- Corresponding Author:
- Shobha R. Desai
Department of Chemistry, S. S. Arts College and T. P. Science Institute, Old P. B. Road, Sankeshwar-591 313
E-mail: dr.shobha.desai@gmail.com
| Date of Submission | 13 April 2010 |
| Date of Revision | 23 July 2011 |
| Date of Acceptance | 13 April 2010 |
| Indian J Pharm Sci, 2011, 73 (4): 478-482 |
Abstract
Various (4-substituted) phenyl-3-β-[(N-benzenesulphonyl/tosyl)-4-(un)substituted anilino]propionylamido-1,3-thiazolidine- 4-ones (3a–x) and 1-β-[(N-benzenesulphonyl/tosyl)-4-(un)substituted anilino]-propionylamido-3-chloro-4-(4- substituted)phenyl-azetidin-2-ones (4a–x) have been synthesised by the cyclocondensation of Schiff bases (2a-x) with thioglycolic acid and chloroacetyl chloride, respectively. The structures of the newly synthesised compounds have been established on the basis of their spectral data and elemental analysis. All compounds were evaluated for antimicrobial activities against Escherichia coli, Bacillus cirroflagellosus, Aspergillus niger and Colletotrichum capsici. Most compounds investigated exhibited significant antifungal activity against Colletotrichum capsici, comparable to that of fluconazole, the standard used.
Keywords
Azetidinones, antimicrobial activity, sulfonamide moiety, thiazolidinones
Survey of the recent literature shows that intensive and indiscriminate use of antibiotics has lead to drug resistant microbial pathogens necessitating the need for the search for new antimicrobial agents with reduced resistance to pathogens and better activity.
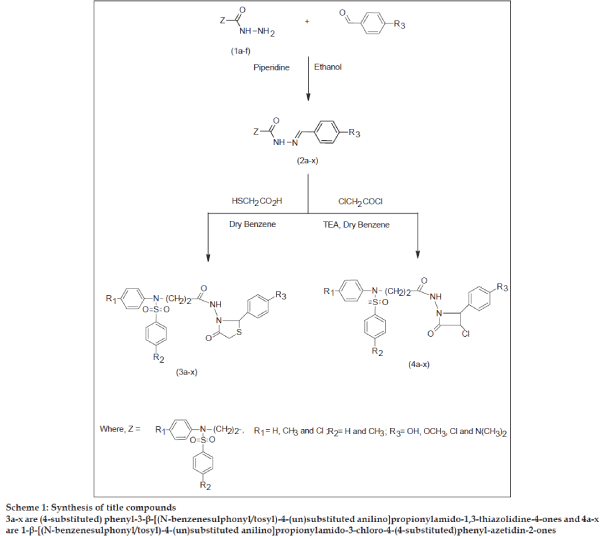
Sulfonamides possess diverse biological and pharmacological activities from antimicrobial[1] to antitumor[2] activities. 4-Thiazolidinones are associated with antibacterial[3] antifungal[4], antitubercular[5] and anticonvulsant[6] activities. β-Lactam compounds e.g., penicillin and cephalosporin are well known antibiotics. Azetidinones are also reported to possess antimicrobial[7], antiinflammatory[8,9], analgesic[9] and CNS depressant[9] activities. Encouraged by these observations, it was contemplated to incorporate the biologically active ‘sulfonamide’ moiety. To enable further evaluation of the potential usefulness of thiazolidinones, azetidinones, and in continuation of our search for nitrogen heterocycles of pharmacological importance[10-13], we report herein the synthesis of some new thiazolidinones (3a-x) and azetidinones (4a-x) with a view to achieve better antimicrobial activity. All the compounds were evaluated for antimicrobial activities against Escherichia coli, Bacillus cirroflagellosus, Aspergillus niger and Colletotrichum capsici. The synthetic route for the title compounds is depicted in Scheme 1.
