- Corresponding Author:
- N. B. Patel
Department of Chemistry, Veer Narmad South Gujarat University, Surat-395 007, India
E-mail: drnavin@satyam.net.in
| Date of Submission | 16 January 2010 |
| Date of Revision | 13 September 2010 |
| Date of Acceptance | 27 September 2010 |
| Indian J. Pharm. Sci., 2010, 72 (5): 613-620 |
Abstract
A novel series of chalcones, pyrimidines and imidazolinone is described; chalcones (4a-o) were prepared from the lead molecule 4-[2-(5-ethylpyridin-2-yl)ethoxy]benzaldehyde. Pyrimidine (5a-o) derivatives were prepared from the reaction of chalcones and guanidine nitrate in alkali media. Imidazolinones (6a-o) were synthesized from reaction of pyrimidine and oxazolone derivatives (prepared by Erlenmeyer azlactone synthesis). The structures of the synthesized compounds were assigned on the basis of elemental analysis, IR, 1 H and 13 C NMR spectral data. All the products were screened against different strains of bacteria and fungi. Most of these compounds showed better inhibitory activity in comparison to the standard drugs.
Keywords
Antibacterial, antifungal, chalcone, imidazolinone, pyrimidine
Chalcones, both natural or synthetic have been reported to exert a variety of biological activities such as antifungal [1], antibacterial [1,2], antimalarial [3,4], antiinfl ammatory [5], and anticancer [6]. Antimicrobial activity of these chalcones is attributed to the presence of a reactive α,β-unsaturated keto function that can be altered depending on the type and position of substituent on the aromatic rings. Pyrimidines, which are important constituents of nucleic acids, are of great importance due to their role in the current chemotherapy of AIDS. Derivatives of pyrimidines are of interest due to their antiHIV [7], antimalarial [8], analgesic [9], and antiinfl ammatory [10], activities.
The basic imidazole nucleus, present in azlactone containing oxazolone moiety, is of great importance for generating penicillin type of drug intermediates and synthetic hormonal compounds. Imidazolones or ketodihydroimidazoles are also known as oxoimidazolines contain a fi ve- membered heterocyclic ring system with nitrogen atoms at positions 1 and 3 and carbonyl group at position 5. Oxoimidazolines have been reported to exhibit antibacterial [11,12], antifungal [13] and antimicrobial activites [14-17]. Imidazolinones have also been reported to possess fungicidal [18,19], herbicidal [19], and vasodilator activities [20].
Recently we have prepared chalcone, pyrimidine and amide derivatives and reported their antibacterial and antifungal activities [21-23]. All these observations and the essential role of heterocyclic chalcone, pyrimidine and imidazolinone derivatives in certain biological importance prompted us to synthesize these compounds for their antimicrobial activity.
Materials and Methods
Laboratory chemicals were supplied by Rankem India Ltd. and Fisher Scientific Ltd. Melting points were determined using the open tube capillary method and were uncorrected. Purity of the compounds was determined by thin layer chromatography (TLC) plates (silica gel G) in the solvent system toluene:ethyl acetate (75:25). The spots were observed by exposure to iodine vapours or by UV light. The IR spectra were obtained on a Perkin-Elmer 1720 FT-IR spectrometer (KBr pellets). The 1H and 13C-NMR spectra were recorded on a Bruker Avance II 400 spectrometer using TMS as the internal standard in CDCl3. Elemental analysis of the newly synthesized compounds were carried out on Carlo Erba 1108 analyzer.
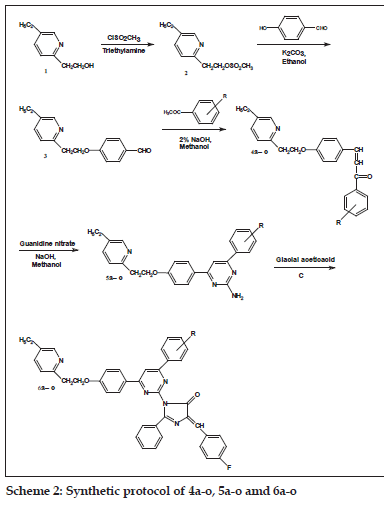
Procedure for the synthesis of 4-[2-(5-ethylpyridin- 2-yl)ethoxy]benzaldehyde (3)
4-[2-(5-Ethylpyridin-2-yl)ethoxy]benzaldehyde (3) was synthesized by the method described in the literature [24,25]. Chalcones and pyrimidines were synthesized and characterized by the reported method [21-23].
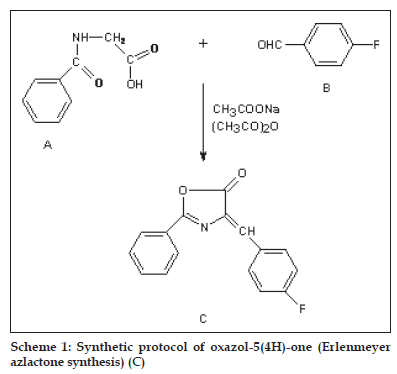
General Process of oxazol-5(4H)-one (Erlenmeyer azlactone synthesis) (C)
A mixture of p-fluoro benzaldehyde (0.33 mol), hippuric acid (0.33 mol) and potassium acetate (0.33 mol) in acetic anhydride (0.83 mol) was refl uxed with stirring for 15 min (reaction progress was monitored by TLC using (3:1 isohexane-ethyl acetate as eluent). The mixture was then cooled down and neutralised by addition of solid potassium carbonate. The solid product was separated by fi ltration, dried and purifi ed by crystallization. The synthetic route has been shown in scheme 1.
