nanoemulgel.
Spreadability: The spreadability of prepared nanoemulgel was determined after 48 h of preparation by measuring the spreading diameter of nanoemulgel between two glass plates. A weight of 500 mg of nanoemulgel was placed within a circle of diameter 1 cm premarked on a glass plate over which a second glass plate was placed. The increase in diameter or spreading of gel as a function of weights was noted. The following equation calculated the spreadability,
S=(m–l)/t
where S is spreadability, m is weight placed on the upper slide, l is the length of the upper slide, and t is the time taken.
Gel hardness: The hardness of the nanoemulgel was determined using a Texture Analyser (QTS Brookfield Texture Analyzer, USA). The cone of the spread test fixture was filled with a specific amount (approximately 40 ml in standard 80 ml beaker) of nanoemulgel. The excess amount of nanoemulgel formulations was removed from the cone holder to obtain an unruffled and flat surface. The texture analyzer was operated at a speed rate of 1 mm/s to a distance of 20 mm. The system recorded the hardness and cohesiveness values. All measurements were done in triplicate at room temperature.
Drug content determination: The amount of raloxifene content in the nanoemulgel formulations was determined by a prevalidated method using High-Performance Liquid Chromatography (HPLC). The HPLC system (SHIMADZU, Kyoto, Japan) was equipped with a diode array UV/VIS detector (SPD- M20A Prominence). The chromatographic parameters for the quantitative determination of raloxifene were as follows; stationary phase: C18 analytical column (4.6×250 mm, 5-micron, Zorbax Eclipse Plus) (Part No: AG993967-902, Make: Agilent Technologies Inc., USA), mobile phase: a mixture of phosphate buffer (10 mM, pH 6.1, adjusted with potassium hydroxide) and acetonitrile at the ratio of 60:40 v/v, flow rate: 1 ml/min, the wavelength of detection: 279 nm, run time: 8 min, retention of raloxifene: around 5.06 min. The method was linear within the range of 0.1 to 50 µg/ml concentration of raloxifene. The required quantity of nanoemulgel was dissolved in methanol and diluted subsequently in the mobile phase to prepare a calculated concentration of 100 µg/ml. The content of raloxifene in the sample was determined by the aforementioned HPLC method against standard drug concentration. The placebo solution prepared in a similar way using excipients was injected into HPLC to detect any unintended co- eluting peak at the retention time of raloxifene.
Ex vivo skin permeation study:
The ex vivo drug permeation study was carried out using Franz diffusion cell (with an effective diffusion area of 1.5 cm2 and receptor volume of 11 ml) using the excised skin of Wistar albino rats. The rat skin was donated by fellow researchers’ group of Pharmaceutical Technology department, Kulliyyah of Pharmacy, International Islamic University Malaysia 25200 after their deliberate sacrifice of studied animal (ethics approval by Institutional Animal Care and Use Committee (IACUC-IIUM), No. 2/2017, 28.11.2017)). The animals' maintenance, care, and study were done following the Helsinki declaration guidelines. The hairs of the dorsal side of the sacrificed rat were removed with a surgical blade. After shaving, the skin was separated, fats and connective tissues were removed with the help of a scalpel. The excised skin was washed with normal saline, checked for any rupturation before use. The skin was mounted on diffusion cell assembly. The stratum corneum side was placed to face the donor compartment, and dermal side faced the receiver compartment. The receptor compartment was filled with a mixture of phosphate buffer (pH 7.4, adjusted with sodium hydroxide): ethanol (60:40 v/v). The cells were placed on a magnetic stirrer rotating at 100 rpm (37° temperature) throughout the study. The nanoemulgel formulation, at a dose of 50 mg of raloxifene was applied onto the membrane of the donor compartment side. At different predetermined time intervals (0.5, 1, 2, 4 and 6 h), samples (1.0 ml aliquots) were collected and replaced immediately with an equal volume of fresh diffusion medium. The concentration of permeated raloxifene in the aliquot was then quantitatively analyzed by a pre-developed and validated HPLC method. A conventional gel of raloxifene in carbopol 934 with the same dosing of nanoemulgel was used as a control for this test. The cumulative drug permeated across the membrane was determined and plotted as a function of time. Steady- state permeability flux (Jss, µg/cm2/h) was calculated from the slope of the linear portion of the curve. Permeability coefficient was also calculated and compared between different formulations concerning the control. These ex vivo study result parameters are derived following standard and established equations[21]. Each sample was studied in triplicate. Statistical analysis by one-way Analysis of Variance (ANOVA) with F test was done to compare the ex vivo study results between test and control samples.
Local toxicity study:
The local toxicity study was done with a slight modification of the method described by Pund et al.[22]. A local toxicity study was carried out with excised abdominal skin of rats donated by fellow researchers, as mentioned in section 2.7.7. After 24 h of treatment by the nanoemulgel, the rat skins were fixed in 10 % formalin, embedded in paraffin wax, stained by eosin and hematoxylin stain. Untreated and treated skin with 37 % v/v nitric acid for 2 h was used as negative control and positive control, respectively. The changes in tissue histology and any sign of abnormalities on the tissue structure were evaluated by an experienced pathologist, blinded to the study.
Results and Discussion
The solubility of raloxifene in different oils is described in Table 2. Sunflower oil exhibited maximum solubility and hence, was selected to develop the formulation. The addition of a high concentration of surfactants in topical preparation can lead to skin irritation. Non-ionic surfactants generally cause lesser skin irritation compared to ionic surfactants. They are also less affected by the change in pH and ionic strength[23,24]. Therefore in this study, we have only screened non-ionic surfactants for the selected oil. Tween 20 was found the most suitable for sunflower oil. On the other hand, Transcutol® P was better as a cosurfactant for the nanoemulsion preparation compared to ethanol. Transcutol® P and other Short to medium-chain alcohols as co-surfactant can reduce the interfacial tension and increase the interface fluidity. They have also been reported to enhance the hydrocarbon tail’s mobility and cause greater permeation of the oil droplets into the skin[25]. The necessary Hydrophilic Lipophilic Balance (HLB) value for oil preparation in water nanoemulsion is greater than 10[26]. The addition of Transcutol® P (HLB: 4) with tween 20 (HLB: 16.7) is theoretically an appropriate blend of low and high HLB surfactants to develop a stable nanoemulsion. The maximum solubility was observed when sunflower oil, tween 20, and Transcutol® P were mixed in a ratio of 1:0.25:0.25 w/w. Therefore, nanoemulsions were formulated with sunflower oil as a lipid component, tween 20 and Transcutol® P as surfactant and cosurfactant, respectively.