Scheme 1: Synthesis of title compounds
3a-x are (4-substituted) phenyl-3-β-[(N-benzenesulphonyl/tosyl)-4-(un)substituted anilino]propionylamido-1,3-thiazolidine-4-ones and 4a-x are 1-β-[(N-benzenesulphonyl/tosyl)-4-(un)substituted anilino]propionylamido-3-chloro-4-(4-substituted)phenyl-azetidin-2-ones
In the present investigation, Schiff bases (2a-x), were obtained by refluxing β-[(N-benzenesulphonyl/ tosyl)-4-(un) substituted anilino]propionic acid hydrazides[14] (1a-f), with 4-substituted benzaldehydes in the presence of piperidine. Schiff bases (2a-x) on cyclocondensation with thioglycolic acid in dry benzene, yielded thiazolidinones (3a-x). Azetidinones (4a-x) were obtained by the cyclocondensation of Schiff bases (2a-x) with chloroacetyl chloride in presence of triethyl amine. The structures of the newly synthesised compounds were confirmed by elemental and spectral (IR, 1HNMR and mass) analysis. After establishing the physicochemical analysis, all the compounds were evaluated for their antimicrobial activities.
Melting points were determined in open capillaries and are uncorrected. IR spectra in KBr were recorded on a Perkin Elmer spectrophotometer and 1HNMR spectra on a Varian 300 MHz NMR spectrometer using TMS as an internal standard (chemical shifts in δ ppm). Mass spectra were recorded on Finnegan Mat 8230 spectrometer. The starting β-[(Nbenzenesulphonyl/ tosyl)-4-(un) substituted anilino] propionic acid hydrazides (1a-f) were prepared by the literature method[14]. The observed melting points were consistent with the melting points reported in the literature.
Preparation of 1-β-[(N-benzenesulphonyl)anilino] propionylamido-2-(4-hydroxy) benzylidene hydrazine (2a): A mixture of 1-β-[(N-benzenesulphonyl)anilino] propionic acid hydrazide (1a, 3.19 g, 0.01 mol) and 4-hydroxy benzaldehyde (1.22 g, 0.01 mol) in 30 ml ethanol with 3–4 drops of piperidine was refluxed for 1 h. The solid separated was filtered, washed with cold ethanol, dried and crystallised from ethanol. Various Schiff bases (2b-x) were prepared by adopting the above procedure. Yields and melting points of all the schiff bases are enumerated in Table 1. 2f: IR (KBr) cm−1: 3200 (b), (NH), 2970 (s) (-CH2-), 1610 (s) and 1570 (m) (>C=C< and >C=N-), 1450 (m) (>C-N), 1680 (s) (>C=O) and 1350 (s) (SO2); 2q: IR KBr) cm−1: 3190 (b), (NH), 2930 (s) (-CH2-), 1610 (s) and 1580 (m) (>C=C< and >C=N-), 1450 (m) (>C-N), 1660 (s) (>C=O) and 1340 (s) (SO2). 2f: 1HNMR (DMSO-d6): d 2.20 (s, 3H, CH3), 2.40 (s, 3H, OCH3), 9.60 (s, 1H, -CONH-), 3.0 (t, 2H, CH2), 3.80 (t, 2H, CH2), 8.10 (s, 1H, -N=CH-) and 6.90–7.70 (m, 12H, ArH); 2 k: 1HNMR (DMSO-d6): d 2.50 (s, 3H, CH3), 10.6 (s, 1H, -CONH-), 3.30 (t, 2H, CH2), 3.90 (t, 2H, CH2), 8.90 (s, 1H, -N=CH-) and 6.92–7.85 (m, 13H, ArH).
Preparation of 2-(4-hydroxy)phenyl-3-β-[(Nbenzenesulphonyl) anilino]propionyl amido-1,3,- thiazolidine-4-one (3a):1-β-[(N-benzenesulphonyl) anilino]propionylamido-2-(4-hydroxy)benzylidene hydrazine (2a, 4.23 g, 0.01 mol), thioglycoloic acid (0.9 g, 0.015 mol) in dry benzene was refluxed for 8–10 h. Excess of solvent was removed under reduced pressure. The residue was washed with saturated solution of sodium bicarbonate followed by water. The powder separated was filtered, washed repeatedly with water, dried and crystallized from ethanol.
