- *Corresponding Author:
- P. M. Mazumder
Department of Pharmaceutical Sciences and Technology, Birla Institute of Technology, Mesra, Jharkhand 835215 India
E-mail: pmitramazumder@bitmesra.ac.in
| Date of Received | 15 November 2021 |
| Date of Revision | 11 May 2022 |
| Date of Acceptance | 06 July 2023 |
| Indian J Pharm Sci 2023;85(4):919-935 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Pulmonary fibrosis treatment with currently available drugs mostly seems inadequate owing to its progressive and irreversible nature. Persistent activation of underlying mechanisms primarily, oxidative-stress and inflammation in lung leads to pulmonary fibrosis progression and subsequently produces sub-therapeutic control even after prolonged drug therapy. Additionally, due to large dose requirements in the treatment of pulmonary fibrosis unavoidable adverse effects are also an important concern. Thus, alternative drug therapy for pulmonary fibrosis, targeting to the aforementioned chief mechanisms is urgently required. In this view, some phytoconstituents were initially screened for antioxidant and anti-inflammatory activities through in vitro testing. Later, an in vivo study was planned to evaluate and compare the efficacy of two selected compounds namely forskolin (20 mg/kg) and rutin (100 mg/kg), individually and in combination against standard drug pirfenidone (50 mg/kg) using bleomycin-triggered pulmonary fibrosis murine model. Assessment parameters including changes in physical and physiological parameters along with alterations in lung injury markers, oxidative-stress, inflammatory status and fibrotic condition were evaluated during the study. Outcomes of the study exhibited, forskolin and rutin co-administration adequately reversed the physical and physiological changes during pulmonary fibrosis. Besides, it synergistically inhibited biochemical alterations in lung with no significant difference as compared to pirfenidone treatment. Further, forskolin and rutin co-administration showed effectively decline in Szapiel’s and Ashcroft scores and maximally diminish mast cell accumulation than that manifested by pirfenidone in lungs. Overall, efficacy of forskolin and rutin combination against pulmonary fibrosis showed promising potential and hence would contribute in the development of a novel effective treatment regimen in future.
Keywords
Pulmonary fibrosis, bleomycin, forskolin, rutin, pirfenidone, synergistic effect
Pulmonary Fibrosis (PF) is a continuous augmentative interstitial lung disease associated with poor prognosis and showing average survival span of around 2-3 y because of progressive restriction in lung function and alveolar spaces[1]. Broadly, inception of PF is considered as a cumulative effect of repetitive Alveolar Epithelial Cell (AEC) injury, abnormal wound healing, fibroblasts proliferation and subsequently their differentiation into myofibroblasts, which leads to accumulate voluminous Extracellular Matrix (ECM) within interstitial spaces and finally materialized as fibrosis[2]. Recently, PF is identified as most frequent and severe type of lung ailment amongst various categories of interstitial lung diseases[3]. Commonly, tobacco smoking, air pollution, Gastroesophageal Reflux Disorder (GERD) and viral infections are measured as the important risk factors in PF[4]. However, the specific aetiology behind the onset of the disease is still ambiguous so, it is also called as Idiopathic PF (IPF).
Drugs of immunosuppressant and antifibrotic categories are usually prescribed by the clinicians for the treatment of PF, frequently accompanied with dose dependent several adverse effects and uncertain improvement in Quality Of Life (QOL) with low survival expectancy in patients[5]. Also, because of progressive nature of this devastating ailment, transplantation of lung is only remained as the ultimate treatment option particularly in the advance stage of PF. Thus, it imposes huge responsibility on the pharmaceutical scientists to develop a new drug or novel treatment strategy on urgent basis, which could at least effectively overcome the progressive condition of PF.
From last few decades, many researchers emphasized that particularly oxidative-stress and chronic inflammation together act as chief underlying mechanisms which could be involved in the pathogenesis of PF[6]. Additionally, it is strongly believed that persistent activation of these mechanisms in lungs are accountable for PF progression[7] and leads to produce sub-therapeutic control with existing drug treatments. Recently, two active phytoconstituents namely forskolin and rutin were demonstrated with considerable potential to inhibit the lung inflammatory responses during asthma in clinical and pre-clinical studies, respectively[8,9]. In addition, rutin has also been recognized with another noticeable property that it could significantly improve wound healing activity[10]. Besides, growing scientific evidences suggested that successive alveolar injury motivate unusual responses which creates disturbance in wound healing process leads to accumulate excess of extracellular matrix at the site of injuries which may incite fibrosis formation instead of normal tissues repairment[2]. Furthermore, these phytoconstituents (forskolin and rutin) were also showed considerable redox balancing and antiinflammatory activities during in vitro testing in our initial studies (data not presented here). In addition, previous systematic research indicated that combined antioxidant therapy might be harmless and effective against the patients those were suffering from IPF as it may provide additive effect on lung functions specifically in vital capacity and carbon monoxide diffusing capacity (DLco) as they compared to single drug therapy[11]. Despite such pharmacological properties, the efficacy of forskolin and rutin as individual and their combination have not been assessed against the PF disease, so far.
Therefore, the present study was aimed to assess and compare the individual and combined effects of forskolin and rutin through various assessment parameters including physical, physiological, biochemical and histopathological examinations by using bleomycin-triggered PF model against standard drug treatment pirfenidone in rats. Additionally, different researchers in their scientific literature had proposed that mast cell might act as an effector cell during the institution of PF[12,13]. Thus, anticipated involvement of mast cell in the development of PF has been also pinpointed in this study.
Materials and Methods
Chemicals and reagents
Bleomycin was purchased from Cipla Ltd. (Maharashtra, India). Rutin was procured from Sigma-Aldrich (Missouri, USA). Forskolin was brought from Healthgenie India Pvt Ltd. (Haryana, India). Pirfenidone was obtained as a gift sample from Chemca Drugs Pvt. Ltd. (Andhra Pradesh, India). Enzyme-Linked Immunosorbent Assay (ELISA) kit for the detection of TNF-α was obtained from Immuno-Tag (G-Bioscience St. Louis, USA). Biochemical assay kits for the detection of Alkaline Phosphatase (ALP), Creatine Kinase (CK) and Lactate Dehydrogenase (LDH) were purchased from Coral Clinical Systems (Goa, India). Remaining other chemical ingredients including reagents were purchased and utilized of highest purity or for analytical purpose and obtainable commercially.
Experimental animals:
Thirty, male, Wistar albino rats of 275-325 g bodyweight and age between 10-12 w were received from the animal house facility, Birla Institute of Technology, Mesra (India). Experimental rats were kept in the propylene cage within maintained environment (Temperature: 25±2°; Relative Humidity: 30-70 %) with 12 h day and night alteration period. All experimental rats were acclimatized for at least week before commencement of study. They were fed with standard rodent meal and allowed free access to drinking water throughout the study duration. The study was approved by Institutional Animal Ethics Committee (Study protocol approval no. 1972/PH/ BIT/55/18/IAEC). Study was carried out in adherence with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (Reg. no. 1968/PO/Re/S/17CPCSEA).
Study design:
Initially, body-weight of all animals was recorded, then under mild anaesthesia each study animal went for survival surgery, where the trachea was surgically exposed through a central line incision on the neck region. For the development of PF model in rats, a single-dose of bleomycin (7.5 IU/ kg) in 0.9 % w/v Normal Saline (NS) as vehicle was instilled intratracheally (IT) via cannula under direct visualization on day-0 of the study[14]. However, normal control group related animals received a single-dose of vehicle (NS) via instillation (IT). At the completion of study, all experimental animals were gently sacrificed through decapitation method. All experimental rats were randomly assigned to the different groups (containing 5 animals each) and allowed to administer the study drugs (orally) for the 21 consecutive d as follows.
Treatment Protocol:
Group-1, administered a single-dose of NS once daily (o.d), after vehicle instillation (IT) and designated as NOS (normal control) group. Group-2, administered a single-dose of forskolin (20 mg/kg) in NS (o.d), after bleomycin instillation (IT) and designated as FOS group. Group-3, administered a single-dose of rutin (100 mg/kg) in NS (o.d), after bleomycin instillation (IT) and designated as RUT group. Group-4, co-administered a single-dose of forskolin (20 mg/kg) and rutin (100 mg/kg) in NS (o.d), after bleomycin instillation (IT) and designated as FOS+RUT group. Group-5, administered a singledose of pirfenidone (50 mg/kg) in NS (o.d), after bleomycin instillation (IT) and designated as PIR (standard treatment) group. Group-6, administered a single-dose of NS (o.d), after bleomycin instillation (IT) and designated as BLM (disease control) group.
In view of allometric scaling of rat metabolism, a daily forskolin dose was derived as 20 mg/kg in rats. It was calculated from the daily recommended human dose equivalent to 200 mg, which was divided by average normal human body-weight (60 kg) and dose conversion factor (0.162) as denoted in the literature[15]. However, doses of the other study drugs including rutin and pirfenidone were adopted from the previous studies[9,16].
Leukocyte infiltration assessment:
After the completion of 21 d dosing, animals were sacrificed and surgically, thoracic chamber was opened immediately and the whole lungs were harvested. Bronchoalveolar Lavage Fluid (BALF) was collected with three successive rinses of lungs by injecting 1 ml Phosphate Buffer Saline (PBS) solution (pH 7.4), pooled and concentrated at 1200 g by centrifugation. For the Total Leukocytes Count (TLC) the cells pellet was re-suspended in freshly prepared PBS solution (pH 7.4) and leukocyte counting was done by using hemocytometer. However, for the Differential Leukocyte Count (DLC), a thin-smear of BALF sample was slightly spread over the slide and subsequent 300 cells were counted in each slide under light microscope (METZ- 877, India) after Giemsa’s staining[17].