| Oils | Solubility (mg/ml), mean±SD (n =3) |
|---|---|
| Palm | 10.14±0.31 |
| Sunflower oil | 23.70±0.40 |
| Olive oil | 7.070±0.18 |
| Lauroglycol | 4.970±0.25 |
Table 2: The Solubility of Raloxifene in Different Oils
Table 2 describes the nanoemulsion compositions with varying oil and Smix system ratios and their physical condition after 72 h of preparation. All the formulations were kept at room temperature and observed for phase separation. After 72 h, it was observed that excluding NE13 to NE16 all other formulations showed phase separation. The presence of an additional amount of tween 20 was a reason behind the phase separation. In NE13 to NE16 formulations, the quantity of Smix remained the same with varying proportions of oil. However, the composition of Smix was varied, which came up with a higher proportion of tween 20 compared to Transcutol® P. Therefore, it can be said that tween 20 in a ratio of 2:1 or 3:1 to Transcutol® P was able to reduce transient interfacial tension of sunflower droplets.
In thermodynamic stability studies, phase separation was observed in the case of NE13 and NE14, whereas NE15 and NE16 were devoid of phase separation, indicating better kinetic stability. Thermodynamic stability is essential to eliminate the possibility of metastable formulations from further processing, which are liable to get phase separation during storage[22]. However, in our research, we evaluated NE13 to NE16 (four nanoemulsions) to find out the reason for the difference in phase separation, regardless of the unstable nature of NE13 and NE14.
Viscosity is an essential parameter for nanoemulsion. It was anticipated earlier that the higher the viscosity, the better the stability of nanoemulsion[27]. In our research, there was no statistically significant difference in viscosity between NE15 and NE16. The lowest viscosity was obtained with NE13 (Table 3). The viscosity of the nanoemulsion depends on the percentage of the aqueous phase and oil content. The lower the oil phase, the lesser the viscosity of nanoemulsion. Nanoemulsion carrier formulations are o/w and so in addition to being less greasy than w/o formulations, often have lower apparent viscosities[28].
| Formulation | Viscosity (Pa s) | pH | Zeta potential | Droplet size (nm) | PDI | Percentage transmittance | Refractive index |
|---|---|---|---|---|---|---|---|
| (Average±SD) | (Average±SD) | (Average±SD) | (Average±SD) | (Average±SD) | (Average±SD) | ||
| NE13 | 10.28±0.16 | 3.93±0.06 | 24.7±0.30 | 181.8±1.6 | 0.241±0.036 | 98.3±1.4 % | 1.39±0.1 |
| NE14 | 11.92±0.80 | 3.83±0.06 | 29.43±1.65 | 208.20±0.98 | 0.249±0.004 | 88±2.3 % | 1.39±0.1 |
| NE15 | 12.80±0.33 | 3.90±0.10 | 33.80±1.35 | 153.10±4.47 | 0.253±0.038 | 98.4±2.2 % | 1.38±0.1 |
| NE16 | 13.06±0.32 | 3.80±0.10 | 28.67±1.40 | 188.67 ±0.55 | 0.217±0.87 | 92±1.7 % | 1.39±0.1 |
Table 3: Viscosity, pH, Zeta Potential, Droplet Size and PDI Values for Nano Emulsion
The pH of all formulations was measured in triplicate and found to in between 3.8 to 4 (Table 3). Though the pH range was low, it was not adjusted, as the nanoemulsion was used as an intermediate for the preparation of the final nanoemulgel formulation to be used topically on the skin. There were no significant differences between the pH of the four nanoemulsions.
Zeta potential values provide information on the repulsive forces between particles in an emulsion system. Theoretically, higher zeta-potential values result in better emulsion stability due to higher repulsive forces than attractive forces between the droplets. In practice, it is considered ¬that ±30 mV has sufficient repulsive forces to attain physical stability[29]. The pH of the formulation also affects zeta potential. The zeta potential shows a negative charge for higher pH and a positive charge for lower pH[30]. All nanoemulsions have positive zeta potential concerning the low pH values (less than 4). The zeta-potential for NE15 was found to be highest (33.80¬±1.35 mV) compared to the other preparations (Table 3). The zeta potential for NE13, NE14, and NE16 were less than 30mV.
In Table 4, it could be observed that the droplet size in all the formulations was in the nano range (<208.20±0.98 nm). We have observed an increase in droplet size with the rise of sunflower oil concentration in the formulation. NE15 has lower oil content compared to NE14 and NE16. Previous researchers have observed that decreasing oil quantity decreases the droplet size of nanoemulsion[31]. Comparing NE15 and NE13, both of which have the same proportion of oil, NE13 had a lesser surfactant ratio in Smix, which was the reason for significantly larger droplet size in NE13. The higher presence of surfactant causes a reduction in surface tension and results in lower droplet size[32]. Smaller droplet size has a better possibility of drug penetration through the skin. Considering these factors of droplet size NE15 was considered the most suitable formulation.
| Formulation code (NE: Carbopol) | Viscosity (Pa s) | pH | Hardness (g) | Cohesiveness | Spreadability (cm) |
|---|---|---|---|---|---|
| NG1 (1:0.5) | 49.14±5.77 | 6.34±0.057 | 58.7±2.88 | -0.22±0.01 | 5.1±0.126 |
| NG2 (1:0.67) | 50.91±10 | 6.61±0.057 | 76.0±2.64 | -0.23±0.03 | 5.01±0.072 |
| NG3 (1:1) | 60.13±10 | 6.65±0.057 | 94.0±4.62 | -0.26±0.05 | 4.99±0.057 |
| NG4 (1:1.5) | 70.34±10 | 6.70±0.057 | 96.7±0.57 | -0.26±0.00 | 4.2±0.057 |
| NG5 (1:2) | 79.01±10 | 6.71±0.057 | 105±2.00 | -0.36±0.11 | 4.2±0.057 |
Table 4: Physicochemical Characteristic of Nanoemulgel (NG)
PDI values of all nanoemulsion were below 0.241, and there was no significant difference between the four formulations. PDI value is indirectly indicative of emulsion stability. Higher PDI means a broad size distribution of droplet, resulting in emulsion instability such as creaming, cracking, or phase separation. In our research low, PDI values for all formulations were considered satisfactory.
