- *Corresponding Author:
- J. K. Joshi
Department of Pharmaceutical Chemistry, Maliba Pharmacy College, Tarsadi, Surat - 394 350, India
E-mail: joshijalpank@yahoo.com
| Date of Submission | 7 April 2006 |
| Date of Revision | 25 April 2007 |
| Date of Acceptance | 17 October 2007 |
| Indian J Pharm Sci 2007, 69 (5): 697-699 |
Abstract
N-aryl anthranilic acid and its derivatives (3a-f) have been synthesized via Ullmann condensation of o-chloro benzoic acid with various substituted anilines (2a-f) in the presence of cupric oxide and anhydrous potassium carbonate. All the synthesized compounds (3a-f) were characterized by mp, TLC, UV, IR, 1 H NMR and mass spectral analysis. All the synthesized compounds (3a-f) were screened for their antiinflammatory activity by carrageenan induced rat paw edema method. All the synthesized compounds (3a-f) showed significant antiinflammatory activity. Compounds 3a and 3c were found to be the most potent compounds.
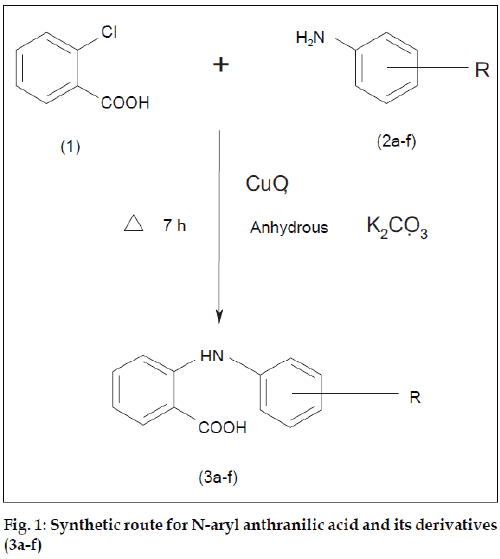
In the present investigation an attempt has been made to synthesize N-aryl anthranilic acid and its derivatives (3a-f) and to evaluate their antiinß ammatory activity. All the compounds (3a-f) were synthesized by Ullmann condensation of o-chlorobenzoic acid with various substituted anilines (2a-f) in the presence of cupric oxide (catalyst) and anhydrous potassium carbonate (for removal of hydrogen halide formed during reaction). Chlorobenzene itself is mostly unreactive, but it is activated by introduction of certain functional groups like carboxylic acid. Several compounds such as mefenamic acid, ß ufenamic acid, clofenamic acid, medofenamic acid and tolfenamate [1-4] have been demonstrated to possess antiinß ammatory [5], analgesic [6], antipyretic [7] activity. Therefore, it was a thought of interest to synthesize and study biological effects of various N-aryl anthranilic acid derivatives.
Melting points of synthesized compounds were determined in open capillary tubes and are uncorrected. Thin layer chromatography was performed on microscopic slides coated with silica gel G, benzene:methanol (4.9:0.1) was used as mobile phase and spots were visualized by overnight exposure to iodine vapour. The structure of compounds was established on the basis of complete spectral analysis. The UV spectra were recorded on Shimadzu UV/Vis spectrophotometer. The IR spectra were recorded in the range of 4000-400 cm-1 using KBr disc on a FTIR RXI Perkin Elmer spectrophotometer. 1H NMR were recorded on a Broker dry 300 KHz spectrophotometer using CDCl3/DMSO-d6 as a solvent with TMS as an internal standard. The FAB mass spectra were recorded on a Joel Sx-102/Da-6000 spectrophotometer data system using xenon as FAB gas.
N-aryl anthranilic acid and its derivatives (3a-f) [8] were synthesized by refluxing a mixture of o-chloro benzoic acid (1 mol) (1), various substituted anilines (1.2 mol) (2a-f), upric oxide (1 g) and anhydrous potassium carbonate (8 g) was heated under reflux for 7 h (fig. 1) The solid thus obtained was suspended in water. Finally title compounds (3a-f) were precipitated with the help of dilute hydrochloric acid, dried and recrystallised from ethanol (95%).