Physical parameter assessment:
Percentage change in body-weight and lung/bodyweight ratio: Initially, the body-weight of individual study animal was recorded and then a weekly change was measured in all the groups[18]. However, to calculate the percentage change in body-weight the initial mean value was considered equivalent to 100 %. At the end of the study, lung/body-weight ratio was also assessed by calculating the ratio between lung and body-weight in each individual for all the study groups.
Exercise Performance Test (EPT):
The physical EPT for experimental animal (rats) was performed analogous to 6 Min Walk Test (6-MWT) which is generally used to assess the lung condition in human[19]. To conduct the EPT in rats, a motorized force running wheel indig-enously developed apparatus of total inner circumference equivalent to 1 m was used which provide unidirectional controlled rotational speed to the wheel. Prior to perform EPT, all the experimental animals were necessarily familiarized to this apparatus to minimized the avoidable errors. During this test, motorized wheel was set at constant rotational speed of 8 rpm in unidirectional movement (clockwise or anti-clockwise). Where, the single animal was gently placed inside the apparatus and the circular movement of wheel force to maintain the running speed correspondingly because of continuous moving surface. In each experiment, apparatus was continuously operated till the complete fatigue condition was achieved and muscle became flaccid to maintained the erect position of test animal inside the apparatus. EPT was performed just before end of the study. Individual’s exercise performance ability was measured through calculating the total distance covered in meter (m) by the test animal. Where, EPT was derived as follows.
Total distance (m)=Wheel speed (rpm)×Running time (min)×Wheel circum¬ference (m)
Physiological parameter assessment:
Arterial blood oxygen saturation test: PF usually develop the condition of hypoxemia (low blood oxygen) which could be determined by measuring the arterial blood oxygen saturation (SpO2) level[17].
To measure the SpO2-level, a non-invasive oximeter of Nisco-med Co., Ltd. (New Delhi, India) has been utilized[20] and the sensor was attached over the peripheral artery at the proximal end of tail in each animal. The value of SpO2-level was recorded in percent (%) which was monitored at weekly intervals during the span of the study.
Biochemical parameter assessment:
Lung injury, oxidative-stress, inflammatory status and fibrosis markers:
Lung injury markers viz., LDH, ALP, CK and Total protein (TP) as well as inflammatory marker such as Tumor necrosis factor-α (TNF-α) were determined in BALF supernatant[21-24]. The estimation of c level was done by utilizing commercially available kits of Choral Clinical Systems (Goa, India). For the measurement of catalytic concentration of ALP, CK and LDH enzymes, an International Federation of Clinical Chemistry (IFCC) method was used with slight modification. However, the TP was assessed through Bradford method[25]. Moreover, the quantification of TNF-α was carried out by using rat specific ELISA kit of Immuno-Tag (G-Bioscience St. Louis, USA) in accordance to the instructions provided by the manufacturer.
For the estimation of oxidative-stress, inflammatory status and fibrotic markers the right lung tissue was utilized and separate lung homogenates were prepared as discussed in our previous research[26]. However, all the biochemical estimations were carried out through spectrophotometry instrument (Bio-Rad multiplate reader). Superoxide Dismutase (SOD) activity was biochemically estimated by prevention of Nitro Blue Tetrazolium (NBT) reduction rate and absorbance change was recorded at 560 nm[27]. Catalase (CAT) activity was interpreted by decomposition of hydrogen peroxide (H2O2) per min at 240 nm absorbance wavelength[28]. Malondialdehyde (MDA) content was estimated indirectly through reaction between Thio-Barbituric Acid (TBA) and Trichloroacetic Acid (TCA) and absorbance of the sample was recorded at 540 nm[29]. Reduced Glutathione (GSH) level in the samples were estimated at 415 nm absorbance[30]. However, Myeloperoxidase (MPO) activity was determined as per the previously established method[31] where, per min absorbance change was noted at 450 nm wavelength. Similarly, Hydroxyproline (HYP) content as a hallmark marker of fibrosis was estimated in lung as discussed earlier[32]. Absorbance of sample was taken at 550 nm. Also, standard-curve equation of 4-hydroxy-L-proline was utilized to the determine the concentration of HYP in different test samples.
Histopathological parameter assessment:
Szapiel’s alveolitis, ashcroft’s fibrosis and mast cell population scoring:
The left portion of the lung was collected and placed in formalin solution (10 % v/v) and fixed in paraffin for histopathological evaluation. The fixed lungs tissue in paraffin wax was sliced in regular thickness of 5 μm and was put on the individual slide, deparaffinized. Three slides each in duplicate of all study groups were separately stained with Haematoxylin and Eosin (H&E), Masson’s Trichrome (MT) and Toluidine Blue (TB) to assess the inflammation and fibrosis grades and total mast cell population within lungs, respectively. For the histopathological examination of lung tissues a Leica microscope (Model: DME, Shanghai, China) was utilized and evaluation of different slides and photomicrographs were captured through computer aided digital camera (Canon Inc., PowerShot S70, Japan). Histopathological evaluations were carried out by independent experienced histologist blinded with the treatment groups. For the histological assessment of alveolar inflammation condition, previously established procedure[33] was utilized to the differentiate and score the inflammatory condition of lung in H&E-stained slide at 100X magnification. Similarly, for the assessment of fibrotic condition of lung, fibrosis scoring scale[34] was utilized in MTstained slide at 100X magnification. However, to measure the total Mast Cell Population (MCTotal) in per mm2 area of lung section, the slide was stained with TB as discussed previously in literature[35,36] and carefully examined at 400X magnification. Positive TB-stained mast cells in each slide were counted in 4 randomly selected fields (0.5 mm2 area each) and presented the MCTotal value per mm2 area by multiplying the average number with 2 as per the established method previously described in the literature[37].
Statistical analysis:
All the study data were calculated as mean±Standard Error of Mean (SEM) with the least criterion set at p<0.05 which was considered to be statistically significant during comparison of the study results.
Statistical evaluation was executed through GraphPad Prism software version 5.0 using 1-way/2- way analysis of variance post-hoc Tukey-Kramer’s multiple comparisons assessment or Bonferroni test as applicable.
Results and Discussion
AInitially, acute oral-toxicity study was carried out for both the investigational phytoconstituents namely forskolin and rutin (test compounds) through fixed dose procedure according to Organisation for Economic Co-operation and Development (OECD)- guidelines no. 420. In this study, male, Wistar rats, on being treated with forskolin and rutin at fixed dose of 2000 mg/kg were supervised for 14 d. However, no apparent symptoms of toxicity were found. Also, physical examination, behavioural pattern, laboratory parameters and histopathological study of heart, liver and kidneys were thoroughly performed. The results of all these parameters in test compounds treated groups were found comparable and showed statistically no significant differences (p<0.05) with normal control. Further, no abnormalities were reported during histopathological study in regards to both the test compounds (forskolin and rutin).
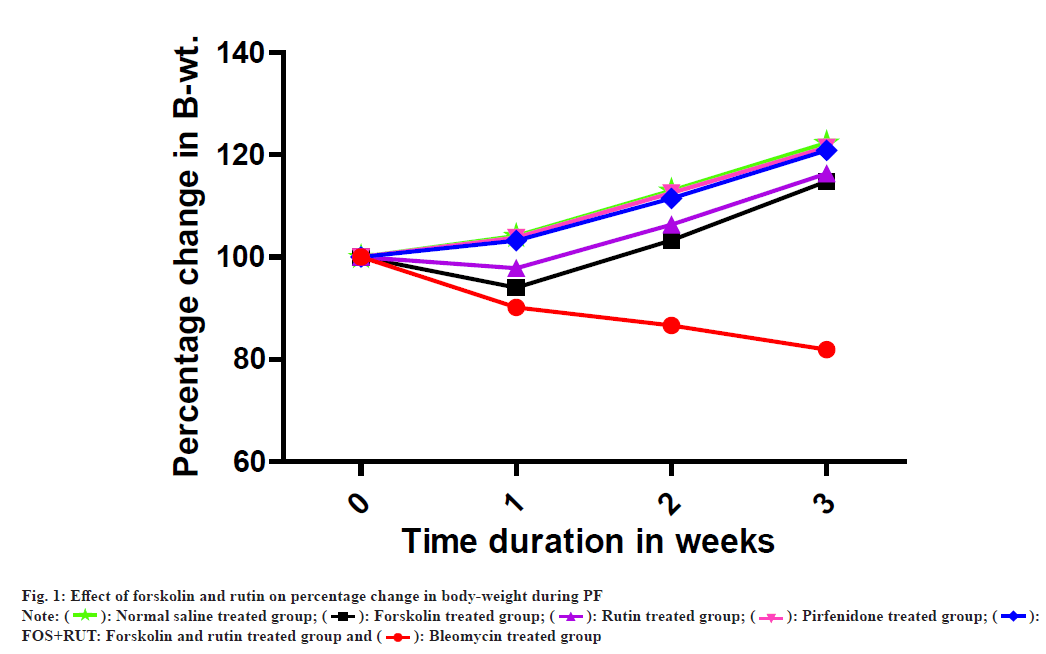
In BLM group, percentage body-weight was observed to be significantly decrease (p<0.001) as compared to normal control (NOS) group which possibly due to development of severe lung injury as lung fibrosis progression is typically associated with gradual loss of body-weight and have also clinical significance in the assessment of PF prognosis[38]. However, administration of forskolin and rutin as an individual treatment in FOS and RUT groups, respectively, both were found to be adequate and offered a positive change in percentage body-weight after bleomycin induced lung challenge with significant gain (p<0.001) in percentage body-weight as compared to disease control (BLM) group. Moreover, FOS and RUT groups both were showed significantly low (p<0.001) value in percentage body-weight as compared to PIR group. Furthermore, treatment with standard drug pirfenidone in PIR group as well as co-administration of forskolin and rutin in FOS+RUT group were presented obvious change with significant increase (p<0.001) in percentage body-weight after administration of bleomycin (IT) as compared to BLM group. In addition, percentage change in body-weight in FOS+RUT and PIR groups were found to be statistically comparable with no significant difference as shown in fig. 1 and Table 1.