The refractive index is measured to determine the isotropic nature of the formulations[33]. It was observed from the results that there was no difference in the refractive index between placebo and formulations. All the resultant values were below 1.39. (Table 3). It is concluded that the raloxifene nanoemulsions formulated by our research work are isotropic. Ideally, nanoemulsions should be an isotropic thermodynamically stable system[34]. These criteria were conformed by the raloxifene nanoemulsions developed in this research. Percentage transmittance of light through the sample determines its transparency. As per the percentage transmittance value shown in Table 3, NE15 and NE13 showed the highest transmittance. If the size remains in the nanoscale range then it should be transparent as indicated by the two formulations. On the contrary, NE14 and NE16 resulted in lower light transmittance (>88 %) due to higher oil content and larger droplet size.
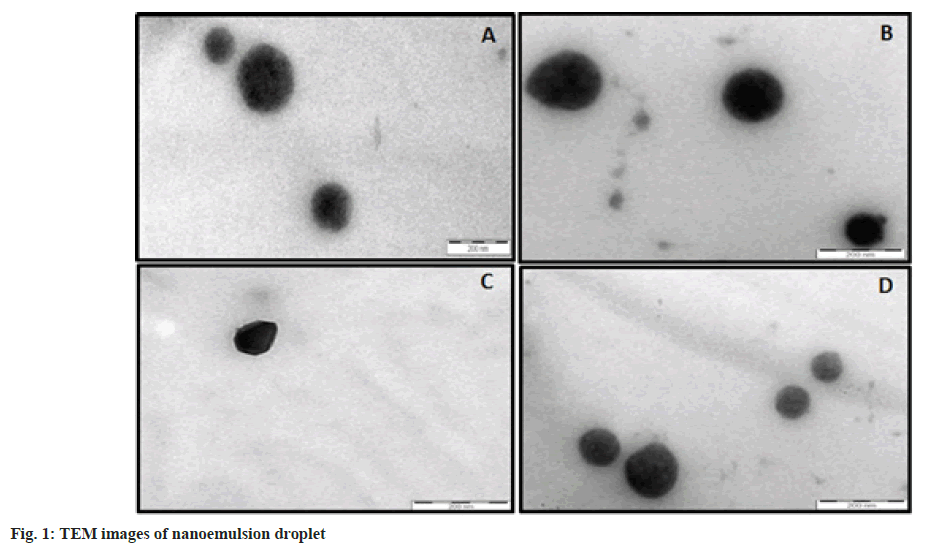
TEM analysis is one of the commonly applied advanced microscopic systems to study the morphology and shape of nano-sized droplets. Positive TEM images are presented in fig. 1. Nanoemulsion droplets area appeared as dark black images in almost spherical shapes. The droplet size measured by TEM was less than 200 nm, which was in close agreement with the sizes measured by zetasizer analysis.
We have chosen NE15 as the most suitable formulation. The zeta potential for NE15 was highest and was thermodynamically stable. The droplet size in NE15 nanoemulsion was the lowest compared to the other preparations. Based on these observations, the nanoemulsion NE15 was selected to develop the nanoemulgel. Five different nanoemulgel formulations NG1, NG2, NG3, NG4 and NG5, were prepared to vary the w/w ratio of raloxifene nanoemulsion and carbopol as 1:0.5, 1:0.67, 1:1, 1:1.5 and 1:2 respectively.
Droplet size and PDI for the developed gels were measured to evaluate whether there was any significant change in mean droplet size when converted from nanoemulsion to nanoemulgel. The droplet size was ranged from 165.8 nm (NG3) to 175.51 nm (NG1). There was no significant difference between 4 nanoemulgels in droplet size. However, the droplet size showed a slight increase from 153.10 nm (NE15) to 165.8 nm (NG3). Such an increase was due to the absorption of gelling polymers on the surface of the oil droplets[22]. The PDI values in the nanoemulgel were ranged from 0.234±0.02 nm to 0.270±0.031 nm. It was concluded that after loading raloxifene nanoemulsion onto the gel, the nano size of the droplets was maintained.
Thermodynamic stability study for different nanoemulgel formulations had come up with mixed results. No sign of phase separation was noted on the nanoemulgel formulation storage after 30 d on benchtop at ambient temperature and inside the freezer. All the formulations were physically stable after repeated three freeze-thaw cycles. However, phase separation was observed for NG1 and NG2 after 30 d of storage at 35°. This separation could be due to the lower content of the gelling agent in the formulations. On the other hand, because of the higher percentage of gelling agents, the gel consistency after 30 d of storage inside the freezer was very high for NG4 and NG5. NG3 was the most stable formulation with appropriate consistency for the external application compared to the others.
The viscosity of the different nanoemulgels was ranged from 49.14±5.77 to 79.01±10 Pa-s (Table 4). It was observed that an increase in gelling polymer (carbopol) ratio increases viscosity. The viscosity of the formulations can be considered satisfactory for its topical use. The pH of the formulations was targeted 6.0-6.8, and the results were found between 6.34 (±0.13) and 6.71 (±0.09) (Table 4). The pH was near to the neutral, which can be considered compatible and nonirritating upon its application on the skin.
Gel hardness is an important parameter to determine while evaluating the gel’s feasibility to fill in the tube and apply it on the skin. Unacceptable hardness would cause a problem during gel filling or application to come out of the rube regardless of extremely high or low. The gel’s hardness depends on the concentration of the gelling polymer, the oil concentration, and the temperature of processing. In our research, all gels have been tested for hardness and cohesiveness by a texture analyzer. The result (Table 4) showed that all the gels had hardness below 105±2.00 g. With such hardness, the gel extrudability would be good, and it would be able to come out of the tube with little pressure. A direct relationship between carbopol concentration and gel hardness was observed. With increasing carbopol ratio concerning nanoemulsion, gel hardness also increased. For instance, NG4 (NE:carbopol, 1:1.5 w/w) and NG5 (NE:carbopol, 1:2 w/w) resulted in significantly higher gel hardness than other nanoemulgel. In another research, Varma et al.[35] had observed increasing extrudability (decreasing hardness) of calcipotriol emulgel with decreasing carbopol concentration. The decrease in carbopol concentration in other ways reduced gel hardness; for instance, NG1 with lowest carbopol content with respect to NE, resulted in the lowest hardness (58.7±2.88 g).