General preparation of the compounds 6a-o
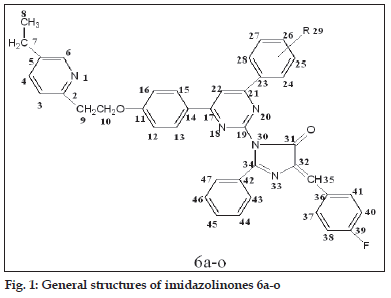
A mixture of 5a-o (0.01 mol) and an appropriate oxazolone (0.01 mol) in 50 ml acetic acid was refl uxed for 6-8 h (reaction progress was monitored by TLC using 7.5:2.5 toluene-ethyl acetate as eluent). After completion of reaction; cooled and was poured into ice cold water. The precipitate was fi ltered and washed with water till pH neutral. The raw product was crystallized from ethanol. The synthetic route is shown in scheme 2 and general structure of 6a-o is represented in fi g. 1.
1-(4-(2,4-dichloro-5-fluorophenyl)-6-(4-(2-(5- ethylpyridin-2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4- (4-fl uorobenzylidene)-2-phenyl-1H-imidazol-5(4H)- one (6a)
Brown solid, m.p. 110-112°, yield 56%, Rf: 0.60; IR (KBr, cm-1) ν: 3062 (Ar-H), 2953, 2833 (-CH2- ), 1795 (-C=O of imidazolinone), 1654 (-C=N imidazolinone), 1612 (-C=N of pyrimidine), 1222, 1036 (C-O-C), 972 (C-F), 743 (C-Cl). 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.17 (t, 3H, -CH3), 2.35(s, 3H, -CH3), 2.51 (q, 2H, -CH2), 3.22 (t, 2H, -CH2), 4.33 (t, 2H, -CH2-O), 7.05-7.55 (m, 16H, Ar-H), 7.31 (s, 1H, -CH), 7.39-8.30 (m, 3H, pyridine-H), 7.81 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.4(C31), 165.3(C19), 164.5(C34), 163.1(C17), 161.0(C21), 126.3-135.5 (C36-C41), 126.3-130.5 (C42-C47), 122.0-157.3 (C2-C6), 118.7-161.4 (C23-C28), 115.0-157.3 (C11-C16), 108.5 (C35), 103.3 (C22), 67.2 (C10), 37.3 (C9), 25.4 (C7), 15.3 (C8). Anal. calcd for C41H29N5O2Cl2F2: C 67.22, H 3.99, N 9.56; found C 67.20, H 3.95, N 9.54.
1-(4-(4-methoxyphenyl)-6-(4-(2-(5-ethylpyridin- 2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4-(4- fl uorobenzylidene)-2-phenyl-1H-imidazol-5(4H)-one (6b)
Pale yellow solid, m.p. 109-113°, yield 60%, Rf: 0.62; IR (KBr, cm-1) ν: 3064 (Ar-H), 2950, 2832 (-CH2-), 1790 (-C=O of imidazolinone), 1653 (-C=N imidazolinone), 1614 (-C=N of pyrimidine), 1225, 1030 (C-O-C). 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.15 (t, 3H, -CH3), 2.30 (s, 3H, -CH3), 2.52 (q, 2H, -CH2) , 3.26 (t, 2H, -CH2), 3.83, (s, 3H, -OCH3), 4.35 (t, 2H, -CH2-O), 7.03-7.55 (m, 18H, Ar-H), 7.15 (s, 1H, -CH), 7.40-8.33 (m, 3H, pyridine-H), 7.89 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.6 (C31), 165.2 (C19), 164.2 (C34), 163.3 (C17), 160.4 (C21), 126.7-135.3 (C36-C41), 126.3- 130.5 (C42-C47), 122.2-157.3 (C2-C6), 115.2-157.1 (C11-C16),114.8-160.6 (C23-C28), 108.1 (C35), 103.5 (C22), 67.2 (C10), 55.3 (C29), 37.4 (C9), 25.1 (C7), 15.3 (C8). Anal. calcd for C42H34N5O3F: C 74.65, H 5.07, N 10.36; found C 74.66, H 5.09, N 10.33.
1-(4-(2,4-dichlorophenyl)-6-(4-(2-(5-ethylpyridin- 2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4-(4- fl uorobenzylidene)-2-phenyl-1H-imidazol-5(4H)-one (6c)
Pale yellow solid, m.p. 105-108°, yield, 62%, Rf: 0.61; IR (KBr, cm-1) ν: 3058 (Ar-H), 2950, 2832 (-CH2-), 1791 (-C=O of imidazolinone), 1653 (-C=N imidazolinone), 1615 (-C=N of pyrimidine), 1225, 1037 (C-O-C), 743 (C-Cl). 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.13 (t, 3H, -CH3), 2.35 (s, 3H, -CH3), 2.54 (q, 2H, -CH2), 3.23 (t, 2H, -CH2), 4.36 (t, 2H, -CH2-O), 7.05-8.03 (m, 17H, Ar-H), 7.32 (s, 1H, -CH), 7.35-8.32 (m, 3H, pyridine-H), 7.83 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.6 (C31), 165.3 (C19), 163.9 (C34), 163.1 (C17), 160.5 (C21), 127.4-135.7 (C23-C28), 126.0-135.5 (C36-C41), 126.2-130.5 (C42-C47), 122.8-157.4 (C2-C6), 114.5-157.6 (C11-C16), 108.7 (C35), 103.5 (C22), 67.7 (C10), 37.7 (C9), 25.1 (C7), 15.1 (C8). Anal. calcd for C41H30N5O2Cl2F: C 68.91, H 4.23, N 9.80; found C 68.92, H 4.25, N 9.81.