The above procedure was followed to prepare 2-(4-OH/OCH3/Cl/N,N-dimethyl amino)phenyl-3-β- [(N-benzenesulphonyl/tosyl)-4-(un) substituted anilino] propionylamido-1,3-thiazolidin-4-ones (3b-x). Yield and melting points of other compounds in the same series are given in Table 1.
| Comp.No., | R1 | R2 | R3 | MP (°) | Yield (%) | Comp. No., | R1 | R2 | R3 | MP (°C) | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2a | H | H | OH | 110-12 | 70 | 3m | CH3 | CH3 | OH | 180-81 | 80 |
| 2b | H | H | OCH3 | 153-55 | 65 | 3n | CH3 | CH3 | OCH3 | 164-65 | 75 |
| 2c | H | H | Cl | 184-86 | 68 | 3o | CH3 | CH3 | Cl | 174-75 | 80 |
| 2d | H | H | N(CH3)2 | 228-29 | 69 | 3p | CH3 | CH3 | N(CH3)2 | >300 | 76 |
| 2e | H | CH3 | OH | 132-33 | 68 | 3q | Cl | H | OH | >300 | 80 |
| 2f | H | CH3 | OCH3 | 172-74 | 70 | 3r | Cl | H | OCH3 | 175-176 | 80 |
| 2 g | H | CH3 | Cl | 165-66 | 78 | 3s | Cl | H | Cl | 162-63 | 70 |
| 2h | H | CH3 | N(CH3)2 | 240-41 | 76 | 3t | Cl | H | N(CH3)2 | 230-31 | 80 |
| 2i | CH3 | H | OH | 183-84 | 75 | 3u | Cl | CH3 | OH | 194-95 | 70 |
| 2j | CH3 | H | OCH3 | 168-69 | 78 | 3v | Cl | CH3 | OCH3 | 172-73 | 75 |
| 2 k | CH3 | H | Cl | 185-86 | 80 | 3w | Cl | CH3 | Cl | 110-11 | 80 |
| 2 l | CH3 | H | N(CH3)2 | 210-11 | 79 | 3x | Cl | CH3 | N(CH3)2 | 230-31 | 69 |
| 2m | CH3 | CH3 | OH | 132-33 | 80 | 4a | H | H | OH | 130-32 | 75 |
| 2n | CH3 | CH3 | OCH3 | 158-59 | 75 | 4b | H | H | OCH3 | 125-26 | 65 |
| 2o | CH3 | CH3 | Cl | 178-79 | 80 | 4c | H | H | Cl | 150-51 | 68 |
| 2p | CH3 | CH3 | N(CH3)2 | 190-91 | 76 | 4d | H | H | N(CH3)2 | 159-60 | 75 |
| 2q | Cl | H | OH | 140-41 | 80 | 4e | H | CH3 | OH | 102-03 | 60 |
| 2r | Cl | H | OCH3 | 145-46 | 85 | 4f | H | CH3 | OCH3 | 165-66 | 70 |
| 2s | Cl | H | Cl | 155-56 | 75 | 4 g | H | CH3 | Cl | 156-57 | 70 |
| 2t | Cl | H | N(CH3)2 | 208-09 | 80 | 4h | H | CH3 | N(CH3)2 | 163-64 | 75 |
| 2u | Cl | CH3 | OH | 105-06 | 80 | 4i | CH3 | H | OH | 98-99 | 70 |
| 2v | Cl | CH3 | OCH3 | 92-93 | 75 | 4j | CH3 | H | OCH3 | 103-04 | 75 |