Cohesiveness measurement of gel gives an idea of the work required to overcome the adhesive and cohesive attractive forces between the gel molecule or gel and the probe. Higher the cohesiveness, the higher the work necessary or the force needed to apply or spread the gel. As per the results shown in Table 4, the highest degree of cohesiveness was measured for NG5, the gel with the highest hardness. Therefore, like hardness, cohesiveness also acted as a function of carbopol concentration in the formulation. Gel hardness/cohesiveness is correlated with rheological properties as well as spreadability. In the subsequent sections, spreadability and rheological evaluation have been discussed.
Spreadability is a composite property that includes the effect of gel viscosity, rheological property and hardness. As Carbopol concentration affects the viscosity and hardness of gel, spreadability can also be explained as a factor of carbopol concentration. The result showed (Table 4) NG1, NG2, and NG3 had significantly better spreadability than NG4 and NG5. Such findings are well correlated with increasing carbopol content, increasing gel hardness. Low concentration of gelling polymer imparts a fluid like nature on the gel resulting in better spreadability. It was also in agreement with viscosity where NG4 and NG5 showed higher viscosity than the other three gels. In short, the spreadability of nanoemulgel formulations decreased with an increase in viscosity and hardness when a higher concentration of the gelling agent (Carbopol 940) is added[36]. Another important point regarding spreadability is the rheological property.
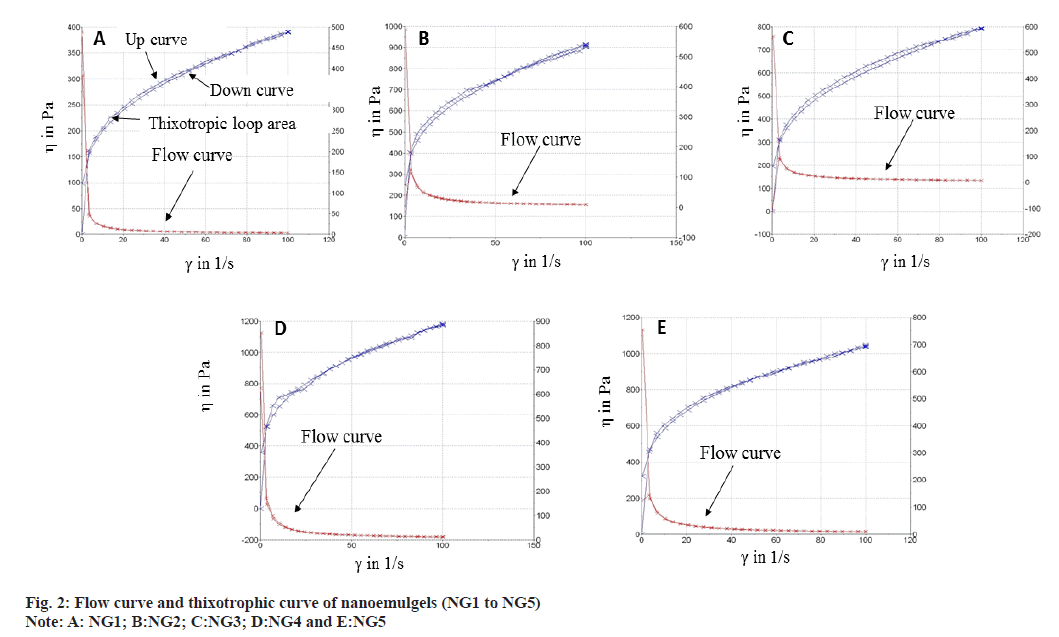
The flow behavior of nanoemulgel formulations was determined by studying the relationships among the shear rate and shear stress (force applied) on the prepared samples. The flow behavior (n) demonstrates the form of fluid. The fluid shows pseudoplastic behavior when n<1, while for n˃1, the fluid will be dilatant. When n=1, the fluid is Newtonian in behavior[37]. The flow behavior (n) of the freshly prepared nanoemulgel formulations (NG 1- NG 5) for topical application was pseudoplastic (non-Newtonian, shear-thinning) with a small yield value. The flow index gives a notion about the flowability of a nanoemulgel formulation from the container. The nanoemulgel acquires a thick base when the value of the flow index is low[38]. The values of the flow index for all the formulations were in the range of ~0.244 and ~0.233. As the n values are less than 1, it could be said that our formulations displayed shear thinning behavior. Shear-thinning behavior is one of the desired properties of topical semisolid formulations, which are typically thick, but has to be thinned when applied on the skin. It could be concluded that the raloxifene nanoemulgel showed a non-Newtonian pseudoplastic behavior. These systems start to flow in accordance with yield value and the viscosity is decreased with increased shear rate (fig. 2). Additionally, the developed nanoemulgels need some force to flow from the container and tube.
Thixotropic property is a time-dependent shear thinning property of a material. It expresses the status of material, whether fluid or gel (which are viscous or thick), after applying a required force to break down its structure and its ability to be reconstructed[39]. The thixotropic property of nanoemulgel was analyzed by up and down the rotational CR ramp. The rheogram of all the nanoemulgel formulations showed thixotropic properties, identified by the differences between the up and down curves (fig. 2). The thixotropic loop area is related to the energy required to break down the thixotropic structure. Higher thixotropic area means higher energy required to break down the materials and more time needed to reconstruct it.