1-(4-(4-hydroxyphenyl)-6-(4-(2-(5-ethylpyridin- 2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4-(4- fl uorobenzylidene)-2-phenyl-1H-imidazol-5(4H)-one (6d)
Yellow solid, m.p. 205-207°, yield, 54%, Rf: 0.63; IR (KBr, cm-1) ν: 3065(Ar-H), 3354 (-OH), 2953, 2833 (-CH2-), 1794 (-C=O of imidazolinone), 1652(-C=N imidazolinone), 1612(-C=N pyrimidine), 1223, 1032(C-O-C). 1H NMR (CDCl3, 400 MHz) δ(ppm):1.20 (t, 3H, -CH3), 2.37 (s, 3H, -CH3), 2.51 (q, 2H, -CH2) , 3.24 (t, 2H, -CH2), 4.34 (t, 2H, -CH2-O), 6.79-7.55 (m, 18H, Ar-H), 7.26 (s, 1H, -CH), 7.37-8.35 (m, 3H, pyridine-H), 7.86 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 169.9 (C31), 165.0 (C19), 164.2 (C34), 163.8(C17), 160.2 (C21), 126.5-135.6(C36-C41), 126.2-130.0 (C42-C47), 122.2-157.3 (C2-C6), 116.4-158.5 (C23-C28), 115.1-157.2 (C11-C16), 108.3 (C35), 103.6 (C22), 67.6 (C10), 37.2 (C9), 25.3 (C7), 15.9(C8). Anal. calcd for C41H32N5O3F: C 74.42, H 4.87, N 10.58; found C 74.40, H 4.85, N 10.55.
1-(4-(2,6-dichloro-5-fluorophenyl)-6-(4-(2-(5- ethylpyridin-2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4- (4-fl uorobenzylidene)-2-phenyl-1H-imidazol-5(4H)- one (6e)
Brown solid, m.p. 110-112°, yield 55%, Rf: 0.61; IR (KBr, cm-1) ν: 3057 (Ar-H), 2958, 2837 (-CH2- ), 1789 (-C=O of imidazolinone), 1653 (-C=N imidazolinone), 1616 (-C=N of pyrimidine), 1223, 1035 (C-O-C), 972 (C-F), 744 (C-Cl) . 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.16 (t, 3H, -CH3), 2.32 (s, 3H, -CH3), 2.56 (q, 2H, -CH2) , 3.26 (t, 2H, -CH2), 4.33 (t, 2H, -CH2-O), 6.81-7.55 (m, 16H, Ar-H), 7.30 (s, 1H, -CH), 7.35-8.34 (m, 3H, pyridine-H), 7.80 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.1 (C31), 165.2 (C19), 164.3 (C34), 163.8 (C17), 160.5 (C21), 126.2-135.5 (C36-C41), 126.1-130.5 (C42-C47), 122.7-157.3 (C2-C6), 118.3-161.4 (C23-C28), 114.6-157.5 (C11-C16), 108.9 (C35), 103.5 (C22), 67.1 (C10), 37.4 (C9), 25.8 (C7), 15.7 (C8). Anal. calcd for C41H29N5O2Cl2F2: C 67.22, H 3.99, N 9.56; found C 67.21, H 3.96, N 9.54.
1-(4-(4-methylphenyl)-6-(4-(2-(5-ethylpyridin- 2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4-(4-fluorobenzylidene)- 2-phenyl-1H-imidazol-5(4H)-one (6f)
Pale yellow solid, m.p. 92-94°, yield 64%, Rf: 0.64; IR (KBr, cm-1) ν: 3062 (Ar-H), 2953, 2836 (-CH2-), 1798 (-C=O of imidazolinone), 1658 (-C=N imidazolinone), 1612 (-C=N of pyrimidine), 1222, 1035 (C-O-C). 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.19 (t, 3H, -CH3), 2.33 (s, 3H, -CH3), 2.54 (q, 2H, -CH2) , 3.20 (t, 2H, -CH2), 4.32 (t, 2H, -CH2-O), 7.05-7.59 (m, 18H, Ar-H), 7.25 (s, 1H, -CH), 7.39- 8.32 (m, 3H, pyridine-H), 7.85 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.3 (C31), 165.5(C19), 164.0(C34), 163.5(C17), 160.9 (C21), 127.4-138.4 (C23-C28), 126.4-135.2 (C36-C41), 126.1- 130.2 (C42-C47), 122.5-157.1 (C2-C6), 114.9-157.4 (C11-C16), 108.0 (C35), 103.2 (C22), 67.5 (C10), 37.5 (C9), 25.5 (C7), 21.2 (C29), 15.4 (C8). Anal. calcd for C42H34N5O2F: C 76.46, H 5.19, N 10.62; found C 76.43, H 5.17, N 10.63.