| 2w | Cl | CH3 | Cl | 148-49 | 80 | 4 k | CH3 | H | Cl | 168-69 | 75 |
| 2x | Cl | CH3 | N(CH3)2 | 200-01 | 69 | 4 l | CH3 | H | N(CH3)2 | 189-90 | 75 |
| 3a | H | H | OH | 168-69 | 75 | 4m | CH3 | CH3 | OH | 115-16 | 80 |
| 3b | H | H | OCH3 | 165-66 | 65 | 4n | CH3 | CH3 | OCH3 | 138-39 | 75 |
| 3c | H | H | Cl | 176-77 | 68 | 4o | CH3 | CH3 | Cl | 150-51 | 80 |
| 3d | H | H | N(CH3)2 | 173-74 | 70 | 4p | CH3 | CH3 | N(CH3)2 | 148-49 | 76 |
| 3e | H | CH3 | OH | 160-61 | 70 | 4q | Cl | H | OH | 120-21 | 80 |
| 3f | H | CH3 | OCH3 | 154-55 | 80 | 4r | Cl | H | OCH3 | 117-18 | 75 |
| 3g | H | CH3 | Cl | 105-06 | 78 | 4s | Cl | H | Cl | 136-37 | 70 |
| 3h | H | CH3 | N(CH3)2 | 175-76 | 76 | 4t | Cl | H | N(CH3)2 | 150-51 | 71 |
| 3i | CH3 | H | OH | 173-74 | 75 | 4u | Cl | CH3 | OH | 134-35 | 75 |
| 3j | CH3 | H | OCH3 | 140-41 | 78 | 4v | Cl | CH3 | OCH3 | 105-06 | 70 |
| 3 k | CH3 | H | Cl | 118-19 | 75 | 4w | Cl | CH3 | Cl | 132-33 | 75 |
| 3 l | CH3 | H | N(CH3)2 | 235-36 | 70 | 4x | Cl | CH3 | N(CH3)2 | 160-61 | 70 |
All the compounds were analysed for C, H and N. The experimental values were within ±0.04% of the calculated value. All the compounds were crystallised from aq ethanol
Table 1: Physicochemical Data
3f: IR (KBr) cm−1: 3220 (b) (NH), 2960 (s) (CH2), 1610 (s) and 1510 (m) (>C=C< and >C=N-), 650 (s) and 700 (sh) (-C-S-C-), 1450 (m) (>C-N), 1720 (m) (>C=O of ring), 1675 (s) (>C=O of side chain) and 1390 (s) of SO2; 3p: 3120 (b) (NH), 2920 (s) (CH2), 1615 (s) and 1450 (m) (>C=C< and >C=N-), 660 (s), 690 (sh) (-C-S-C), 1470 (m) (-C-N), 1670 (w) (>C=O of ring), 1620 (w) (>C=O of side chain) and 1400 (w) (SO2). 3a: 1HNMR: (DMSO-d6): d 10.5 (s, 1H, OH), 9.40 (s, 1H, >CONH), 2.95 (t, 2H, CH2), 3.5 (s, 1H, N-CH), 3.75 (s, 2H, -S-CH2), 3.95 (t, 2H, CH2), 6.95-7.70 (m, 14H, ArH). 3p: MS (m/z, Rel. Abund): 553 (M+, 13), 508 (63), 274 (50), 179 (52), 157 (85), 137 (63), 120 (66) 101 (66) 79 (100) and 23 (31). Physicochemical properties of all the thiazolidinones are enumerated in Table 1.