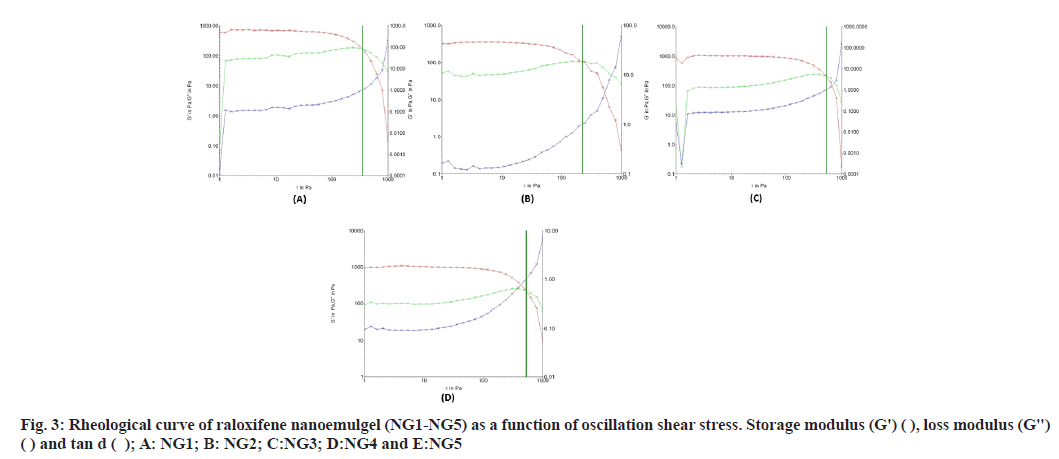
The stress sweep curve was used to determine the Linearity of the Viscoelastic Region (LVR), and is presented in fig. 3 (NG3). The rheological data, G′ (elastic or storage modulus), G″ (viscous or loss modulus) and tan δ of shear stress of nanoemulgel were measured. When G ′ value is more than that of G″, the behavior of a material is solid like, but when the G″ value is more than that of G′, the material behaves like a liquid. From fig. 3, it could be observed that the G′ for all the formulations is higher than G″, indicating the raloxifene nanoemulgel formulations are more towards ‘solid-like’ than ‘liquid-like’ materials.
The average content of raloxifene in the nanoemulgel formulation was 94.80 % (±1.49), 95.65 % (±2.23), 99.07 % (±1.25), 91.41 % (±2.06) and 91.00 % (±2.24) for NG1, NG2, NG3, NG4 and NG5, respectively. The results indicate that raloxifene got uniformly distributed throughout the preparation and loss of drug was not significant during formulating nanoemulgel. Drug loading found to be maximum in NG3. The average drug loading in the spiked solution was 102.18 % (±1.87) and in HPLC, no peak was detected at the retention time of raloxifene, either blank or placebo solution.
An ex vivo permeation study compared the drug permeation between conventional suspension and formulated nanoemulgel. The study results, presented in terms of the Cumulative Amount of Drug Permeated (CADP) through rat skin, steady- state permeation flux (Jss), and permeation coefficient are described in Table 5. It is observed that each of the Raloxifene nanoemulgel formulations (NG1- NG5) showed a statistically significant (p˂0.05) higher maximum cumulative release than the control sample (conventional RLX gel). There was a minimum of 2-folds increase in CADP value (8.14 µg/cm2) obtained from nanoemulgel (NG5) from that of the control sample (4.15 µg/cm2). A significant increase of almost 19 times in CADP and percentage cumulative drug permeated was obtained from NG 3 compared to the control sample. In the case of Jss also, there was a significant difference between the nanoemulgel and the control sample. Essential factors behind higher drug permeation from nanoemulgel include nano size of the droplet, hydration of stratum corneum, presence of oil and presence of surfactant. Nanosize of oil droplet containing the solubilised drug has better penetrability through the skin by virtue of their smaller size and higher surface area[40]. Hydration of the stratum corneum layer can cause swelling and opening of the tight junction of the skin, thereby promoting drug permeation[41]. The presence of an aqueous phase in nanoemulsion may help drug permeation in this way. The presence of sunflower oil was also an essential factor. When the gel is applied topically, it releases the oil droplets from the gel network, which permeate inside the stratum corneum of the skin and immediately deliver the drug[42]. Sunflower oil acts as a permeation enhancer due to the presence of a higher proportion of natural tocopherols as well as terpenes in it. In addition, sunflower oil contains monounsaturated and polyunsaturated fatty acid, a mixture of linoleic acid (66 %) and oleic acid (23 %). These fatty acids (oleic acid) increase the fluidity of the lipid portion of the stratum corneum[43,44]. The third factor was the presence of Smix, i.e. a mixture of a non-ionic surfactant (Tween® 20) and co-solvent (Transcutol® P) in the nanoemulgel formulation. As per previous theories, non-ionic surfactants like the “Tween” family follows two mechanisms. Initially, they penetrate the intercellular spaces of stratum corneum and enhance the fluidity followed by solubilization of the lipid component of the barrier[45]. They can also bind with the keratin filament of the intercellular spaces causing rupture of corneocyte. The other component of Smix, Transcutol® P, also helps in drug permeation. The previous study showed that Transcutol® P, diethylene glycol monoethyl ether acted as a transdermal permeation enhancer for clonazepam[46].
| Formulation | Cumulative amount permeated at 6 h (µg/cm2) | Flux (µg/cm2//h) | Permeation coefficient (cm/h)×103 | Percentage Cumulative drug permeated |
|---|---|---|---|---|
| NG1 | 41.8 | 6.5 | 2.2 | 0.21 |
| NG2 | 18.66 | 2.84 | 0.9 | 0.0933 |
| NG3 | 76.72 | 11.84 | 3.9 | 0.3836 |
| NG4 | 8.14 | 1.26 | 0.4 | 0.04 |
| NG5 | 10.61 | 1.6 | 0.5 | 0.053 |
| Control | 4.15 | 0.6 | 0.2 | 0.02 |
Table 5: Ex Vivo Permeation Parameters of Nanoemulgel (NG) Through Rat Skin
A comparison of ex vivo permeation parameters between different nanoemulgel showed that NG3 performed the best among all formulations. CADP and flux values of NG3 were 72.22 μg/cm2 and 11.84 μg/cm2/h respectively, whereas that of for NG1 were 41.80 μg/cm2 and 6.50 μg/cm2/h respectively. All nanoemulgel showed a lag time of 12-15 min. The differences in ex vivo permeation between different formulations were attributed to the correlation of various factors such as droplet size, viscosity, a ratio of nanoemulsion:gel base. NG3 had the lowest droplet size (165.8 nm) and viscosity of 60.13±10 Pa-s. The advantage of the nanosize droplet acted in favor of NG1. High viscosity hinders drug release from the gel.