1-(4-(1-phenyl)-6-(4-(2-(5-ethylpyridin-2-yl)ethoxy) phenyl)pyrimidin-2-yl)-4-(4-fl uoro- benzylidene)-2- phenyl-1H-imidazol-5(4H)-one (6g)
Brown solid, m.p. 86-88° yield 53%, Rf: 0.58; IR (KBr, cm-1) ν: 3063 (Ar-H), 2957, 2836 (-CH2-), 1794 (-C=O of imidazolinone), 1655 (-C=N imidazolinone), 1615 (-C=N of pyrimidine), 1229, 1034 (C-O-C). 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.14 (t, 3H, -CH3), 2.34 (s, 3H, -CH3), 2.51 (q, 2H, -CH2) , 3.22 (t, 2H, -CH2), 4.35 (t, 2H, -CH2-O), 7.05-7.85 (m, 19H, Ar- H), 7.23 (s, 1H, -CH), 7.32-8.40 (m, 3H, pyridine-H), 7.82 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.8(C31), 165.9 (C19), 164.3(C34), 163.0(C17), 160.5 (C21), 127.5-133.0 (C23-C28), 126.1- 135.1 (C36-C41), 126.4-130.5 (C42-C47), 122.2-157.3 (C2-C6), 114.2-157.6 (C11-C16), 108.1 (C35), 103.6 (C22), 67.3 (C10), 37.6 (C9), 25.7 (C7), 15.3 (C8). Anal. calcd for C41H32N5O2F: C 76.26, H 5.00, N 10.85; found C 76.21, H 5.01, N 10.82.
1-(4-(4-fluorophenyl)-6-(4-(2-(5-ethylpyridin-2- yl)ethoxy)phenyl)pyrimidin-2-yl)-4-(4-fluorobenzylidene)- 2-phenyl-1H-imidazol-5(4H)-one (6h)
Dark brown solid, m.p. 160-162°, yield 57%, Rf: 0.63; IR (KBr, cm-1) ν: 3068 (Ar-H), 2955, 2832 (-CH2-), 1793 (-C=O of imidazolinone), 1653 (-C=N imidazolinone), 1618 (-C=N of pyrimidine), 1226, 1039 (C-O-C), 974 (C-F). 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.20 (t, 3H, -CH3), 2.36 (s, 3H, -CH3), 2.56 (q, 2H, -CH2) , 3.23 (t, 2H, -CH2), 4.36 (t, 2H, -CH2-O), 7.05-8.15 (m, 18H, Ar-H), 7.28 (s, 1H, -CH), 7.37-8.38 (m, 3H, pyridine-H), 7.86 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.4 (C31), 165.6 (C19), 164.6 (C34), 163.6 (C17), 160.3 (C21), 126.0-135.0 (C36-C41), 126.1-130.7 (C42-C47), 122.3-157.7 (C2-C6), 116.0-162.9 (C23-C28), 114.3-157.6 (C11-C16), 108.6 (C35), 103.5 (C22), 67.5 (C10), 37.4 (C9), 25.6 (C7), 15.1 (C8). Anal. calcd for C41H31N5O2F2: C 74.19, H 4.71, N 10.55; found C 74.15, H 4.69, N 10.50.
1-(4-(2,4-fluorophenyl)-6-(4-(2-(5-ethylpyridin- 2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4-(4-fluoro benzylidene)-2-phenyl-1H-imidazol-5(4H)-one (6i)
Brown solid, m.p. 105-107° yield 59%, Rf: 0.64; IR (KBr, cm-1) ν: 3066 (Ar-H), 2953, 2833 (-CH2-), 1795 (-C=O of imidazolinone), 1654 (-C=N imidazolinone), 1613 (-C=N of pyrimidine), 1225, 1035 (C-O-C), 975 (C-F). 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.22 (t, 3H, -CH3), 2.39 (s, 3H, -CH3), 2.58 (q, 2H, -CH2) , 3.19 (t, 2H, -CH2), 4.37 (t, 2H, -CH2-O), 6.74-7.55 (m, 17H, Ar-H), 7.30 (s, 1H, -CH), 7.35-8.35 (m, 3H, pyridine-H), 7.84 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.7 (C31), 165.7 (C19), 164.3 (C34), 163.3 (C17), 160.4 (C21), 105.2-164.5 (C23-C28), 126.3-135.6 (C36-C41), 126.3-130.6 (C42-C47), 122.2-157.5 (C2-C6), 114.5-157.7 (C11-C16), 108.3 (C35), 103.7 (C22), 67.2 (C10), 37.4 (C9), 25.8 (C7), 15.9 (C8). Anal. calcd for C41H30N5O2F3: C 72.24, H 4.44, N 10.27; found C 72.21, H 4.42, N 10.22.
1-(4-(4-bromophenyl)-6-(4-(2-(5-ethylpyridin- 2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4-(4- fl uorobenzylidene)-2-phenyl-1H-imidazol-5(4H)-one (6j)
Brown solid, m.p. 102-104° yield 63%, Rf: 0.61; IR (KBr, cm-1) ν: 3063 (Ar-H), 2954, 2836 (-CH2-), 1794 (-C=O of imidazolinone), 1656 (-C=N imidazolinone), 1610 (-C=N of pyrimidine), 1225, 1033 (C-O-C), 864 (C-Br) . 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.18 (t, 3H, -CH3), 2.31 (s, 3H, -CH3), 2.53 (q, 2H, -CH2) , 3.24 (t, 2H, -CH2), 4.33 (t, 2H, -CH2-O), 7.05-7.78 (m, 18H, Ar-H), 7.31 (s, 1H, -CH), 7.41-8.39 (m, 3H, pyridine-H), 7.87 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.4 (C31), 165.3 (C19), 164.7 (C34), 163.2 (C17), 160.5 (C21), 123.1-132.1 (C23-C28), 126.6-135.4 (C36-C41), 126.6-130.3 (C42-C47), 122.2-157.6 (C2-C6), 115.2-157.2 (C11-C16), 108.4 (C35), 103.5 (C22), 67.2 (C10), 37.2 (C9), 25.4 (C7), 15.6 (C8). Anal. calcd for C41H31N5O2FBr: C 67.96, H 4.31, N 9.66; found C 67.90, H 4.30, N 9.68.