Preparation of 1-β-[(N-benzenesulphonyl)anilino] propionylamido-3-chloro-4-(4-hydroxy) phenylazetidine- 4-one (4a): To a mixture of 1-β-[(Nbenzenesulphonyl) anilino]propionylamido-2-(4- hydroxy)benzylidene hydrazine (2a, 4.23 g, 0.01 mol) and triethylamine (0.5 g, 0.005 mol) dissolved in dry benzene (80 ml), chloroacetyl chloride (0.569 g, 0.005 mol) in benzene (50 ml) was added drop wise with stirring for 1 h and the reaction mixture was stirred further for 2 h. Triethylamine hydrochloride thus separated was filtered off and the filtrate was concentrated under reduced pressure. Viscous liquid thus obtained was digested with a mixture of n-hexane and diethyl ether (1:1; 3×30 ml). The resulting solid was then digested with ethanol for 1 h, the solution was concentrated, treated with animal charcoal and filtered. On standing crystals of azetidinone were separated. The above procedure was used to prepare remaining azetidinones (4b-x). Yield and melting points of all the azetidinones are enumerated in Table 1. 4f: IR (KBr) cm−1: 3200 (b) (NH), 2985 (s) (CH2), 1610 (s) and 1570 (m) (>C=C< and >C=N), 1450 (m) (>C=N), 1680 (>C=O of ring), 1610 (s) (>C=O of side chain) and 1350 (s) (SO2); 4q: IR (KBr) cm−1: 3190 (b) (NH), 2980 (s) (CH2), 1620 (s) and 1580 (m) (>C=C< and >C=N-), 1450 (m), (-C-N-), 1760 (s) (>C=O of ring), 1670 (s) (>C=O of side chain) and 1350 (w) (SO2). 4b: 1HNMR (DMSO-d6): δ 2.2 (s, 3H, OCH3), 9.80 (s, 1H, CONH), 3.0 (t, 2H, CH2), 3.85 (t, 2H, CH2), 2.60 (d, 1H, Cl-CH-), 4.0 (d, 1H, Cl-C-CH) and 6.80–7.76 (m, 14H, ArH); 4q: 10.5 (s, 1H, OH), 9.40 (s, 1H, CONH), 2.95 (t, 2H, CH2), 3.90 (t, 2H, CH2), 2.60 (d, 1H, Cl-CH-), 4.30 (d, 1H, Cl-C-CH) and 6.9–7.70 (m, 13H, ArH). 4q: MS (m/z, Rel. Abund): 534 (M+, 42), 530 (100), 532 (48), 388 (33), 154 (94), 136 (79), 102 (92), 77 (44), and 39 (15).
Evaluation of antimicrobial activity: All the newly synthesised compounds were evaluated for their antimicrobial activity against Gram negative bacterium Escherichia coli, Gram positive bacterium Bacillus cirroflagellosus and fungi Aspergillus niger and Colletotrichum capsici by cup plate method[15]. Cotrimoxazole (Ciplin DS containing trimethoprim 500 mg and sulphamethoxazole 800 mg) and fluconazole were used as standards and DMF as solvent control. All the test samples and the standards were tested at a concentration of 100 μg. The results are expressed as relative percent inhibitions (with respect to the standard, values given in parenthesis).
Amongst the compounds tested, all the compounds have shown minimal antibacterial activity against Bacillus cirroflagellosus (in the range of 15–30%), except 2 k (57.82) and 2r (48.97). Some of the compounds exhibited moderate to significant antibacterial activity against Escherichia coli., 2 k (100), 2p (67.68), 3e (100), 3g (67.7), 3m (67.7), 3o (79.9), 4 k (93.06), 4o (93.06). Rest of the compounds were moderate with (40–60%). Some of the compounds exhibited moderate to significant antifungal activity against Aspergillus niger, 2b (72.2), 2h (59.84), 2n (72.2), 2q (78.7), 2u (72.2), 2v, 2w and 2x (78.76), 3i (78.7), 4m (78.7), 4v (78.7). Remaining compounds exhibited minimum to moderate activity (25–48%).
Most compounds exhibited significant antifungal activity against Colletotrichum capsici., Amongst all the compounds, 2v has exhibited maximum activity 944.44%. Enumerating in the decreasing order of their activities, 2f, 4e, 4f and 4o (302.02); 3v (299.39); 4n and 4s (261); 3s (242.42); 2c, 2i, 3i, 4c and 4m (206.2); 3b, 4a (188.88); 2l, 2r, 3o and 4g (156.56); 2q, 2u, 3p, 3j and 4v (126.7); 2x, 3a, 3e, 3w and 4K (100). Rest of the compounds exhibited moderate activity (45–75%).
Conversion of hydrazides (1a-f) into schiff bases (2ax), decreased the antifungal activity. No significant change in the antimicrobial activity of thiazolidinones (3a-x). However the conversion of schiff bases into azetidinones (4a-x) enhanced the antifungal activity against Colletotrichum capsici noticeably. From the observations, it is concluded that, most of the compounds exhibited most significant antifungal activity against C. capsici comparable to that of the standard, fluconazole.