The viscosity of NG3 was relatively lower than NG5 and NG6, which did not hinder drug release. Considering the composition, oil percentage in the formulation was decreasing from NG1 to NG6 due to increased carbopol gel base concentration. Sunflower oil is a permeation enhancer promoted higher drug permeation in NG3 than NG4 and NG5 where its’ presence was relatively lower in concentration. Increasing concentration of oleic acid, one of the major ingredients of sunflower oil increased transdermal permeation of piroxicam from the gel[8]. However, after a certain concentration oil can form aggregate, which hinders the drug release and permeation through the skin[8]. In this research, we assumed that NG1 and NG2 resulted in lower permeation parameters than NG3 due to relatively higher oil concentration. Based on the ex vivo permeation study, NG3 was considered the most suitable outcome of the research.
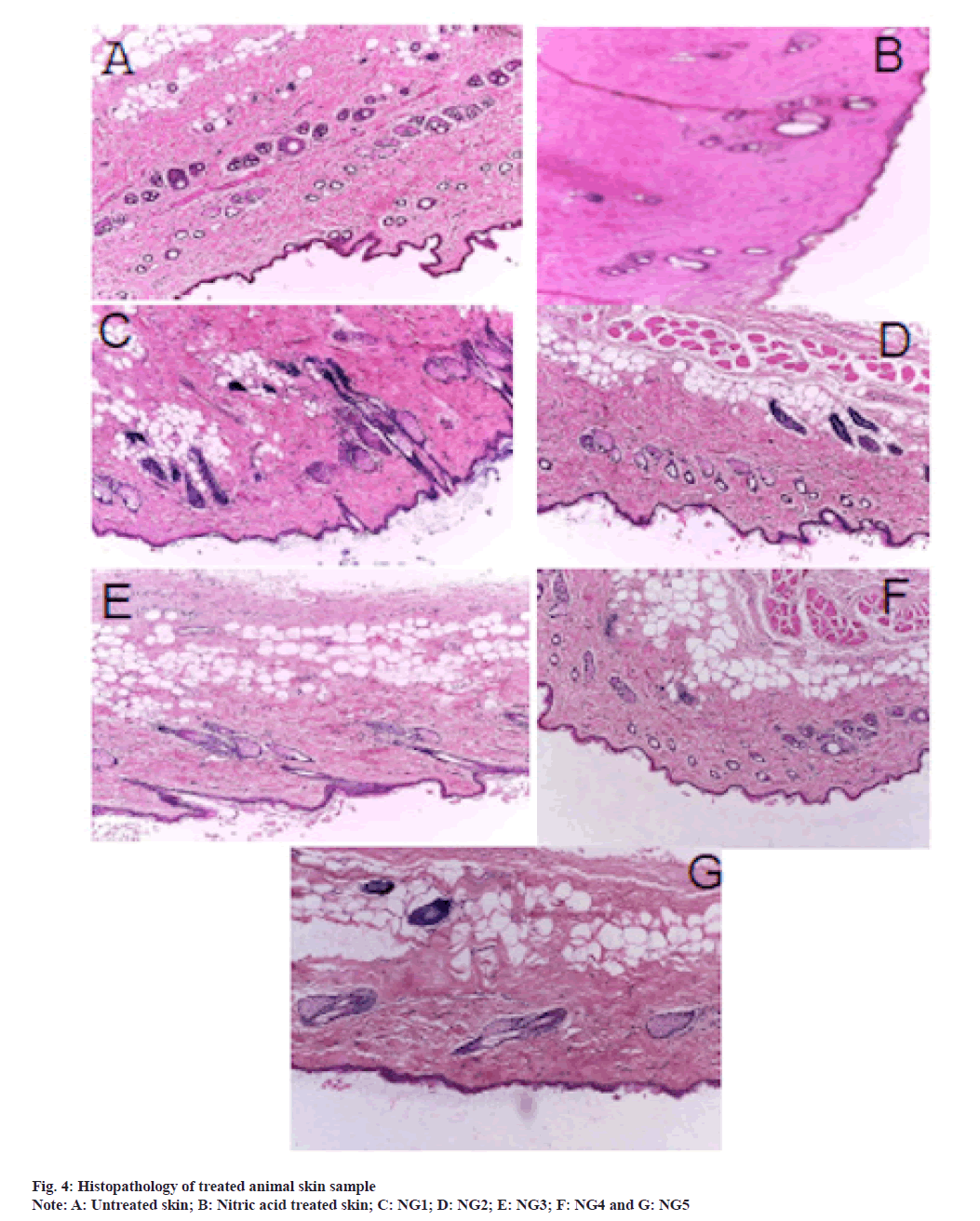
Histopathology of rat skin samples was carried out to assess the local toxicity caused by RLX nanoemulgel. The presence of surfactants may cause skin damage and irritation. Therefore, it was important to determine whether the surfactant type and concentration, employed in our formulation cause local toxicity to the skin or not. Seven sections of rat skin were subjected to histopathological study, as shown in fig. 4. After 6 h of exposure, the sample treated skin showed no toxicity sign and was comparable with the untreated skin (negative control). No significant change in skin morphology was observed in the nanoemulgel treated skin. The stratum corneum did not separate and remain intact unlike the positive control, where the skin was treated with nitric acid.
Raloxifene nanoemulsion was developed using sunflower oil as lipid component. To overcome the short retention time of the nanoemulsion on skn, it was converted into gel by incorporating carbopol. The most suitable nanoemulsion was selected based on the lowest droplet size (<208.02 nm) and the highest zeta potential with acceptable thermodynamic stability. Increasing oil content showed an increase in droplet size and a decrease in zeta potential. Selected nanomeulsion was loaded onto carbopol based gel platform. One drawback of the study is the use of Carbopol 940 as gel forming agent, which is restricted to use by the pharmaceutical industry now-a-days due to high residual benzene content. Therefore, replacing carbopol 940 with carbopol 980 while maintaining the gel viscosity could further the study.
The most suitable nanoemulgel, coded by NG3, was obtained in a 1:1 weight ratio of nanoemulsion and carbopol gel. The exact composition of NG3 could be written as sunflower oil:Smix:carbopol in a ratio of 6.25:43.75:50 %. Conversion of nanoemulsion to gel enhances retention on the skin due to enhanced viscosity. At the resultant viscosity, the hardness and spreadability of the gel were acceptable. An increase in carbopol concentration increased gel hardness and viscosity when reduced drug release. Cumulative drug permeation through rat skin showed a complex relationship with gel viscosity, droplet size, oil and surfactant ratio. Higher gel viscosity hindered drug release. Cumulative drug permeation was increased up to 19 times from the developed nanoemulgel compared to conventional gel. Sunflower oil and surfactant ratio can modify the drug permeation flux. Local toxicity study did not show any significant morphological change on rat skin after one time shortterm exposure (24 h). Although used in minimum quanity as neutralizer in gel, triethanolamine may incur skin toxicity on repeated and prolonged exposure. Hence, a repeated dose toxicity study could be done as future development of the formulation.