| W | NOS Group | FOS Group | RUT Group | FOS+RUT Group | PIR Group | BLM Group |
|---|---|---|---|---|---|---|
| 1 | 104.21±1.97 | 94.03±1.57***,$$$ | 97.81±1.44***,$$$ | 103.23±1.60*** | 103.84±1.12*** | 90.14±1.25### |
| 2 | 113.02±1.76 | 103.27±2.0***,$$$ | 106.37±1.28***,$$$ | 111.48±1.56*** | 112.57±1.50*** | 86.63±1.23### |
| 3 | 122.38±1.62 | 114.83±1.45***,$$$ | 116.34±1.27***,$$$ | 120.91±1.96*** | 121.50±1.68*** | 81.89±1.47### |
Note: NOS: Normal Saline treated group; BLM: Bleomycin treated group; FOS: Forskolin treated group; RUT: Rutin treated group; FOS+RUT: Forskolin and rutin treated group and PIR: Pirfenidone treated group; ###p<0.001 BLM vs. NOS; ***p<0.001 (FOS, RUT, FOS+RUT and PIR) vs. BLM; $$$p<0.001 FOS vs. PIR and $$$p<0.001 RUT vs. PIR. All values are given as mean±SEM, (n=5). All values are given as mean±SEM for n=5. Statistical analysis was executed through 2-way ANOVA post-hoc Bonferroni test
Table 1: Effect of Forskolin and Rutin Treatments On Percentage Change In Body-Weight
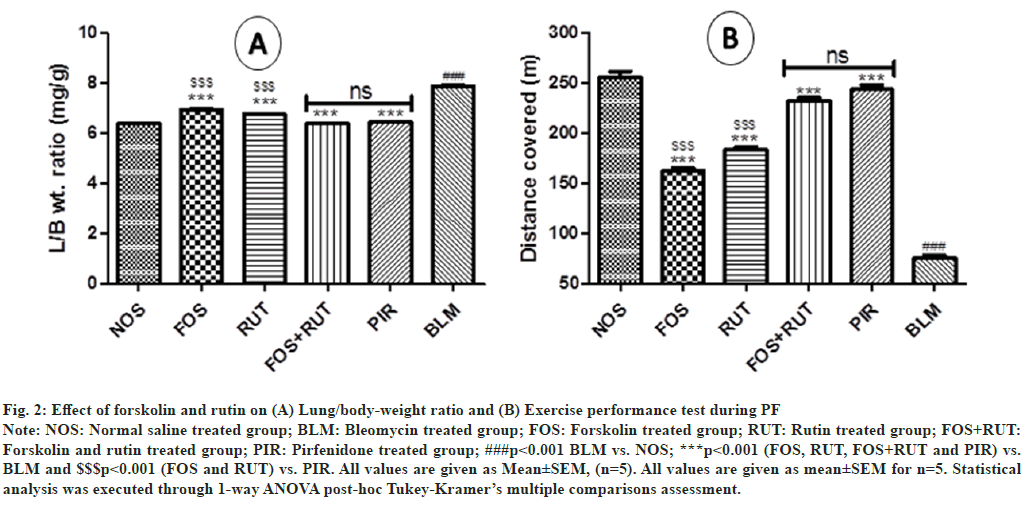
In disease control (BLM) group, administration of bleomycin via IT route produced significant increase (p<0.001) in Length/Beam (L/B) ratio as compared to normal control (NOS) group which indicated that development of excess lung inflammatory conditions was occurred post bleomycin instillation. However, treatment with study drugs including forskolin and rutin as individual and their combination in FOS, RUT and FOS+RUT groups, respectively after bleomycin administration, presented significant lowering (p<0.001) in L/B ratio as compared to BLM group. Moreover, FOS and RUT groups were showed significantly high (p<0.001) value of L/B ratio with respect to PIR group, which reflected that forskolin (20 mg/kg) and rutin (100 mg/kg) as individual treatment, both were comparatively inadequate to lowered the L/B ratio due to generation of intense lung inflammation during PF, as demonstrated by pirfenidone (50 mg/kg) treatment. However, coadministration of forskolin and rutin (20 mg/kg+100 mg/kg) in FOS+RUT group efficiently abolished the bleomycin induced lung inflammation and causes considerable reduction in L/B ratio and the result was found to be comparable with no significant difference to PIR group as shown in fig. 2(A).
Fig. 2: Effect of forskolin and rutin on (A) Lung/body-weight ratio and (B) Exercise performance test during PF
Note: NOS: Normal saline treated group; BLM: Bleomycin treated group; FOS: Forskolin treated group; RUT: Rutin treated group; FOS+RUT:
Forskolin and rutin treated group; PIR: Pirfenidone treated group; ###p<0.001 BLM vs. NOS; ***p<0.001 (FOS, RUT, FOS+RUT and PIR) vs.
BLM and $$$p<0.001 (FOS and RUT) vs. PIR. All values are given as Mean±SEM, (n=5). All values are given as mean±SEM for n=5. Statistical
analysis was executed through 1-way ANOVA post-hoc Tukey-Kramer’s multiple comparisons assessment.
EPT value in respect to distance covered (m) in disease control (BLM) group was found to be significantly low (p<0.001) as compared to the normal control (NOS) group. It imitated that the lung condition was deeply compromised and subsequently affected the physical activity due to severity of PF disease induced after bleomycin challenge through instillation (IT) in rats. However, after bleomycin administration, treatment with forskolin and rutin as individual and their combination in FOS, RUT and FOS+RUT groups, respectively, reflected significant increase (p<0.001) in EPT value as compared to BLM group. Moreover, in FOS and RUT groups, EPT value was found to be significantly low (p<0.001) with respect to PIR group. It exhibited that forskolin (20 mg/ kg) and rutin (100 mg/kg) as individual treatment, both were comparatively inadequate to enhance the EPT value due to compromised lung condition during PF, as showed by pirfenidone (50 mg/kg) treatment. However, co-administration of forskolin and rutin (20 mg/kg+100 mg/kg) in FOS+RUT group adequately reversed the bleomycin induced degraded lung condition and causes substantial improvement in EPT value and the result was found to be comparable with no significant difference to PIR group as shown in fig. 2(B).
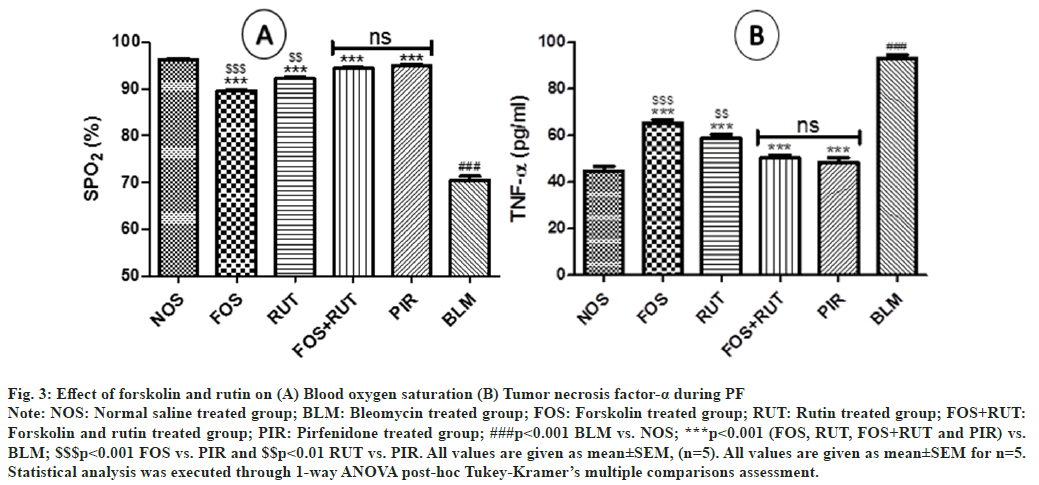
In the study, when disease control (BLM) group was compared with normal control (NOS) group, a significant decrease (p<0.001) in SpO2-level was observed. This was possibly due to compromised lung function and in turn gaseous exchange ability was also reduced after bleomycin instillation (IT). However, treatment with forskolin and rutin as individual and their combination in FOS, RUT and FOS+RUT groups, respectively after bleomycin administration, reflected significant increase (p<0.001) in SpO2-level as compared to BLM group. Moreover, in FOS and RUT groups, SpO2-level was found to be significantly low (p<0.001 and p<0.01, respectively) with respect to PIR group. It showed that forskolin (20 mg/kg) and rutin (100 mg/kg) as individual treatment, both were comparatively inadequate to maintained the SpO2-level due to severe declined gaseous exchange ability of lung during PF, as manifested by pirfenidone (50 mg/kg) treatment. However, co-administration of forskolin and rutin (20 mg/kg+100 mg/kg) in FOS+RUT group adequately overturned the bleomycin associated deprived gaseous exchange ability of lung and causes noticeable increase in SpO2-level and the result was found to be comparable with no significant difference to PIR group as shown in fig. 3(A).
Fig. 3: Effect of forskolin and rutin on (A) Blood oxygen saturation (B) Tumor necrosis factor-α during PF
Note: NOS: Normal saline treated group; BLM: Bleomycin treated group; FOS: Forskolin treated group; RUT: Rutin treated group; FOS+RUT:
Forskolin and rutin treated group; PIR: Pirfenidone treated group; ###p<0.001 BLM vs. NOS; ***p<0.001 (FOS, RUT, FOS+RUT and PIR) vs.