Acknowledgements
The authors thank the Chairman, Department of Chemistry, Karnatak University, Dharwad, for providing the necessary facilities. The authors also thank the Heads of RSIC-CDRI, Lucknow, TIFR-Mumbai, RSIC-IIT, Mumbai for spectral analysis. Authors SRD and UVL are thankful to UGC and CSIR respectively for the financial help in the form of FIP and SRF.
References
- Dogruer DS, Urlu S, Onkol T, Ozcelik B, Sahin MF. Synthesis of some pyridazine derivatives carrying urea, thiourea, and sulfonamide moieties and their antimicrobial activity. Turk J Chem 2010; 34:57-65.
- Ghorab MM, Ragab FA, Hamed MM. Design, synthesis and anticancer evaluation of novel tetrahydroquinoline derivatives containing sulfonamide moiety. Eur J Med Chem 2009;44:4211-7.
- Mehta PD, Sengar NP, Subrahmanyam EV, Satyanarayana D. Synthesis and biological activity studies of some thiazolidinones and azetidinones. Indian J Pharm Sci 2006;68:103-6.
- Güzeldemirci NU, Ilhan E, Küçükbasmaci O, SatanaD . Synthesis and antimicrobial evaluation of new 3-alkyl/aryl-2-[((α,α-diphenyl-α-hydroxy)acetyl)hydrazono]-5-methyl-4-thiazolidinones. Arch Pharm Res 2010;33:17-24.
- Pawar RP, Andurkar NM, Vibhute YB. Studies on synthesis and antibacterial activity of some new schiff bases, 4-thiazolidinones and 2-azetidinones. J Indian ChemSoc 1999;76:271.
- Ulusoy N, Ergenç N, Ekinci AC, Özer H. Synthesis and anticonvulsant activity of some new arylidenehydrazides and 4-thiazolidinones. MonatsheftefürChemie 1996;127:1197-202.
- Bhusare SR, Shinde AB, Pawar RP, Vibhute YB. Synthesis and antimicrobial activity of heterocyclic Schiff bases, 4-thiazolidinones and 2-azethidinones. Indian J Pharm Sci 2004;66:228-31.
- Kale R, Shrimali M, Bhalla TN, Barthwal JP. Synthesis and anti-inflammatory activity of indolylazetidinones. Indian J Pharm Sci1990;52:129-34.
- Gurupadayya BM, Gopal M, Padmashali B, Manohara YN. Synthesis and pharmacological evaluation of azetidin-2-ones and thiazolidin-4-ones encompassing benzothiazole. Indian J Pharm Sci 2008;70:572-7.
- Laddi UV, Desai SR, Somannavar YS, Bennur RS. Bennur SC. Synthesis, antiinflammatory and biological activities of some new 2-mercapto-5-substituted-1,3,4-oxadiazoles. Indian Drugs 1997;34:666-73.
- Laddi UV, Desai SR, Somannavar YS, Bennur RS, Bennur SC. Antiinflammatory activity of 3-substituted-4-amino-5-piperidino-1,2,4-triazoles. Indian Drugs 1998;35:509-13.
- Laddi UV, Desai SR, Somannavar YS, Bennur RS, Bennur SC. Some new 1,2,4-triazoles as non steroidalantiinflammatory agents. Indian J Chem 1998;37:461-7.
- Laddi UV, Desai SR, Somannavar YS, Bennur RS, Bennur SC. Synthesis, antimicrobial and antitubercular activity of N-bridgehead heterocycles. Indian J Chem 2001;40:828-34.
- Desai SR, Laddi UV, Bennur RS, Bennur SC. Synthesis and Pharmacological Activities of Some New 3-Substituted-4-Amino-5-Mercapto-1,2,4Triazoles. Indian J Pharm Sci 2011;73:115-20.
- Seely HW, Van Demark PJ. Microbes in Action. USA: Freeman W.H and Co.; 1972.