The study was carried out according to the rules and regulation laid down by the Institutional Animal Ethics Committee. Animals (rats) were weighed, numbered and marked on one of the hind paw just beyond tibio-tarsal junction to ensure accurate paw volume. The paw volume of each rat was determined by mercury displacement method [9-12]. The animals were divided into two groups, one as control and other as test, each comprising of two rats. To one group (test) was injected N,N-dimethylformamide solution of synthesized compounds intraperitonialy and to the second group (control) was injected N,N-dimethylformamide solution. 0.1 ml of 1% w/v carrageenan was injected in the plantar region of the paw of control as well as synthesized compoundtreated group after 30 min. The rat paw volume of legs of control and synthesized compound treated rats were noted at 15, 30, 45, 60, 75, 90, 105 and 120 min interval after carrageenan challenge. The % inhibition [13-16] of paw volume in drug-treated animals taking control as reference was calculated at 60 min interval.
O-chlorobenzoic acid on reaction with various substituted aniline derivatives (2a-f) produced N-aryl anthranilic acid derivatives (3a-f) in good yield. The structure of compound 3a has been proved by UV, IR, 1H NMR and mass spectral data. The UV spectra showed two absorption maxima at 298 nm and 388 nm, respectively. The IR absorption due to (C=O), (-OH) and (-NH-) appeared at 1710-1678 cm-1, 3300-2600 cm-1 and 3280-3110 cm-1, respectively. The 1H NMR spectra of this compound in DMSO-d6 exhibited following signals: (δ in ppm) 6.692-7.353 ( m, 9H, Ar-H), 7.961 (s, 1H, N-H), 9.639 (s, 1H, -COOH). The mass spectrum showed a fairly intense molecular ion peak at m/z 213 (M+), conforming the molecular formula C13H11O2N of this compound. The structure of other compounds (3b-f) was also proved by the same manner.
The reaction conditions however were depended upon aromatic substituents (3a-f). Reflux time for completion of reaction varied between 6-8 h. A dilute solution of hydrochloric acid in water was best suitable for reprecipitation of synthesized compounds. The complete structure was elucidated on the basis of physical, chemical and spectral studies. The physical and spectral data are presented in Tables 1 and 2.
| Compound no. | Substitution | Molecular formula (Mol. Wt.) | Meltingpoint (Reported)° | % yield |
|---|---|---|---|---|
| 3a | -H | C13H11O2 N (213) |
181-183 (180-182)8 |
36.14 |
| 3b | p-CH3 | C14H13O2N (227) |
176-178 | 17.05 |
| 3c | p-OCH3 | C14H13O3N (243) |
178-180 | 35.15 |
| 3d | o-CH3 | C14H13O2N (227) |
184-185 | 20.45 |
| 3e | o-OCH3 | C14H12O3N (243) |
174-175 | 16.85 |
| 3f | m-CH3 | C14H13O2N (227) |
132-134 | 21.96 |
Table 1: Physical Characteristics Of Synthesized Compounds (3a-f)
| Compd. number | UV (λmax in nm), (N,N-dimethylformamide as a solvent) | IR in KBr (cm-1) | Mass(M+), (M+1) | 1H NMR (δ ppm value) |
|---|---|---|---|---|
| 3a | 298,388 | 1251 (C-O), 1502 & 1597 (Ar-H), 1672 (C=O), 3400 - 2400 (-OH), 3334 (N-H) | 213,214 | δ 6.692 – 7.353 (m, 9H, Ar-H),δ 7.961 (s, 1H, N-H), δ 9.639 (s, 1H, -COOH) |
| 3b | 298, 390 | 1238 (C-O), 1450 & 1520 (Ar-H), 1693 (C=O), 3300 - 2500 (-OH), 3326 (N-H) | 227, 228 | δ 2.242 (s, 1H, -CH3), δ 6.645 - 7.315 (m, 8H, Ar-H), δ 7.918 (s, 1H, N-H), δ 9.544 (s, 1H, -COOH) |
| 3c | 293, 382 | 1172 (C-O), 1514 & 1587 (Ar-H), 1689 (C=O), 3300 - 2400 (-OH), 3326 (N-H) | 243, 244 | δ 3.735 (s, 3H, -OCH3), δ 6.602 - 7.277 (m, 8H, Ar-H), δ 7.900 (s, 1H, N-H), δ 9.417 (s, 1H, -COOH) |