BLM; $$$p<0.001 FOS vs. PIR and $$p<0.01 RUT vs. PIR. All values are given as mean±SEM, (n=5). All values are given as mean±SEM for n=5.
Statistical analysis was executed through 1-way ANOVA post-hoc Tukey-Kramer’s multiple comparisons assessment.
Bleomycin instillation (IT) in BLM group triggered significant increase (p<0.001) in TLC value as compared to NOS group due to aggravation of inflammatory responses within lungs. However, treatment with forskolin, rutin and their combination in FOS, RUT and FOS+RUT groups respectively, after bleomycin instillation caused significant decrease (p<0.001) in TLC value as compared to BLM group. Moreover, in FOS and RUT groups, TLC value was remained significantly high (p<0.001 and p<0.01, respectively) as compared to PIR group. However, in between FOS+RUT and PIR groups, TLC value was differed insignificantly. When DLC was measured in the BLM group, percentage of lymphocyte and neutrophil were found significantly increased (p<0.001), whereby, percentage of macrophage was significantly decreased (p<0.001) as compared to normal control. But, in FOS, RUT and FOS+RUT groups, percentage of macrophage was found significantly increased (p<0.001) and percentage of neutrophil and lymphocyte were significant decrease (p<0.001 and p<0.01, respectively). Moreover, in comparison to standard drug treated PIR group, in FOS and RUT groups, percentage of macrophage, lymphocyte and neutrophil were differed significantly. However, the combination treatment of forskolin showed statistically comparable results for these parameters to standard drug pirfenidone treatment after bleomycin instillation (IT). Besides, it was detected that percentage of eosinophil and basophil among all the treatment groups did not differ significantly. The obtained values of TLC and DLC from different experimental groups in BALF are presented in Table 2.
| Parameters | NOS | FOS | RUT | FOS+RUT | PIR | BLM |
|---|---|---|---|---|---|---|
| Total Leukocyte Count (×106/ml) | 0.45±0.02 | 0.82±0.03***,$$$ | 0.69±0.02***,$$ | 0.52±0.03*** | 0.56±0.02*** | 1.39±0.03### |
| M (%) | 83.00±1.20 | 54.06±1.96***,$$$ | 62.66±1.44***,$$$ | 75.53±1.51*** | 79.86±1.53*** | 35.26±1.72### |
| L (%) | 10.60±0.45 | 16.40±1.05$$ | 13.86±1.12** | 12.40±1.43*** | 11.33±0.52*** | 20.00±0.59### |
| N (%) | 3.73±0.41 | 25.53±1.21***,$$$ | 19.93±1.97***,$$$ | 8.80±0.58*** | 5.86±0.40*** | 40.26±1.21### |
| E (%) | 2.20±0.31 | 3.06±0.51 | 2.73±0.27 | 2.60±0.54 | 2.33±0.52 | 3.40±0.46 |
| B (%) | 0.46±0.09 | 0.93±0.30 | 0.80±0.25 | 0.66±0.18 | 0.60±0.13 | 1.66±0.13 |
Note: M: Macrophage; L: Lymphocyte; N: Neutrophil; E: Eosinophil; B: Basophil; NOS: Normal; BLM: Diseased; FOS: Forskolin treated; RUT: Rutin treated; FOS+RUT: Forskolin and rutin treated and PIR: Pirfenidone treated; #: BLM vs. CSS; *: (FOS, RUT, FOS+RUT and PIR) vs. BLM; $: (FOS, RUT and FOS+RUT) vs. PIR; #,*,$p<0.05; ##**$$p<0.01 and ###,***,$$$p<0.001. All values are given as mean±SEM, (n=5)
Table 2: Leukocyte Infiltration Counts Measured In Bronchoalveolar Lavage Fluid
The results related to lung injury markers showed that treatments including forskolin (20 mg/kg), rutin (100 mg/kg), forskolin and rutin combination (20 mg/ kg+100 mg/kg) as well as standard drug pirfenidone (50 mg/kg) were sufficed to decrease all parameters TP, ALP, LDH and CK significantly (p<0.001) as compared to disease control (BLM) group. However, in comparison to pirfenidone treated group (PIR), all parameters-TP, ALP, LDH and CK were found to be remained significantly elevated (p<0.001, p<0.001, p<0.001 and p<0.01 respectively) with the forskolin treatment (as alone) after bleomycin instillation (IT). Similarly, rutin treatment (as alone) was also not be qualified to entirely controlled all the parameters-CK and LDH (except TP and ALP) and were continued significantly high (p<0.001) as compared to standard drug pirfenidone treated group (PIR). Interestingly, forskolin (20 mg/kg) and rutin (100 mg/kg) combination treatment showed efficacy equivalent to pirfenidone (50 mg/kg) treatment and synergistically lowered all the parameter assessed for the evaluation of lung tissue injury after bleomycin challenge in rats. The obtained values of TP, LDH, ALP and CK parameters in BALF samples and their statistical comparison have been presented in Table 3.
| NOS | FOS | RUT | FOS+RUT | PIR | BLM | |
|---|---|---|---|---|---|---|
| TP (μg/ml) | 141.33±2.37 | 455.83±3.29***,$$$ | 303.67±2.23***,$$$ | 192.17±2.42*** | 170.02±2.76*** | 1307.76±9.89### |
| LDH (U/l) | 39.66±1.22 | 46.33±0.97***,$$$ | 43.66±0.62***,$$$ | 41.73±0.48*** | 40.86±0.83*** | 55.33±0.62### |
| ALP (U/l) | 149.46±2.39 | 212.06±1.69***,$$$ | 191.13±1.92*** | 172.11±2.41*** | 164.27±2.39*** | 285.01±2.33### |
| CK (U/l) | 115.32±3.59 | 141.12±2.66***,$$ | 132.25±2.46*** | 120.95±2.53*** | 125.32±2.32*** | 198.31±2.17### |
Note: TP: Total Protein; LDH: Lactate Dehydrogenase; ALP: Alkaline Phosphatase; CK: Creatinine Kinase; #: BLM vs. CSS; *: (FOS, RUT, FOS+RUT and PIR) vs. BLM; $: (FOS, RUT and FOS+RUT) vs. PIR; NOS: Normal; BLM: Diseased; FOS: Forskolin treated; RUT: Rutin treated; FOS+RUT: Forskolin and rutin treated; PIR: Pirfenidone treated; #,*,$p<0.05; ##,**,$$p<0.01; ###,***,$$$p<0.001; All values are given as Mean±SEM, (n=5)
Table 3: Biochemical Assays of Lung Injury Markers Estimated In Bronchoalveolar Lavage Fluid
The results showed that oxidative-stress parameters including GSH, SOD and CAT values were decreased significantly (p<0.001) however, MDA and NO content were increased significantly (p<0.001) after bleomycin instillation (IT) as compared to normal control. This reflected that enhanced oxidative-stress condition was developed within the lungs, possibly due to creation of imbalanced environment between oxidant and anti-oxidant molecules led by bleomycin challenge. However, treatment with all the study drugs namely forskolin (20 mg/kg) and rutin (100 mg/ kg) as alone and their combination (20 mg/kg+100 mg/kg) as well as standard drug pirfenidone (50 mg/ kg), were qualified to reversed the obtained results after bleomycin administration (IT) with significant differences (p<0.001). Moreover, test compounds (forskolin and rutin) as alone treatments were found to be inadequate to attained the statistically equivalent results in all assessed oxidative-stress related parameters (except SOD in rutin treatment) than that manifested by standard drug pirfenidone treatment. Importantly, co-administration of forskolin (20 mg/ kg) and rutin (100 mg/kg) synergistically brought comparable results with no significant differences to standard drug pirfenidone (50 mg/kg) treatment after bleomycin instillation (IT) in aspects to all assessed parameters related to oxidative-stress carried out in the lungs. The obtained values of SOD, MDA, GSH, CAT and NO parameters and their statistical comparison have been presented in Table 4.
| NOS | FOS | RUT | FOS+RUT | PIR | BLM | |
|---|---|---|---|---|---|---|
| SOD (U/mg protein) | 1.79±0.02 | 1.51±0.04**,$$ | 1.56±0.06*** | 1.76±0.04*** | 1.69±0.02*** | 1.25±0.02### |
| MDA (nM/mg protein) | 0.09±0.01 | 0.22±0.02***,$$$ | 0.17±0.01***,$$ | 0.10±0.01*** | 0.11±0.01*** | 0.63±0.02### |
| CAT (U/min/mg tissue) | 18.08±0.34 | 12.97±0.34***,$$$ | 14.61±0.91***,$ | 17.35±0.65*** | 17.21±0.35*** | 7.90±0.20### |
| GSH (nM/mg protein) | 275.98±3.97 | 214.36±2.16***,$$$ | 226.37±3.66***,$$$ | 270.05±1.96*** | 266.40±2.27*** | 160.31±1.33### |
| NO (μM/mg protein) | 0.61±0.01 | 0.74±0.03***,$$$ | 0.68±0.01***,$$$ | 0.62±0.02*** | 0.63±0.01*** | 1.05±0.03### |
Note: SOD: Superoxide dismutase; CAT: Catalase; MDA: Malondialdehyde; GSH: Reduced glutathione; NO: Nitric oxide; #: BLM vs. CSS; *: (FOS, RUT, FOS+RUT and PIR) vs. BLM; $: (FOS, RUT and FOS+RUT) vs. PIR; NOS: Normal; BLM: Diseased; FOS: Forskolin treated; RUT: Rutin treated; FOS+RUT: Forskolin and rutin treated; PIR: Pirfenidone treated; #,*,$p<0.05; ##,**,$$p<0.01 and ###,***,$$$p<0.001. All values are given as Mean±SEM, (n=5)
Table 4: Biochemical Assays of Oxidative-Stress Markers In Lung Homogenate
In this study, two inflammatory markers namely tumor necrosis factor-α (TNF-α) and myeloperoxidase (MPO) were analyzed in BALF and lung tissues, respectively. In the assessment of TNF-α level in BLM group, it was found to be significantly increased (p<0.001) as compared to control (NOS) group, which indicated that inflammatory condition was extensively developed within lung after instillation of bleomycin (IT) in rats. However, treatment with forskolin and rutin as an individual and their combination in FOS, RUT and FOS+RUT groups, respectively after bleomycin administration, reflected significant decrease (p<0.001) in TNF-α level as compared to disease control (BLM) group. Moreover, in FOS and RUT groups, TNF-α level was found to be remained significantly high (p<0.001) with respect to PIR group, which exhibited that forskolin (20 mg/kg) and rutin (100 mg/kg) as individual treatment, both were comparatively inadequate to minimize the proinflammatory cytokine (TNF-α) level as presented by pirfenidone (50 mg/kg) treatment during bleomycin- triggered PF. However, treatment with forskolin and rutin (20 mg/kg+100 mg/kg) combination in FOS+RUT group, synergistically reversed the TNF-α level and causes substantial reduction, after bleomycin administration (IT) and result of TNF-α level was found to be comparable to PIR group as shown in fig. 3(B).