1-(4-(3,4-dichlorophenyl)-6-(4-(2-(5-ethylpyridin- 2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4-(4- fl uorobenzylidene)-2-phenyl-1H-imidazol-5(4H)-one (6k)
Brown solid, m.p. 75-78° yield 61%, Rf: 0.62; IR (KBr, cm-1) ν: 3060 (Ar-H), 2952, 2834 (-CH2-), 1798 (-C=O of imidazolinone), 1658 (-C=N imidazolinone), 1609 (-C=N of pyrimidine), 1220, 1034 (C-O-C), 748 (C-Cl) . 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.20 (t, 3H, -CH3), 2.36 (s, 3H, -CH3), 2.50 (q, 2H, -CH2) , 3.23 (t, 2H, -CH2), 4.35 (t, 2H, -CH2-O), 7.05-7.86 (m, 17H, Ar-H), 7.21 (s, 1H, -CH), 7.33-8.35 (m, 3H, pyridine-H), 7.82 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.6 (C31), 165.0 (C19), 164.1 (C34), 163.1 (C17), 161.0 (C21), 127.0-133.8 (C23-C28), 126.7-135.4 (C36-C41), 126.5-130.6 (C42-C47), 122.1-157.4 (C2-C6), 114.3-157.3 (C11-C16), 108.5 (C35), 103.0 (C22), 67.1 (C10), 37.2 (C9), 25.7 (C7), 15.1 (C8). Anal. calcd for C41H30N5O2Cl2F: C 68.91, H 4.23, N 9.80; found C 68.88, H 4.20, N 9.79.
1-(4-(4-chlorophenyl)-6-(4-(2-(5-ethylpyridin- 2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4-(4-fluoro benzylidene)-2-phenyl-1H-imidazol-5(4H)-one (6l)
Pale yellow solid, m.p. 135-138°, yield 67%, Rf: 0.58; IR (KBr, cm-1) ν: 3061(Ar-H), 2951, 2837(- CH2-), 1792(-C=O imidazolinone), 1652 (-C=N imidazolinone), 1615(-C=N pyrimidine), 1227, 1037(C-O-C), 742(C-Cl) . 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.15(t, 3H, -CH3), 2.34(s, 3H, -CH3), 2.52(q, 2H, -CH2) , 3.22(t, 2H, -CH2), 4.34 (t, 2H, -CH2-O), 7.05-7.98(m, 18H, Ar-H), 7.32(s, 1H, -CH), 7.36-8.29(m, 3H, pyridine-H), 7.82 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 171.2(C31), 164.9(C19), 164.3 (C34), 163.2 (C17), 160.4 (C21), 128.9-134.3 (C23-C28), 126.4-135.4 (C36-C41), 126.6-130.0 (C42-C47), 122.2-157.4 (C2-C6), 114.5- 157.6 (C11-C16), 108.2 (C35), 103.6 (C22), 67.8 (C10), 37.8 (C9), 25.1 (C7), 15.5 (C8). Anal. calcd for C41H31N5O2ClF: C 72.40, H 4.59, N 10.30; found C 72.39, H 4.57, N 10.32.
1-(4-(3-methoxyphenyl)-6-(4-(2-(5-ethylpyridin- 2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4-(4- fl uorobenzylidene)-2-phenyl-1H-imidazol-5(4H)-one (6m)
Yellow solid, m.p. 84-87° yield 59%, Rf: 0.63; IR(KBr, cm-1) ν: 3063(Ar-H), 2952, 2831(-CH2- ), 1797 (-C=O imidazolinone), 1657 (-C=N imidazolinone), 1616 (-C=N pyrimidine), 1220, 1036 (C-O-C). 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.18(t, 3H, -CH3), 2.36(s, 3H, -CH3), 2.57(q, 2H, -CH2) , 3.24(t, 2H, -CH2), 3.73(s, 3H, -OCH3), 4.36(t, 2H, -CH2-O), 6.73-7.55(m, 18H, Ar-H), 7.24(s, 1H, -CH), 7.40-8.35(m, 3H, pyridine-H), 7.84 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.0 (C31), 165.2 (C19), 164.4(C34), 163.8(C17), 160.3(C21), 126.3-135.5(C36-C41), 126.4-130.6(C42-C47), 122.3-157.3 (C2-C6), 111.2-161.1(C23-C28), 114.5-157.6(C11-C16), 108.1(C35), 103.4(C22), 67.2 (C10), 55.8(C29), 37.3(C9), 25.2(C7), 15.7(C8). Anal. calcd for C42H34N5O3F: C 74.65, H 5.07, N 10.36; found C 74.63, H 5.04, N 10.34.
1-(4-(3-fluorophenyl)-6-(4-(2-(5-ethylpyridin- 2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4-(4-fluoro benzylidene)-2-phenyl-1H-imidazol-5(4H)-one (6n)
Brown solid, m.p. 115-118°, yield 56%, Rf: 0.64; IR (KBr, cm-1) ν: 3067(Ar-H), 2955, 2835(-CH2-), 1795 (-C=O imidazolinone), 1653 (-C=N imidazolinone), 1613(-C=N pyrimidine), 1225, 1030(C-O-C), 973(C-F) . 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.16(t, 3H, -CH3), 2.38(s, 3H, -CH3), 2.56(q, 2H, -CH2), 3.24 (t, 2H, -CH2), 4.35 (t, 2H, -CH2-O), 6.93-7.55 (m, 18H, Ar-H), 7.21 (s, 1H, -CH), 7.39-8.38 (m, 3H, pyridine-H), 7.87 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.4 (C31), 165.8 (C19), 164.9 (C34), 163.0 (C17), 160.0 (C21), 126.0-135.2 (C36-C41), 126.7-130.7 (C42-C47), 122.3-157.4 (C2-C6), 115.5-163.4 (C23-C28), 114.3-157.6 (C11-C16), 108.7 (C35), 103.5 (C22), 67.4 (C10), 37.2 (C9), 25.8 (C7), 15.3 (C8). Anal. calcd for C41H31N5O2F2: C 74.19, H 4.71, N 10.55; found C 74.20, H 4.72, N 10.53.