| 3d | 292, 385 | 1328 (C-O), 1454 & 1641 (Ar-H), 1741 (C=O), 3200 - 2570 (-OH), 3311 (N-H) | 227, 228 | δ 2.176 (s, 3H, -CH3), δ 6.645 - 7.304 (m, 8H, Ar-H), δ 7.930 (s, 1H, N-H), δ 9.449 (s, 1H, -COOH) |
| 3e | 290, 388 | 1244 (C-O), 1610 & 1506 (Ar-H), 1650 (C=O), 3400 – 2450 (-OH), 3355 (N-H) | 243, 244 | δ 3.788 (s, 3H, -OCH3), δ 6.676 - 7.375 (m, 8H, Ar-H), δ 7.937 (s, 1H, N-H), δ 9.591 (s, 1H, -COOH) |
| 3f | 296, 380 | 1164 (C-O), 1454 & 1598 (Ar-H), 1683 (C=O), 3200 - 2450 (-OH), 3336 (N-H) | 227, 228 | δ 2.345 (s, 3H, -CH3), δ 6.702 - 7.351 (m, 8H, Ar-H), δ 8.030 (s, 1H, N-H), δ 9.261 (s, 1H, -COOH) |
Table 2: Spectral Characteristics of Synthesized Compounds (3a-f)
Using 100 mg/kg body weight concentration in N,N-dimethylformamide, the synthesized compounds (3a-f) were tested in vivo for antiinflammatory activity taking N,N-dimethylformamide as a control (Table 3). The result showed that the activity depends upon type and position of the substituents. For example compound 3c is more active among others. It can be concluded from antiinflammatory activity that the Naryl anthranilic acid derivatives showed good activity and may be explored for inflammatory disease.
| Compound no. | Dose (mg/kg body weight) | % inhibition of paw volumeat 60 min interval |
|---|---|---|
| 3a | 100 | 60.01% |
| 3b | 100 | 55.56% |
| 3c | 100 | 68.54% |
| 3d | 100 | 44.44% |
| 3e | 100 | 48.43% |
| 3f | 100 | 51.64% |
| N,N-dimethylformamide (Control) | 100 | -------- |
Table 3: Antiinflammatory Activity Of Synthesized Compounds (3a-f)
Acknowledgements
We thank Mr. D. R. Shah (Director, Maliba Pharmacy College, Surat) for providing laboratory facility and chemical reagents. We also thank Dr. M. T. Chhabria (Assistant Professor, L. M. College of Pharmacy, Ahmedabad) for providing FTIR spectra of the synthesized compounds. We are grateful to Central Drug Research Institute, Lucknow for providing facility of 1H NMR and mass spectra.
References
- Cioli, V., Putzolu, S., Rossi, V. and Carrondine, C., Toxicol. Appl. Pharmacol., 1979, 50, 283.
- Robert, T.S. and Ronald, J.V., Amer. J. Med., 1989, 86, 449.
- Allan, H.P. and Fletcher, M., Drugs, 1990, 50, 1.
- Tammata, V.K., Narutkar, M.M., Crinder, A.M. and Khan, M.A., Pharma. Res., 1993, 10, 1191.
- Furukawa, S. and Oshitak, M., Jpn. Kokai Tokyo Koyo, Jp., 1987, 62, 71, through Chem. Abstr., 1987, 107, 77826j.
- Takehiko, N., Yoshiyasu, F. and Akinobu, N., Eur. Pat. Appli. Ep., 1987, 237, 289, through Chem. Abstr., 1988, 108, 37857j.
- Machon, Z. and Krystyna, W., Acta. Pol. Pharma., 1985, 42, 516.
- Mann, F.G. and Saunders, B.C., Practical Organic Chemistry, 4th edition, new impression, 303.
- Collier, H.O.J., Dinnen, L.C. and Johnsan, C.A., Brit. J. Pharmacol. Chemother., 1968, 32, 295.
- Ghosh, M.N., Editors, In; Toxicology study in fundamental Pharmacology , Scientific Book Agency, Calcutta, 1984, 320.
- Penn, G.B. and Ashford, A. J. Pharm. Pharmacol., 1963, 15, 798.
- Kulkarni, S.K., In; Handbook of Experimental Pharmacology, Vallabh Prakashan, Delhi, 1987, 65.
- Rathod, I.S. and Baheti, K.G., Indian J. Pharm. Sci., 2005, 67, 593.
- Winter, C.A., Risley, E.A. and Nuss, G.W., Proc. Soc. Exp. Biol. Med., 1962, 111, 544.
- Singh, H. and Gosh, M., J. Pharm. Pharmacol., 1968, 20, 316.
- Vogel, G., In; Drug Discovery and Evaluation, Springer-Verlag, New York, 2002, 725.