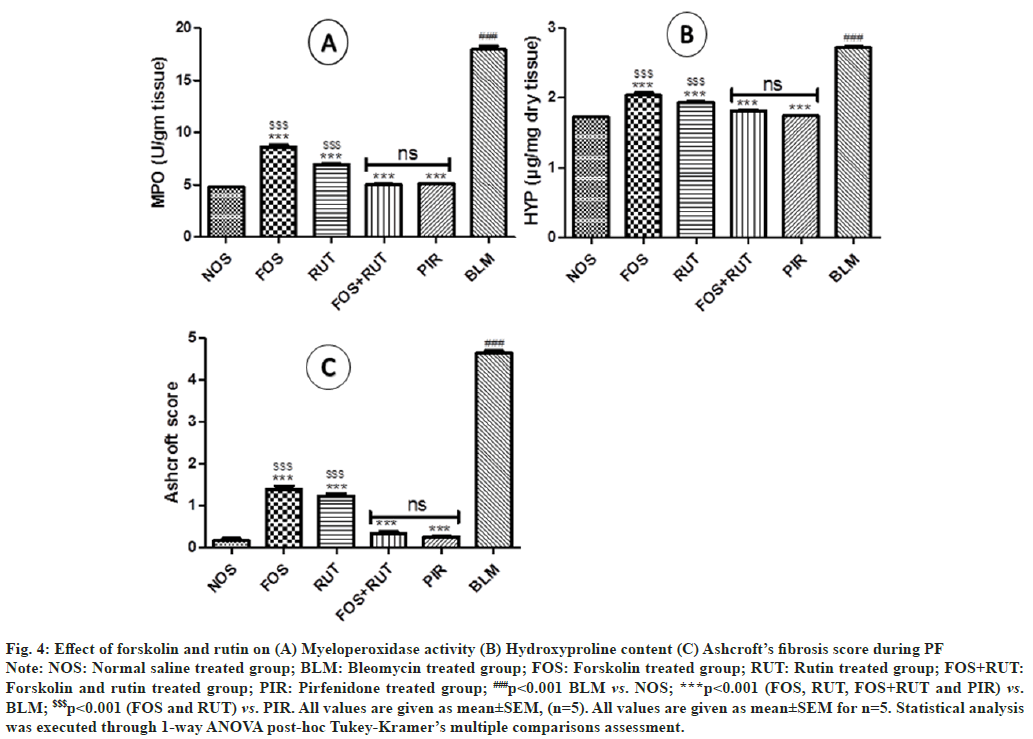
In comparison to NOS group, MPO activity was measured apparently high (p<0.001) in the lung homogenate of BLM group indicates excess activation of inflammatory responses and in turn increased neutrophil infiltration within lungs. However, treatment with forskolin and rutin as an individual and their combination in FOS, RUT and FOS+RUT groups, respectively after bleomycin administration, executed significant reduction (p<0.001) in MPO activity as compared to BLM group. Moreover, in FOS and RUT groups, MPO activity was found to be continued significantly high (p<0.001) with respect to PIR group, which exhibited that forskolin (20 mg/ kg) and rutin (100 mg/kg) as individual treatment, both were comparatively insufficient to abate the MPO activity as presented by pirfenidone (50 mg/ kg) treatment during bleomycin-triggered PF. Remarkably, co-administration of forskolin and rutin in FOS+RUT group, exhibited synergistic effect and produced comparable result of MPO activity with no significant difference to standard drug pirfenidone treated PIR group as shown in fig. 4(A).
Fig. 4: Effect of forskolin and rutin on (A) Myeloperoxidase activity (B) Hydroxyproline content (C) Ashcroft’s fibrosis score during PF
Note: NOS: Normal saline treated group; BLM: Bleomycin treated group; FOS: Forskolin treated group; RUT: Rutin treated group; FOS+RUT:
Forskolin and rutin treated group; PIR: Pirfenidone treated group; ###p<0.001 BLM vs. NOS; ***p<0.001 (FOS, RUT, FOS+RUT and PIR) vs.
BLM; $$$p<0.001 (FOS and RUT) vs. PIR. All values are given as mean±SEM, (n=5). All values are given as mean±SEM for n=5. Statistical analysis
was executed through 1-way ANOVA post-hoc Tukey-Kramer’s multiple comparisons assessment.
In general, Hydroxyproline (HYP) content is considered as a hallmark fibrosis marker and hence it was analyzed in lung tissues of the experimental rats. In the lung tissue of BLM group, HYP content was found noticeably high (p<0.001) as compared to NOS group, representing marked fibrosis development post bleomycin instillation. However, treatment with all study interventions including forskolin, rutin (as alone) and their combination in FOS, RUT and FOS+RUT groups, respectively sufficiently attenuated the lung fibrosis triggered through bleomycin as the HYP content in lung were found significantly reduced (p<0.001) in comparison to BLM group. Moreover, the extent of reduction in HYP content by individual treatment of forskolin and rutin in FOS and RUT groups were found to be not so effective as achieved through standard drug pirfenidone treatment in PIR group after bleomycin administration (IT), although, both FOS and RUT groups showed significantly high (p<0.001) content of HYP in lung samples. Notably, co-administration of forskolin and rutin in FOS+RUT group showed that HYP content was decreased synergistically and the value was does not differ significantly as compared to standard drug pirfenidone treated PIR group after bleomycin challenged as shown in fig. 4(B).
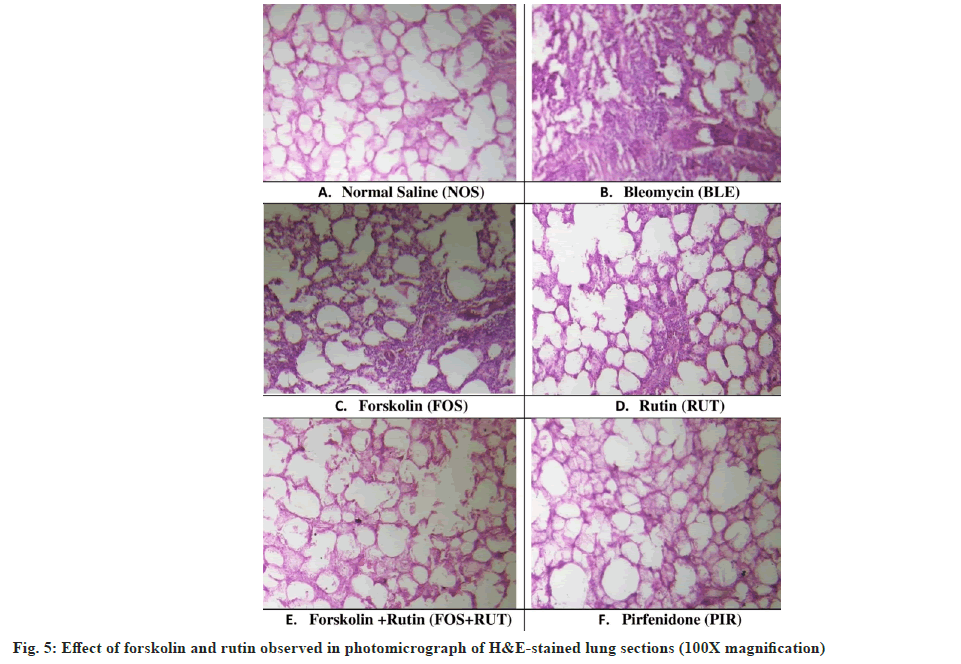
The histopathological changes observed in H&Estained slides of different groups are shown in fig. 5. In NOS group, the lung sections showed regular shape with normal alveolar architecture, continuous thin walls with prominent vasculature structures without any evidence of rupture in wall and no pathological alterations were observed (fig. 5A). On the other hand, intratracheal administration of bleomycin in BLM group led to drastic morphological alteration in lung including alveolar wall thickening, moderate to severe hemorrhage and collapsed alveolar space (fig. 5B). However, lung section obtained from FOS and RUT treated rats presented considerable inhibition of lung inflammation, comparatively less compromised alveolar space and irregularity in shapes with slight septal wall thickening, although some architectural ruptures were present (fig. 5C and fig. 5D). However, in lung sections received from FOS+RUT and PIR groups, showed substantial decrease in lung inflammation along with marked improvement in alveolar space without septal wall thickening around the alveolar structures (fig. 5E and fig. 5F).