1-(4-(3,4-difluorophenyl)-6-(4-(2-(5-ethylpyridin- 2-yl)ethoxy)phenyl)pyrimidin-2-yl)-4-(4- fl uorobenzylidene)-2-phenyl-1H-imidazol-5(4H)-one (6o)
Brown solid, m.p. 137-140° yield 58%, Rf: 0.62; IR (KBr, cm-1) ν: 3064 (Ar-H), 2956, 2835 (-CH2-), 1794 (-C=O of imidazolinone), 1653 (-C=N imidazolinone), 1613 (-C=N of pyrimidine), 1223, 1037 (C-O-C), 970 (C-F) . 1H NMR (CDCl3, 400 MHz) δ(ppm): 1.23 (t, 3H, -CH3), 2.34 (s, 3H, -CH3), 2.51 (q, 2H, -CH2) , 3.25 (t, 2H, -CH2), 4.39 (t, 2H, -CH2-O), 7.01-7.92 (m, 17H, Ar-H), 7.27 (s, 1H, -CH), 7.38-8.33 (m, 3H, pyridine-H), 7.82 (s, 1H, pyrimidine-H). 13C NMR (100 MHz, CDCl3) δ(ppm): 170.6 (C31), 165.2 (C19), 164.3 (C34), 163.2 (C17), 160.4 (C21), 126.2-135.5 (C36-C41), 126.3-130.5 (C42-C47), 122.3-157.4 (C2-C6), 117.6-150.0 (C23-C28), 114.2-157.5 (C11-C16), 108.8 (C35), 103.7 (C22), 67.8 (C10), 37.5 (C9), 25.0 (C7), 15.1 (C8). Anal. calcd for C41H30N5O2F3: C 72.24, H 4.44, N 10.27; found C 72.26, H 4.42, N 10.25.
Results and Discussion
The MICs of synthesized compounds were carried out by broth microdilution method as described by Rattan [26]. Antibacterial activity was screened against two gram positive (Staphylococcus aureus MTCC 96, Streptococcus pyogenus MTCC 443) and two gram negative (Escherichia coli MTCC 442, Pseudomonas aeruginosa MTCC 2488) bacteria, ampicillin was used as a standard antibacterial agent. Antifungal activity was screened against three fungal species Candida albicans MTCC 227, Aspergillus niger MTCC 282 and Aspergillus clavatus MTCC 1323, greseofulvin was used as a standard antifungal agent. All MTCC cultures were collected from Institute of Microbial Technology, Chandigarh, India.
The minimum bactericidal concentrations (MBCs) of 4a-o, 5a-o and 6a-o are shown in Table 1. From the screening data, most of the compounds possessed better antibacterial activity (MBC, 50-250 μg/ml) against S. aureus; chalcones 4b, 4f and 4h with 4-OCH3, 4-CH3 and 4-F group showed MBC value in the range between 62.5-100 μg/ml against E. coli; 4h at 100 μg/ml against P. aeruginosa; 4f and 4h bearing 4-CH3 and 4-F at 100-150 μg/ml while remaining 4b, 4d and 4n having 4-OCH3, 4-OH and 3-F showed comparable activity against S. aureus with ampicillin. The remaining chalcones showed less activity against all four bacterial species. The pyrimidine 5b and 5i containing 4-OCH3 and 2,4-F possessed more activity of 62.5 μg/ml against E. coli; 5h having 4-F at 100 μg/ml against S. aureus and S. pyogeneus; 5i containing 2,4-F at 150 μg/ ml against S. aureus; 5l having 4-Cl at 100 μg/ml against P. aeruginosa and at 150 μg/ml against S. aureus. The remaining pyrimidines displayed less activity against all four bacterial species comparable to ampicillin. Imidazolinones 6j having 4-Br at 100 μg/ml against E. coli and 250 μg/ml against S. aureus; 6a, 6d, 6f, 6h, 6i, 6j, 6k, 6n and 6o containing 2,4-Cl, 5-F, 4-OH, 4-CH3, 4-F, 2,4-F, 4-Br, 3,4-Cl, 3-F and 3,4-F grpups at 200-250 μg/ml against S. aureus were comparable with ampicillin. Minimum fungicidal concentrations (MFCs) are shown in Table 2. Most of the compounds possessed very good antifungal activity against C. albicans; their MFC range between 100-500 μg/ml. In chalcones, 4c, 4d, 4g, 4h, 4m and 4n containing 2,4-Cl, 4-OH, phenyl, 4-F, 3-OCH3 and 3-F at 200- 500 μg/ml; 5o having 3,4-F at 200 μg/ml against C. albicans, while remaining pyrimidines are less active against A. niger and A. clavatus compared with greseofulvin. Imidazolinones, 6c containing 2,4-Cl displayed more at 100 μg/ml against C. albicans, A. niger and A. clavatus with greseofulvin and nystatin; 6e and 6h having 2,6-Cl, 5-F and 4-F at 250 μg/ ml against C. albicans, while other imidazolinones show moderate activity against C. albicans with greseofulvin. Remainning imidazolinones are less active against two fungal A. niger and A. clavatus.