Acknowledgements:
We are thankful to the International Islamic University Malaysia, Malaysia, for providing financial support via the Research Initiation Grant Scheme (RIGS 16-121- 0825). We are also grateful to SVKM’s NMIMS for extending the support to complete this article.
Conflict of interest:
The authors disclosed no conflict of interest.
References
- Jepps OG, Dancik Y, Anissimov YG, Roberts MS. Modeling the human skin barrier—towards a better understanding of dermal absorption. Adv Drug Deliv Rev 2013;65(2):152-68.
[Crossref] [Google Scholar] [PubMed]
- Khurana S, Jain NK, Bedi PM. Nanoemulsion based gel for transdermal delivery of meloxicam: Physico-chemical, mechanistic investigation. Life Sci 2013;92(6-7):383-92.
[Crossref] [Google Scholar] [PubMed]
- Severino P, Fangueiro JF, Ferreira SV, Basso R, Chaud MV, Santana MH, et al. Nanoemulsions and nanoparticles for non-melanoma skin cancer: Effects of lipid materials. Clin Transl Oncol 2013;15(6):417-24.
[Crossref] [Google Scholar] [PubMed]
- Choudhury H, Gorain B, Chatterjee B, K Mandal U, Sengupta P, K Tekade R. Pharmacokinetic and pharmacodynamic features of nanoemulsion following oral, intravenous, topical and nasal route. Curr Pharm Des 2017;23(17):2504-31.
[Crossref] [Google Scholar] [PubMed]
- Shafiq-un-Nabi S, Shakeel F, Talegaonkar S, Ali J, Baboota S, Ahuja A, et al. Formulation development and optimization using nanoemulsion technique: A technical note. AAPS Pharmscitech 2007;8(2):E12-7.
[Crossref] [Google Scholar] [PubMed]
- Alexander A, Khichariya A, Gupta S, Patel RJ, Giri TK, Tripathi DK. Recent expansions in an emergent novel drug delivery technology: Emulgel. J Control Release 2013;171(2):122-32.
[Crossref] [Google Scholar] [PubMed]
- Sengupta P, Chatterjee B. Potential and future scope of nanoemulgel formulation for topical delivery of lipophilic drugs. Int J Pharm 2017;526(1-2):353-65.
[Crossref] [Google Scholar] [PubMed]
- Dhawan B, Aggarwal G, Harikumar SL. Enhanced transdermal permeability of piroxicam through novel nanoemulgel formulation. Int J Pharm Invsting 2014;4(2):65-76.
[Crossref] [Google Scholar] [PubMed]
- Wais MO, Samad AB, Nazish IR, Khale AN, Aqil MO, Khan MO. Formulation development ex vivo and in vivo evaluation of nanoemulsion for transdermal delivery of glibenclamide. Int J Pharm Pharm Sci 2013;5(4):747-54.
- Pratap SB, Brajesh K, Jain SK, Kausar S. Development and characterization of a nanoemulsion gel formulation for transdermal delivery of carvedilol. Int J Drug Dev Res 2012;4(1):151-61.
- Arianto A, Cindy C. Preparation and evaluation of sunflower oil nanoemulsion as a sunscreen. Open Access Maced J Med Sci 2019;7(22):3757.
- Yazgan H, Ozogul Y, Durmuş M, Balikçi E, Gökdoğan S, Uçar Y, et al. Effects of oil-in-water nanoemulsion based on sunflower oil on the quality of farmed sea bass and gilthead sea bream stored at chilled temperature (2±2°C). J Aquatic Food Prod Technol 2017;26(8):979-92.
- Shadman S, Hosseini SE, Langroudi HE, Shabani S. Evaluation of the effect of a sunflower oil-based nanoemulsion with Zataria multiflora Boiss. essential oil on the physicochemical properties of rainbow trout (Oncorhynchus mykiss) fillets during cold storage. LWT Food Sci Technol 2017;79:511-7.
- Babanejad N, Farhadian A, Omrani I, Nabid MR. Design, characterization and in vitro evaluation of novel amphiphilic block sunflower oil-based polyol nanocarrier as a potential delivery system: Raloxifene-hydrochloride as a model. Mater Sci Eng C 2017;78:59-68.
[Crossref] [Google Scholar] [PubMed]
- Burra M, Jukanti R, Janga KY, Sunkavalli S, Velpula A, Ampati S, Jayaveera KN. Enhanced intestinal absorption and bioavailability of raloxifene hydrochloride via lyophilized solid lipid nanoparticles. Adv Powder Technol 2013;24(1):393-402.
- Mahmood S, Mandal UK, Chatterjee B. Transdermal delivery of raloxifene HCl via ethosomal system: Formulation, advanced characterizations and pharmacokinetic evaluation. Int J Pharm 2018;542(1-2):36-46.
[Crossref] [Google Scholar] [PubMed]
- Elsheikh MA, Elnaggar YS, Gohar EY, Abdallah OY. Nanoemulsion liquid preconcentrates for raloxifene hydrochloride: Optimization and in vivo appraisal. Int J Nanomed 2012:3787-802.
[Crossref] [Google Scholar] [PubMed]
- Puro D, Athawale R, Pandya A. Design, optimization and characterization of nanostructured lipid carriers of raloxifene hydrochloride for transdermal delivery. Nanosci Nanotechnol Asia 2020;10(1):57-67.
- Nagai N, Ogata F, Otake H, Nakazawa Y, Kawasaki N. Design of a transdermal formulation containing raloxifene nanoparticles for osteoporosis treatment. Int J Nanomed 2018;13:5215.
[Crossref] [Google Scholar] [PubMed]
- Ali HH, Hussein AA. Oral nanoemulsions of candesartan cilexetil: Formulation, characterization and in vitro drug release studies. Aaps Open 2017;3(1):1-6.