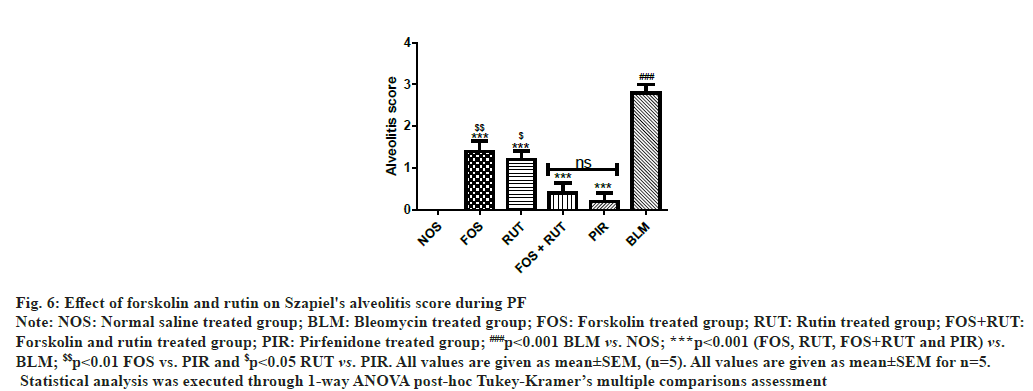
Through scoring in the lung section received from the animals of BLM group, showed development of critical lung inflammation as the high alveolitis score was obtained, which showed statistically highly significant (p<0.001) in comparison to NOS group. However, after treatment with study interventions in FOS, RUT and FOS+RUT subsequently lowered the alveolitis score in lungs. Moreover, both FOS and RUT groups, showed significantly high (p<0.01 and p<0.05, respectively) alveolitis score as compared to PIR group. Particularly, co-administration of forskolin and rutin in FOS+RUT group offered obvious declined alveolitis score with no significant difference as compared to pirfenidone treated PIR group as shown in fig. 6.
Fig. 6: Effect of forskolin and rutin on Szapiel's alveolitis score during PF
Note: NOS: Normal saline treated group; BLM: Bleomycin treated group; FOS: Forskolin treated group; RUT: Rutin treated group; FOS+RUT:
Forskolin and rutin treated group; PIR: Pirfenidone treated group; ###p<0.001 BLM vs. NOS; ***p<0.001 (FOS, RUT, FOS+RUT and PIR) vs.
BLM; $$p<0.01 FOS vs. PIR and $p<0.05 RUT vs. PIR. All values are given as mean±SEM, (n=5). All values are given as mean±SEM for n=5.
Statistical analysis was executed through 1-way ANOVA post-hoc Tukey-Kramer’s multiple comparisons assessment
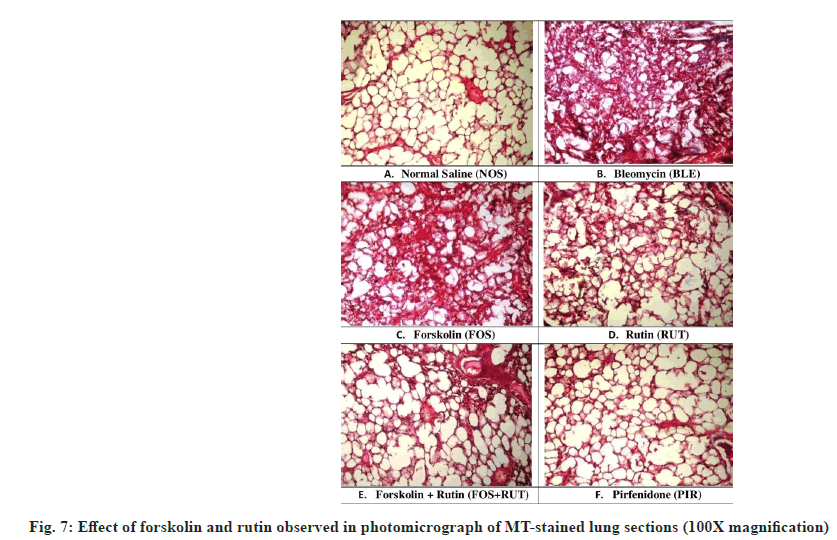
Fibrillar collagen deposition is considered as an indicator of lung fibrosis normally visualized in purple color after MT staining. MT-stained slides of different groups are depicted in fig. 7. MTstained lung section obtained from NOS group presented ideal architecture of lung with no traces of collagen deposition (fig. 7A). However, microscopic examination of lung section received from BLM group revealed distorted architecture including increased thickening of alveolar septa, excess of collagen deposition and fibroplasia were observed (fig. 7B). Individual treatment with forskolin and rutin in FOS and RUT groups, respectively, promptly attenuated fibrotic changes and collagen deposition as developed after bleomycin instillation (IT). However, architectural alteration in lung sections of FOS and RUT groups, were extended between mild to moderate degree of septal wall thickening and collagen deposition at interstitial spaces (fig. 7C and fig. 7D). Moreover, treatment with coadministration of forskolin and rutin in FOS+RUT group and pirfenidone in PIR group, were observed to be markedly decreases collagen deposition and fibrotic changes and also maintained the intact blood vasculature without septal wall broadening and retained the normal alveolar structures (fig. 7E and fig. 7F). All these data supported the view that combination treatment of forskolin and rutin provided an impressive impact almost similar to pirfenidone treated group, in limiting the pathological condition and collagen accumulation as well as architectural lung damage during bleomycin triggered PF in rats.
When Ashcroft’s fibrosis scoring was conducted for the lung section pertained to different study interventions, BLM group presented maximum fibrotic score and was found significantly high (p<0.001) in comparison to NOS group. However, FOS, RUT and FOS+RUT group produced significant reduction (p<0.001) in fibrosis score as compared to the result obtained in BLM group. Moreover, individual treatment with forskolin and rutin in FOS and RUT groups, fibrotic score was found to be significantly high (p<0.001) as compared to pirfenidone treated PIR group. Noticeably, fibrotic score recorded in the lung sections obtained from the FOS+RUT group was found to be apparently decreased and the obtained result of fibrotic score was observed statistically comparable to PIR group. The Ashcroft quantitative pathological fibrotic score related to all study interventions and their statistical comparisons are shown in fig. 4.
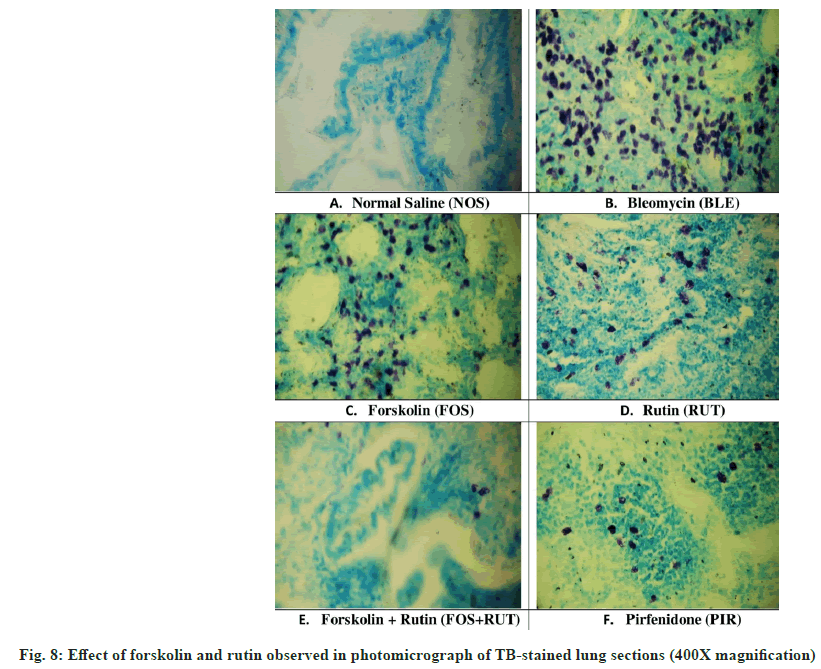
In TB-stained lung sections, mast cells were prominently observed in round shape with reddishpurple color and quite detectable due to clear contrast with blue background as shown in fig. 8. In the current study, lung section obtained from NOS group, presented absence of mast cell (fig. 8A) in lung parenchyma without any sign of inflammatory condition. However, fibrotic lung parenchyma related to BLM group, showed significant increase in the MCTotal score (fig. 8B), which suggested that maximum inflammatory condition was developed. Moreover, individual treatment of forskolin and rutin in FOS and RUT groups, both were found to be deficient to reduce the MCTotal score (fig. 8C and fig. 8D) in lung parenchyma of bleomycin pre-treated rats. Besides, combination treatment in FOS+RUT group showed profound restriction to mast cell migration and accumulation in lung parenchyma even more than that presented by pirfenidone treatment in PIR group (fig. 8E and fig. 8F), possibly due to potentiation of anti-inflammatory effect due to their co-administration.