In overall microbial analysis; all compounds are comparable with S. aureus and C. albicans. Chalcones having methoxy, methyl, hydroxy and fluoro groups, pyrimidines with methoxy, difluoro and chloro substitutents and imidazolinones having, chloro, dichloro, fl uoro and difl uoro are more active group against S. aureus and C. albicans. Imidazolinone 6c 2,4-Cl is comparable with both nystatin and greseofulvin.
| Compounds R | Minimal bactericidal concentration μg/ml | |||||
|---|---|---|---|---|---|---|
| Gram negative | Gram positive | |||||
| E. coli | P.aeruginosa | S.aureus | S.pyogenus | |||
| 4b | 4-OCH3 | 100 | 150 | 250 | 250 | |
| 4d | 4-OH | 150 | 200 | 250 | 250 | |
| 4f | 4-CH3 | 100 | 150 | 100 | 250 | |
| 4h | 4-F | 62.5 | 100 | 150 | 150 | |
| 4n | 3-F | 250 | 500 | 250 | 500 | |
| 5b | 4-OCH3 | 62.5 | 150 | 250 | 250 | |
| 5c | 2,4-Cl | 500 | 500 | 250 | 500 | |
| 5f | 4-CH3 | 200 | 200 | 250 | 250 | |
| 5h | 4-F | 250 | 250 | 100 | 100 | |
| 5i | 2,4-F | 62.5 | 150 | 150 | 200 | |
| 5j | 4-Br | 250 | 250 | 200 | 200 | |
| 5k | 3,4-Cl | 250 | 250 | 250 | 250 | |
| 5l | 4-Cl | 250 | 100 | 150 | 250 | |
| 5n | 3-F | 500 | 500 | 250 | 250 | |
| 6a | 2,4-Cl,5-F | 500 | 500 | 250 | 250 | |
| 6d | 4-OH | 500 | 500 | 250 | 500 | |
| 6f | 4-CH3 | 125 | 200 | 250 | 250 | |
| 6h | 4-F | 150 | 250 | 250 | 250 | |
| 6i | 2,4-F | 500 | 500 | 250 | 250 | |
| 6j | 4-Br | 100 | 150 | 250 | 250 | |
| 6k | 3,4-Cl | 500 | 500 | 200 | 200 | |
| 6n | 3-F | 500 | 250 | 250 | 200 | |
| 6o | 3,4-F | 500 | 500 | 250 | 500 | |
| Ampicillin | 100 | 100 | 250 | 100 | ||
Table 1: Antibacterial Activity Of Compound 4a-O, 5a-O And 6a-O.
| Compounds | R | Minimal fungicidal concentration μg/ml | ||
|---|---|---|---|---|
| C. albicans | A. niger | A. clavatus | ||
| 4c | 2,4-Cl | 500 | 500 | 1000 |
| 4d | 4-OH | 500 | 500 | 1000 |
| 4g | Phenyl | 200 | 500 | 500 |
| 4h | 4-F | 250 | >1000 | >1000 |
| 4m | 3-OCH3 | 500 | 500 | 500 |
| 4n | 3-F | 500 | 1000 | 1000 |
| 5a | 2,4-Cl,5-F | 500 | 500 | 1000 |
| 5b | 4-OCH3 | 500 | >1000 | >1000 |
| 5c | 2,4-Cl | 500 | >1000 | >1000 |
| 5d | 4-OH | 500 | 500 | 1000 |
| 5e | 2,6-Cl,5-F | 500 | 500 | 500 |
| 5f | 4-CH3 | 500 | 500 | 500 |
| 5g | Phenyl | 500 | 500 | 500 |
| 5h | 4-F | 500 | 250 | 250 |
| 5i | 2,4-F | 500 | 1000 | 1000 |
| 5l | 4-Cl | 500 | 500 | 500 |
| 5n | 3-F | 500 | 1000 | 1000 |
| 5o | 3,4-F | 200 | 200 | 200 |
| 6c | 2,4-Cl | 100 | 100 | 100 |
| 6e | 2,6-Cl,5-F | 250 | >1000 | >1000 |
| 6f | 4-CH3 | 500 | 1000 | 1000 |
| 6h | 4-F | 250 | 1000 | 1000 |
| 6j | 4-Br | 500 | 1000 | 1000 |
| 6k | 3,4-Cl | 500 | 1000 | 1000 |
| 6m | 3-OCH3 | 500 | 500 | 500 |
| 6n | 3-F | 500 | 250 | 500 |
| Nystatin | 100 | 100 | 100 | |
| Greseofulvin | 500 | 100 | 100 | |
Table 2: Antifungal Activity Of Compound 4a-O, 5a-O And 6a-O
Acknowledgements
The authors thank Professor and Head, Department of Chemistry for extending laboratory facilities; the librarian, Veer Narmad South Gujarat University, Surat for library facilities and D. Rajani, Microcare Laboratory, Surat for carrying out antimicrobial activity. We also thank the CDRI, Lucknow for elemental analysis and SAIF, Chandigarh for 1H NMR and 13C NMR spectral analysis.
References
- Tomar V, Bhattacharjee G, Kamaluddina, Kumar A. Synthesis and antimicrobial evaluation of new chalcones containing piperazine or 2,5-dichlorothiophene moiety. Bioorg Med ChemLett 2007;17:5321-4.