- Dixit P, Jain DK, Dumbwani J. Standardization of an ex vivo method for determination of intestinal permeability of drugs using everted rat intestine apparatus. J Pharmacol Toxicol Methods 2012;65(1):13-7.
[Crossref] [Google Scholar] [PubMed]
- Pund S, Pawar S, Gangurde S, Divate D. Transcutaneous delivery of leflunomide nanoemulgel: Mechanistic investigation into physicomechanical characteristics, in vitro anti-psoriatic and anti-melanoma activity. Int J Pharm 2015;487(1-2):148-56.
[Crossref] [Google Scholar] [PubMed]
- Al Abood RM, Talegaonkar S, Tariq M, Ahmad FJ. Microemulsion as a tool for the transdermal delivery of ondansetron for the treatment of chemotherapy induced nausea and vomiting. Colloids and Surf B Biointerfaces 2013;101:143-51.
[Crossref] [Google Scholar] [PubMed]
- Kawakami K, Yoshikawa T, Hayashi T, Nishihara Y, Masuda K. Microemulsion formulation for enhanced absorption of poorly soluble drugs: II. In vivo study. J Control Release 2002;81(1-2):75-82.
[Crossref] [Google Scholar] [PubMed]
- Tenjarla S. Microemulsions: An overview and pharmaceutical applications. Crit Rev Ther Drug Carrier Syst 1999;16(5):461-521.
[Crossref] [Google Scholar] [PubMed]
- Ali MS, Alam MS, Alam N, Siddiqui MR. Preparation, characterization and stability study of dutasteride loaded nanoemulsion for treatment of benign prostatic hypertrophy. Iran J Pharm Res 2014;13(4):1125-40.
[Google Scholar] [PubMed]
- Bali V, Ali M, Ali J. Study of surfactant combinations and development of a novel nanoemulsion for minimising variations in bioavailability of ezetimibe. Colloids Surf B Biointerfaces 2010;76(2):410-20.
[Crossref] [Google Scholar] [PubMed]
- Gurpreet K, Singh SK. Review of nanoemulsion formulation and characterization techniques. Indian J Pharm Sci 2018;80(5):781-9.
- Win T, Rajagopal J, Mandal UK, Sengupta P, Chatterjee B. Incorporation of carbopol to palm olein based analgesic cream: Effect on formulation characteristics. Latin Am J Pharm 2017;36(11):2144-52.
- Malhotra A, Coupland JN. The effect of surfactants on the solubility, zeta potential, and viscosity of soy protein isolates. Food Hydrocolloids 2004;18(1):101-8.
- Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm 2007;66(2):227-43.
[Crossref] [Google Scholar] [PubMed]
- Chatterjee B, Gorain B, Mohananaidu K, Sengupta P, Mandal UK, Choudhury H. Targeted drug delivery to the brain via intranasal nanoemulsion: Available proof of concept and existing challenges. Int J Pharm 2019;565:258-68.
[Crossref] [Google Scholar] [PubMed]
- Choudhury H, Gorain B, Karmakar S, Biswas E, Dey G, Barik R, et al. Improvement of cellular uptake, in vitro antitumor activity and sustained release profile with increased bioavailability from a nanoemulsion platform. Int J Pharm 2014;460(1-2):131-43.
[Crossref] [Google Scholar] [PubMed]
- Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015;5:123-7.
[Crossref] [Google Scholar] [PubMed]
- Varma VN, Maheshwari PV, Navya M, Reddy SC, Shivakumar HG, Gowda DV. Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharm J 2014;22(6):591-9.
[Crossref] [Google Scholar] [PubMed]
- Ramasamy T, Khandasami US, Ruttala H, Shanmugam S. Development of solid lipid nanoparticles enriched hydrogels for topical delivery of anti-fungal agent. Macromol Res 2012;20:682-92.
- Phaechamud T, Mahadlek J, Charoenteeraboon J, Choopun S. Characterization and antimicrobial activity of N-methyl-2-pyrrolidone-loaded ethylene oxide-propylene oxide block copolymer thermosensitive gel. Indian J Pharm Sci 2012;74(6):498.
[Crossref] [Google Scholar] [PubMed]
- Arora R, Aggarwal G, Harikumar SL, Kaur K. Nanoemulsion based hydrogel for enhanced transdermal delivery of ketoprofen. Adv Pharm 2014;2014:1-2.
- Lee CH, Moturi V, Lee Y. Thixotropic property in pharmaceutical formulations. J Control Release 2009;136(2):88-98.
[Crossref] [Google Scholar] [PubMed]
- Elmataeeshy ME, Sokar MS, Bahey-El-Din M, Shaker DS. Enhanced transdermal permeability of Terbinafine through novel nanoemulgel formulation; development, in vitro and in vivo characterization. Future J Pharm Sci 2018;4(1):18-28.
- Akhter S, Jain GK, Ahmad FJ, Khar RK, Jain N, Khan ZI, et al. Investigation of nanoemulsion system for transdermal delivery of domperidone: Ex vivo and in vivo studies. Curr Nanosci 2008;4(4):381-90.
- Chellapa P, Mohamed AT, Keleb EI, Elmahgoubi A, Eid AM, Issa YS, et al. Nanoemulsion and nanoemulgel as a topical formulation. IOSR J Pharm 2015;5(10):43-7.
- Sinha VR, Kaur MP. Permeation enhancers for transdermal drug delivery. Drug Dev Indu Pharm 2000;26(11):1131-40.
[Crossref] [Google Scholar] [PubMed]
- Sanap GS, Dama GY, Hande AS, Karpe SP, Nalawade SV, Kakade RS, et al. Preparation of transdermal monolithic systems of indapamide by solvent casting method and the use of vegetable oils as permeation enhancer G. Int J Green Pharm 2008;2(2).
- Shokri J, Nokhodchi A, Dashbolaghi A, Hassan-Zadeh D, Ghafourian T, Jalali MB. The effect of surfactants on the skin penetration of diazepam. Int J Pharm 2001;228(1-2):99-107.
[Crossref] [Google Scholar] [PubMed]
- Mura P, Faucci MT, Bramanti G, Corti P. Evaluation of transcutol as a clonazepam transdermal permeation enhancer from hydrophilic gel formulations. Eur J Pharm Sci 2000;9(4):365-72.
[Crossref] [Google Scholar] [PubMed]