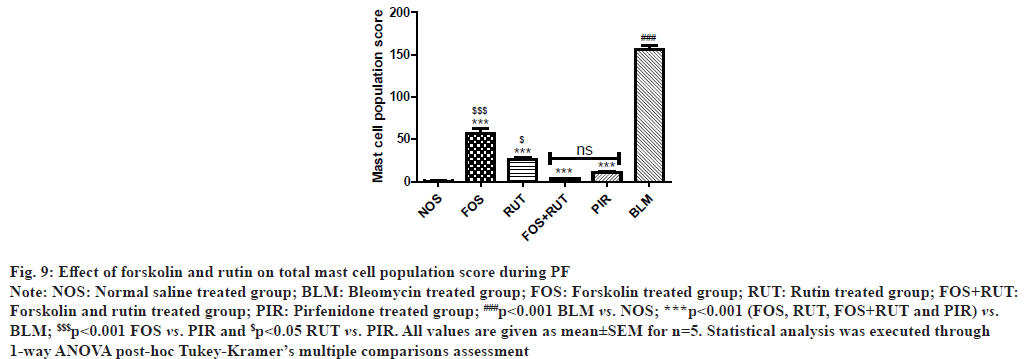
In microscopic examination of TB-stained lung sections, BLM group exhibited significantly increased (p<0.001) in MCTotal score in comparison to NOS group. However, treatment with forskolin and rutin as alone and their combination in FOS, RUT and FOS+RUT groups, respectively caused significant decrease (p<0.001) in MCTotal score as compared to BLM group. Moreover, individual treatment with forskolin and rutin in FOS and RUT groups, respectively, after bleomycin instillation reflected that MCTotal score was remained significantly high (p<0.001 and p<0.05, respectively) as compared to standard drug pirfenidone treated PIR group. Interestingly, the MCTotal score obtained in FOS+RUT group was drastically decreased and the result demonstrated that co-administration of forskolin and rutin could diminished mast cell aggregation more effectively than that manifested by PIR group. MCTotal scores related to all study interventions are shown in fig. 9.
Fig. 9: Effect of forskolin and rutin on total mast cell population score during PF
Note: NOS: Normal saline treated group; BLM: Bleomycin treated group; FOS: Forskolin treated group; RUT: Rutin treated group; FOS+RUT:
Forskolin and rutin treated group; PIR: Pirfenidone treated group; ###p<0.001 BLM vs. NOS; ***p<0.001 (FOS, RUT, FOS+RUT and PIR) vs.
BLM; $$$p<0.001 FOS vs. PIR and $p<0.05 RUT vs. PIR. All values are given as mean±SEM for n=5. Statistical analysis was executed through
1-way ANOVA post-hoc Tukey-Kramer’s multiple comparisons assessment
PF is a lung disease categorized by persistent worsening and irreversible condition, intact with fibroblasts and myofibroblast disarray and over production of Extracellular Matrix (ECM), occurs through aberrant AECs injuries, ECM re-modelling, uncontrolled fibroblast activation, proliferation and differentiation into myofibroblast, which ultimately causes lung dysfunction and leads to death.
In clinical practice, change in body-weight is usually considered as significant measurable parameter for the assessment of prognosis in PF patients, because continuous body-weight loss is an important characteristic of PF progression[38]. In this view, percent change in body-weight with respect to initial value was recorded in all study groups. It was observed that instillation of bleomycin (IT) caused drastic reduction in body-weight, particularly during 1st w after administration. However, forskolin and rutin co-administration presented an ability to manage the body-weight loss during PF as effectively as implied by standard drug pirfenidone treatment. Similarly, L/B ratio provides an idea about the severity of PF condition and reflects the extent of inflammation in lungs[35]. According to this, L/B ratio was calculated for each group, and it was found that the value of L/B ratio differs significantly between disease and normal control groups. However, individual treatment with forskolin or rutin assisted in lowering the lung inflammation, although unconventional reduction in L/B ratio was found with the forskolin and rutin combination treatment and the L/B ratio value showed no significant difference with standard drug pirfenidone effect in rats.
A lung function defect, directly affects physical activities and subsequently causes exercise constraint due to dyspnea, which is the most perceptible and devastating symptom of PF[39]. Also, the compromised lung function associated with PF condition are usually assessed through 6-MWT in a clinical setup, as the individual exercise performance considerably decreases corresponding to the severity of PF[19]. Similarly, in this study the rundown exercise performance due to severity of PF condition was assessed by EPT (analogous to 6-MWT) in individual animal of each group. It was found that bleomycin administration (IT) drastically reduced the EPT value in experimental rats. However, individual treatment with forskolin or rutin uplifted the exercise performance to some extent in bleomycin pre-treated rats. Notably, forskolin and rutin combination treatment substantially improved EPT value statistically equivalent to standard drug pirfenidone treatment as demonstrated by individual rats.
Alteration in the integrity of alveolar-capillary membrane normally affects gaseous exchange process in lungs[20]. Correspondingly, reduction in functionality or insufficiency of lung reflects lowering in SpO2-level due to decline gaseous exchange activity[39]. Therefore, the determination of SpO2-level in blood could be considered as an important indicator to evaluate the lung efficiency in regards to gaseous exchange ability. Also, a previous study reflected that IT instillation of bleomycin, decreases the oxygen saturation content in rats[40]. In current study, similarly it was observed that lung insult through bleomycin administration significantly reduced the SpO2-level in rats. However, individual treatment with FOR and RUT, showed slight improvement in SpO2-level in bleomycin pre-treated rats. Importantly, their combination treatment was found quite effective and enabled to improve the SpO2-level with almost same extent as served by standard drug pirfenidone treatment in this study. Infiltration of leukocyte in BALF, reveals the degree of activation of inflammatory responses and in turn inflammatory cells migration.
Estimation of tissue injury markers in BALF is important tools to evaluate the progression of PF condition as they directly reflect the disease severity. In previous studies LDH, CK, ALP and TP are identified and measured as important early markers of lung tissue injury[22-24]. The obtained results related to lung injury markers in this study, showed all study treatments including forskolin and rutin and their combination as well as standard drug pirfenidone were served to decrease these elevated lung injury markers in BALF after intra-tracheal bleomycin administration. However, in comparison to pirfenidone, all assessed lung injury markers were found to be markedly high after forskolin or rutin administration. Both, individual treatments were attributed less efficacy to entirely controlled all these elevated parameters post bleomycin instillation (IT). Interestingly, forskolin and rutin combination treatment synergistically qualified to lowered all the lung injury markers and showed efficacy equivalent to pirfenidone treatment in bleomycin challenged rats.
Interaction of several cytokines develop a complex network, which may involve in the pathogenesis of PF[41]. In previous studies, various researchers measured the TNF-α level in BALF as an important cytokine due to its critical role in the process of inflammation as well as fibrosis activity and also having potency to excite collagen synthesis by activating latent growth factors (Transforming growth factor TGF-β) and fibroblasts proliferation[42]. Therefore, in this study the TNF-α level was estimated in bleomycin-triggered PF condition as well as after treatment with forskolin and rutin in rats. Where, the results showed that after bleomycin instillation a significant increase in TNF-α level was occurred. However, individual treatment of forskolin or rutin qualified to suppress the TNF-α, although, their coadministration provided advance efficacy against bleomycin-triggered PF and produced comparable result as presented by pirfenidone treatment in rats. Besides, these alterations associated to PF, forskolin and rutin combination treatment also presented synergistic effect and offered additive efficacy which were found statistically comparable to standard drug pirfenidone treatment in modulation of bleomycintriggered oxidative-stress, inflammation and fibrosis in lungs.
Practically, bleomycin-triggered PF showed predominant inflammatory phase initially characterized by increased inflammatory cells accumulation and their pro-fibrotic mediators release and enhanced oxidative-stress, which ultimately leads to lung parenchymal injury post bleomycin instillation in rodents. Post inflammatory phase a fibrotic condition normally develops as secondary phase within next 2 w, which is categorized by excess ECM deposition and leads to development of fibrosis in lungs[43]. In this study, bleomycin altered the lung morphology with excess of inflammatory cells infusion within interstitial space around the alveoli, thickening of septal wall in peri-bronchial and peri-alveolar area and extreme reduction in alveolar spaces within lungs (fig. 5). Also, instillation of bleomycin (IT) caused intense collagen deposition within interstitial spaces and subsequently collapsed alveolar walls are seen in the lung parenchyma (fig. 7). However, treatment with forskolin or rutin as alone, sufficed to reduce the lung damaging process, but their combination treatment significantly lessened the collagen deposition within interstitial spaces, which indicates its shielding effect against bleomycintriggered PF. Moreover, anti-inflammatory and antifibrotic action of combination treatment was found to be quite significant as reflected by Szapiel’s alveolitis (fig. 6) and Ashcroft’s fibrosis scores (fig. 4C), respectively.
In general, various pro-fibrotic mediators are chiefly stored in mast cells and profoundly releases upon activation during cellular injuries and through chemoattractant activity, the population of mast cell characteristically enhances at the site of injury. Besides, many researchers anticipated that mast cell act as main effector cell and may be involved in the pathogenesis of inflammatory lung diseases[17]. Similarly, previous study showed that during lung fibrotic condition, number of mast cell upsurges and consequently instigates fibrosis by the release of histamine, renin and growth factors locally[18]. A previous study suggested that estimation of mast cell population in lung parenchyma could be used as distinct and important investigational tool for the histopathological study in PF. Alterations in MCTotal score with increase in numbers, indicates enhanced inflammatory condition of the lung and also specifies the degree of tissue remodeling as well as functionality of lung[42]. Thus, assessment of MCTotal scoring was carried out in this study. Where, the combination treatment of forskolin and rutin executed synergistic inhibition of mast cell aggregation in lung tissues, reflected extensive control over activation of inflammatory responses after bleomycin instillation (IT). This study also revealed the facts about involvement of mast cell in the development of PF, as highest score of MCTotal was observed in disease control (BLM) group. Therefore, the anticipated role of mast cell for the development of PF has been quite confirmed. Henceforth, aiming to decrease the mast cell aggregation in lung may be considered as an effective strategy in preventing the PF progression. Overall, efficacy of forskolin and rutin combination against PF showed promising potential and hence would contribute in the development of a novel effective treatment regimen in future.
Acknowledgement:
Author(s) would like to express gratitude and acknowledge Birla Institute of Technology, Mesra (India) for providing their infrastructural resources and technical help for the completion of this experimental study and preparation of the manuscript.
Financial Support:
This research was conducted without any grant and financial support.
Conflict of Interest:
There are no conflicts of interest.
References
- Kalchiem-Dekel O, Galvin JR, Burke AP, Atamas SP, Todd NW. Interstitial lung disease and pulmonary fibrosis: a practical approach for general medicine physicians with focus on the medical history. J Clin Med 2018;7(12):476.