- Prasad YR, Kumar PR, Smiles J, Babu PA. QSAR studies on chalcone derivatives as antibacterial agents against Bacillus pumilis. Arkivoc 2008;11:266-76.
- Dominguez JN, Leon C, Rodrigues J, Dominguez NG, Gut J, Rosenthal PJ. Synthesis and antimalarial activity of sulfonamide chalcone derivatives. Farmaco 2005;60:307-11.
- Mishra N, Arora P, Kumar B, Mishra LC, Bhattacharya A, Awasthi SK, et al. Synthesis of novel substituted 1,3-diaryl propenone derivatives and their antimalarial activity in vitro. Eur J Med Chem 2008;43:1530-5.
- Kalirajan R, Sivakumar SU, Jubie S, Gowramma B, Suresh B. Synthesis and Biological evaluation of some heterocyclic derivatives of Chalcones. Int J Chem Res 2009;1:27-34.
- Modzelewska A, Pettit C, Achanta G, Davidson NE, Huang P, Khana SR. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg Med Chem 2006;14;3491-5
- Gadhachanda VR, Wu B, Wang Z, Kuhen KL, Caldwell J, Zondler H, et al. 4-Amino- pyrimidines as novel HIV-1 inhibitors. Bioorg MedChemLett 2007;17:260-5.
- Agarwal A, Srivastava K, Puri SK, Chauhana PMS. Antimalarial activity and synthesis of new trisubstitutedpyrimidines. Bioorg Med ChemLett 2005;15:3130-2.
- Chhabria MT, Bhatt HG, Raval HG, Oza PM. Synthesis and biological evaluation of some 5-ethoxycarbonyl-6- isopropylamino-4-(substitutedphenyl)aminopyrimidines as potent analgesic and anti-inflammatory agents. Bioorg Med ChemLett 2007;17:1022-4.
- Venu TD, Khanum SA, Firdouse A, Manuprasad BK, Shashikanth S, Mohamed R, et al. Synthesis and anti-inflammatory activity of 2-(2-aroylaroxy)-4,6-dimethoxy Pyrimidines. Bioorg Med ChemLett 2008;18:4409-12.
- Desai NC, BhavsarAM, Baldaniya BB. Synthesis and antimicrobial activity of 5-Imidazolinone derivatives. Indian J Pharm Sci 2009;71:90-4.
- Solankee A, Solankee S, Patel G. Potential antibacterial agents: 5-imidazolones derivatives. Rasayan J Chem 2008;1:228-31.
- El-Sayed Ali T, Abdel-Aghfaar Abdel-Aziz S, Metwali El-Shaaer H, Ismail Hanafy F, Zaky El-Fauomy A. Synthesis of Some New 4-oxo-4H-Chromene Derivatives Bearing Nitrogen Heterocyclic Systems as Antifungal Agents. Turk J Chem 2008;32:365-74.
- Mistry RN, Desai KR. Studies on Synthesis of Some Novel Heterocyclic Azlactone Derivatives and Imidazolinone Derivatives and their Antimicrobial Activity. E J Chem 2005;2:42-51.
- Benkli K, Karaburun AC, Gundo du- Karaburun N, Demirayak S, Guven K. Synthesis and Antimicrobial Activities of Some New Nitroimidazole Derivatives. Arch Pharm Res 2003;26:773-7.
- De B, Gupta JK, Saravanan VS. Synthesis of some oxazolinones and imidazolinones and their antimicrobial screening. Acta Pharm 2005;55:287-96.
- Amir M, Kumar A, Ali I, Khan SA. Synthesis and pharmaceutically important 1,3,4- thiadiazole and imidazolinone derivatives as antimicrobials. Indian J Chem 2009;48B:1288-93
- Ding M, Zeng G, Liu Z. Synthesis and Fungicidal Activities of 4H-Imidazolin-4-ones Containing Sulfur Substituent. Phosphorus Sulfur Silicon 2002;177:1315-21.
- Huang X, Liu Z, Yang F, Ding M. Synthesis and Properties of Novel Imidazolone Derivatives Containing a Sulfur Atom. Phosphorus Sulfur Silicon 2007;182:939-50.
- Demirayak S, Karaburun AC, Kayagil I, Erol K, Sirmagul B. Some Pyridazinone and Phtha- lazinone Derivatives and Their Vasodilator Activities. Arch Pharm Res 2004;27:13-8.
- Patel NB, Patel HR. Synthesis and pharmacological studies of 5-ethyl pyridin-2-ethanol analogs derivatives. ARKIVOC 2009;12:302-21.
- Patel NB, Patel HR. Characterization and pharmacological evaluation of new pyridine analogs. Arabian J Chem Article in press, doi:10.1016/j. arabjc.2010.07.028
- Patel NB, Patel HR. Design and Synthesis of 2-(5-ethyl-pyridine-2-yl) ethanol Analogs as Potential Microbial Agents. Int J Drug Design 2010;1:93-106.
- Gaonkar SL, Rai KM, Prabhuswamy B. Synthesis and antimicrobial studies of a new series of 2-{4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl}-5-substituted-1,3,4-oxadiazoles. Eur J Med Chem 2006;41:841-6.
- Momose Y, Meguro K, Ikeda H, Hatanaka C, Satoru OI, SohdaT. Studies on Antidiabatic agents: Synthesis and biological activity of pioglitazone and related compounds. Chem Pharm Bull 1991;39:1440-5.
- Rattan A. Antimicrobials in laboratory medicine. 1st ed. New Delhi: Churchill Livingstone; 2000. p. 85.