[Crossref] [Google Scholar] [PubMed]
- Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med 2014;190(8):867-78.
[Crossref] [Google Scholar] [PubMed]
- Sgalla G, Biffi A, Richeldi L. Idiopathic pulmonary fibrosis: Diagnosis, epidemiology and natural history. Respirology. 2016;21(3):427-37.
[Crossref] [Google Scholar] [PubMed]
- Wuyts WA, Agostini C, Antoniou KM, Bouros D, Chambers RC, Cottin V, et al. The pathogenesis of pulmonary fibrosis: A moving target. Eur Respir J 2013;41(5):1207-18.
[Crossref] [Google Scholar] [PubMed]
- Fujimoto H, Kobayashi T, Azuma A. Idiopathic pulmonary fibrosis: Treatment and prognosis. Clin Med Insights Circ Respir Pulm Med 2015;9:CCRPM-S23321.
[Crossref] [Google Scholar] [PubMed]
- Cheresh P, Kim SJ, Tulasiram S, Kamp DW. Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta 2013;1832(7):1028-40.
[Crossref] [Google Scholar ] [PubMed]
- van der Vliet A, Janssen-Heininger YM, Anathy V. Oxidative stress in chronic lung disease: From mitochondrial dysfunction to dysregulated redox signaling. Mol Aspects Med 2018;63:59-69.
[Crossref] [Google Scholar] [PubMed]
- Huerta M, Urzua Z, Trujillo X, Gonzalez-Sanchez R, Trujillo-Hernandez B. Forskolin compared with beclomethasone for prevention of asthma attacks: A single-blind clinical trial. J Int Med Res 2010;38(2):661-8.
[Crossref] [Google Scholar] [PubMed]
- Lv HY, Chen J, Wang T. Rutin has anti-asthmatic effects in an ovalbumin-induced asthmatic mouse model. Tropic J Pharm Res 2017;16(6):1337-47.
- Chen LY, Huang CN, Liao CK, Chang HM, Kuan YH, Tseng TJ, et al. Effects of rutin on wound healing in hyperglycemic rats. Antioxidants 2020;9(11):1122.
[Crossref] [Google Scholar] [PubMed]
- Kandhare AD, Mukherjee A, Ghosh P, Bodhankar SL. Efficacy of antioxidant in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. EXCLI J 2016;15:636-51.
[Crossref] [Google Scholar] [PubMed]
- Moon TC, St Laurent CD, Morris KE, Marcet C, Yoshimura T, Sekar Y, et al. Advances in mast cell biology: new understanding of heterogeneity and function. Mucosal Immunol 2010;3(2):111-28.
[Crossref] [Google Scholar] [PubMed]
- Veerappan A, O'Connor NJ, Brazin J, Reid AC, Jung A, McGee D, et al. Mast cells: a pivotal role in pulmonary fibrosis. DNA Cell Biol 2013;32(4):206-18.
[Crossref] [Google Scholar] [PubMed]
- Nikbakht J, Hemmati AA, Arzi A, Mansouri MT, Rezaie A, Ghafourian M. Protective effect of gallic acid against bleomycin-induced pulmonary fibrosis in rats. Pharmacol Rep 2015;67(6):1061-7.
[Crossref] [Google Scholar] [PubMed]
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016;7(2):27.
[Crossref] [Google Scholar] [PubMed]
- Song X, Yu W, Guo F. Pirfenidone suppresses bleomycin‑induced pulmonary fibrosis and periostin expression in rats. Exp Ther Med 2018;16(3):1800-6.
[Crossref] [Google Scholar] [PubMed]
- Geng X, Dufu K, Hutchaleelaha A, Xu Q, Li Z, Li CM, et al. Increased hemoglobin–oxygen affinity ameliorates bleomycin‐induced hypoxemia and pulmonary fibrosis. Physiol Rep 2016;4(17):e12965.
[Crossref] [Google Scholar] [PubMed]
- Mansouri MT, Vardanjani HR, Hemmati AA, Tabandeh MR, Rezaie A, Pashmforosh M, et al. Zingerone attenuates bleomycin-induced pulmonary fibrosis in rats. Jundishapur J Nat Pharm Prod 2019;14(1):e80098.
- Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 2017;8(1):14532.
[Crossref] [Google Scholar] [PubMed]
- Zhou Z, Kandhare AD, Kandhare AA, Bodhankar SL. Hesperidin ameliorates bleomycin-induced experimental pulmonary fibrosis via inhibition of TGF-beta1/Smad3/AMPK and IkappaBalpha/NF-kappaB pathways. EXCLI J 2019;18:723-45.
[Crossref] [Google Scholar] [PubMed]
- Huang C, Wu X, Wang S, Wang W, Guo F, Chen Y, et al. Combination of Salvia miltiorrhiza and ligustrazine attenuates bleomycin-induced pulmonary fibrosis in rats via modulating TNF-α and TGF-β. Chin Med 2018;13:1-0.
[Crossref] [Google Scholar] [PubMed]
- Raish M, Ahmad A, Ansari MA, Ahad A, Al-Jenoobi FI, Al-Mohizea AM, et al. Sinapic acid ameliorates bleomycin-induced lung fibrosis in rats. Biomed Pharmacother 2018;108:224-31.
[Crossref] [Google Scholar] [PubMed]
- Zhou M, Zou BM, Li T, Lu C, Chen W, Yang WN. Study on changes in inflammatory cytokines and the relationship to multiple organ injury in rats with aspiration lung injury after fire-arm injury. Chin Crit Care Med 2005;17(12):732-5.
[Google Scholar] [PubMed]
- Behnia R, Molteni A, Waters CM, Panos RJ, Ward WF, Schnaper HW, et al. Early markers of ventilator-induced lung injury in rats. Ann Clin Lab Sci 1996;26(5):437-50.
[Google Scholar] [PubMed]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72(1-2):248-54.
[Crossref] [Google Scholar] [PubMed]
- Abdullah SM, Mazumder PM. An experimental model to induce homogeneous and progressive pulmonary fibrosis in rats. Indian J Pharm Edu Res 2021;751-64.
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Bio 1984;21(2):130-2.
[Google Scholar] [PubMed]
- Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Ana Biochem 1970;34(1):30-8.
[Crossref] [Google Scholar] [PubMed]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8.
[Crossref] [Google Scholar] [PubMed]
- Jollow DJ, Mitchell JR, Zampaglione NA, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974;11(3):151-69.
[Crossref] [Google Scholar] [PubMed]
- Goldblum SE, Wu KM, Jay MI. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol 1985;59(6):1978-85.
[Crossref] [Google Scholar] [PubMed]
- Edwards CA, O'Brien Jr WD. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta 1980;104(2):161-7.
[Crossref] [Google Scholar] [PubMed]
- Szapiel SV, Elson NA, Fulmer JD, Hunninghake GW, Crystal RG. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis 1979;120(4):893-9.
[Crossref] [Google Scholar] [PubMed]
- Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 1988;41(4):467-70.
[Crossref] [Google Scholar] [PubMed]
- Turgut NH, Kara H, Elagoz S, Deveci K, Gungor H, Arslanbas E. The protective effect of naringin against bleomycin-induced pulmonary fibrosis in Wistar rats. Pulmonary medicine. 2016;2016:7601393.
[Crossref] [Google Scholar] [PubMed]
- Andersson CK, Andersson-Sjöland A, Mori M, Hallgren O, Pardo A, Eriksson L, et al. Activated MCTC mast cells infiltrate diseased lung areas in cystic fibrosis and idiopathic pulmonary fibrosis. Respir Res 2011;12(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Nakatsuka Y, Handa T, Kokosi M, Tanizawa K, Puglisi S, Jacob J, et al. The clinical significance of body weight loss in idiopathic pulmonary fibrosis patients. Respiration 2018;96(4):338-47.
[Crossref] [Google Scholar] [PubMed]
- Triantafillidou C, Manali E, Lyberopoulos P, Kolilekas L, Kagouridis K, Gyftopoulos S, et al. The role of cardiopulmonary exercise test in IPF prognosis. Pulm Med 2013;2013:514817.
[Crossref] [Google Scholar] [PubMed]
- Collins JA, Rudenski A, Gibson J, Howard L, o’Driscoll R. Relating oxygen partial pressure, saturation and content: the haemoglobin–oxygen dissociation curve. Breathe 2015;11(3):194-201.
[Crossref] [Google Scholar] [PubMed]
- Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: decisive role of Bax, Nrf2, NF-κB, Muc5ac, TNF-α and IL-1β. Chem Biol Interact 2015;237:151-65.
[Crossref] [Google Scholar] [PubMed]
- Antoniou KM, Alexandrakis MG, Siafakas NM, Bouros D. Cytokine network in the pathogenesis of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2005;22(2):91-104.
[Google Scholar] [PubMed]
- Zhao Y, Tian B, Sadygov RG, Zhang Y, Brasier AR. Integrative proteomic analysis reveals reprograming tumor necrosis factor signaling in epithelial mesenchymal transition. J Proteomics 2016;148:126-38.
[Crossref] [Google Scholar] [PubMed]
- Della Latta V, Cecchettini AN, Del Ry S, Morales MA. Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions. Pharmacol Res 2015;97:122-30.
[Crossref] [Google Scholar] [PubMed]
 ): Normal saline treated group; (
): Normal saline treated group; ( ): Forskolin treated group; (
): Forskolin treated group; ( ): Rutin treated group; (
): Rutin treated group; (  ): Pirfenidone treated group; (
): Pirfenidone treated group; ( ): FOS+RUT: Forskolin and rutin treated group and (
): FOS+RUT: Forskolin and rutin treated group and ( ): Bleomycin treated group
): Bleomycin treated group